Abstract
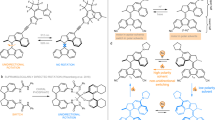
Artificial molecular motors and machines constitute a critical element in the transition from individual molecular motion to the creation of collective dynamic molecular systems and responsive materials. The design of artificial light-driven molecular motors operating with high efficiency and selectivity constitutes an ongoing fundamental challenge. Here we present a highly versatile synthetic approach based on Rieche formylation that boosts the quantum yield of the forward photoisomerization reaction while reaching near-perfect selectivity in the steps involved in the unidirectional rotary cycle and drastically reducing competing photoreactions. This motor is readily accessible in its enantiopure form and operates with nearly quantitative photoconversions. It can easily be functionalized further and outperforms its direct predecessor as a reconfigurable chiral dopant in cholesteric liquid crystal materials.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The online version of this paper provides Supplementary Information, encompassing supplementary figures, general methods, detailed experimental and analytical data, NMR spectra and SFC chromatograms, as well as all other supporting data for the study. Source data are provided with this paper. All the unprocessed data are available from the corresponding author upon reasonable request. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 2170238 (4st), CCDC 2170239 (Z-1st), CCDC 2170240 (E-1st), CCDC 2264532 (Z-S1st) and CCDC 2264533 (E-S1st). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Kinbara, K. & Aida, T. Toward intelligent molecular machines: directed motions of biological and artificial molecules and assemblies. Chem. Rev. 105, 1377–1400 (2005).
Goodsell, D. S. Bionanotechnology: Lessons from Nature (Wiley, 2004).
Balzani, V., Credi, A., Raymo, F. M. & Stoddart, J. F. Artificial molecular machines. Angew. Chem. Int. Ed. 39, 3348–3391 (2000).
Sauvage, J. P. et al. (eds) Molecular Machines and Motors (Springer, 2001).
Browne, W. R. & Feringa, B. L. Making molecular machines work. Nat. Nanotechnol. 1, 25–35 (2006).
Kassem, S. et al. Artificial molecular motors. Chem. Soc. Rev. 46, 2592–2621 (2017).
Feng, Y. et al. Molecular pumps and motors. J. Am. Chem. Soc. 143, 5569–5591 (2021).
Coskun, A., Banaszak, M., Astumian, R. D., Stoddart, J. F. & Grzybowski, B. A. Great expectations: can artificial molecular machines deliver on their promise? Chem. Soc. Rev. 41, 19–30 (2012).
Brandt, J. R., Salerno, F. & Fuchter, M. J. The added value of small-molecule chirality in technological applications. Nat. Rev. Chem. 1, 0045 (2017).
Koumura, N., Zijistra, R. W. J., Van Delden, R. A., Harada, N. & Feringa, B. L. Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999).
Roke, D., Wezenberg, S. J. & Feringa, B. L. Molecular rotary motors: unidirectional motion around double bonds. Proc. Natl Acad. Sci. USA 115, 9423–9431 (2018).
Kudernac, T. et al. Electrically driven directional motion of a four-wheeled molecule on a metal surface. Nature 479, 208–211 (2011).
Van Delden, R. A. et al. Unidirectional molecular motor on a gold surface. Nature 437, 1337–1340 (2005).
Chen, J., Wezenberg, S. J. & Feringa, B. L. Intramolecular transport of small-molecule cargo in a nanoscale device operated by light. Chem. Commun. 52, 6765–6768 (2016).
Chen, S. et al. Photoactuating artificial muscles of motor amphiphiles as an extracellular matrix mimetic scaffold for mesenchymal stem cells. J. Am. Chem. Soc. 144, 3543–3553 (2022).
Wang, J. & Feringa, B. L. Dynamic control of chiral space. Science 331, 1429–1423 (2011).
Wezenberg, S. J., Croisetu, C. M., Stuart, M. C. A. & Feringa, B. L. Reversible gel–sol photoswitching with an overcrowded alkene-based bis-urea supergelator. Chem. Sci. 7, 4341–4346 (2016).
Chen, J. et al. Artificial muscle-like function from hierarchical supramolecular assembly of photoresponsive molecular motors. Nat. Chem. 10, 132–138 (2018).
Li, Q. et al. Macroscopic contraction of a gel induced by the integrated motion of light-driven molecular motors. Nat. Nanotechnol. 10, 161–165 (2015).
Foy, J. T. et al. Dual-light control of nanomachines that integrate motor and modulator subunits. Nat. Nanotechnol. 12, 540–545 (2017).
Eelkema, R. et al. Nanomotor rotates microscale objects. Nature 440, 163–163 (2006).
Orlova, T. et al. Revolving supramolecular chiral structures powered by light in nanomotor-doped liquid crystals. Nat. Nanotechnol. 13, 304–308 (2018).
Danowski, W. et al. Unidirectional rotary motion in a metal–organic framework. Nat. Nanotechnol. 14, 488–494 (2019).
Castiglioni, F. et al. Modulation of porosity in a solid material enabled by bulk photoisomerization of an overcrowded alkene. Nat. Chem. 12, 595–602 (2020).
Klok, M. et al. MHz unidirectional rotation of molecular rotary motors. J. Am. Chem. Soc. 130, 10484–10485 (2008).
Pollard, M. M., Meetsma, A. & Feringa, B. L. A redesign of light-driven rotary molecular motors. Org. Biomol. Chem. 6, 507–512 (2008).
Ruangsupapichat, N., Pollard, M. M., Harutyunyan, S. R. & Feringa, B. L. Reversing the direction in a light-driven rotary molecular motor. Nat. Chem. 3, 53–60 (2011).
Sheng, J. et al. Designing P-type bi-stable overcrowded alkene-based chiroptical photoswitches. Chem. Sci. 14, 4328–4336 (2023).
Qu, D. H. & Feringa, B. L. Controlling molecular rotary motion with a self-complexing lock. Angew. Chem. Int. Ed. 49, 1107–1110 (2010).
Faulkner, A., Van Leeuwen, T., Feringa, B. L. & Wezenberg, S. J. Allosteric regulation of the rotational speed in a light-driven molecular motor. J. Am. Chem. Soc. 138, 13597–13603 (2016).
Geertsema, E. M., Van Der Molen, S. J., Martens, M. & Feringa, B. L. Optimizing rotary processes in synthetic molecular motors. Proc. Natl Acad. Sci. USA 106, 16919–16924 (2009).
Klok, M., Browne, W. R. & Feringa, B. L. Kinetic analysis of the rotation rate of light-driven unidirectional molecular motors. Phys. Chem. Chem. Phys. 11, 9124–9131 (2009).
Pooler, D. R. S., Lubbe, A. S., Crespi, S. & Feringa, B. L. Designing light-driven rotary molecular motors. Chem. Sci. 12, 14964–14986 (2021).
García-López, V., Liu, D. & Tour, J. M. Light-activated organic molecular motors and their applications. Chem. Rev. 120, 79–124 (2020).
Aprahamian, I. The future of molecular machines. ACS Cent. Sci. 6, 347–358 (2020).
Sheng, J., Pooler, D. R. S. & Feringa, B. L. Enlightening dynamic functions in molecular systems by intrinsically chiral light-driven molecular motors. Chem. Soc. Rev. 52, 5875–5891 (2023).
Conyard, J. et al. Ultrafast dynamics in the power stroke of a molecular rotary motor. Nat. Chem. 4, 547–551 (2012).
Conyard, J., Cnossen, A., Browne, W. R., Feringa, B. L. & Meech, S. R. Chemically optimizing operational efficiency of molecular rotary motors. J. Am. Chem. Soc. 136, 9692–9700 (2014).
Wiley, T. E., Konar, A., Miller, N. A., Spears, K. G. & Sension, R. J. Primed for efficient motion: ultrafast excited state dynamics and optical manipulation of a four stage rotary molecular motor. J. Phys. Chem. A 122, 7548–7558 (2018).
Greb, L. & Lehn, J. M. Light-driven molecular motors: imines as four-step or two-step unidirectional rotors. J. Am. Chem. Soc. 136, 13114–13117 (2014).
Roke, D., Sen, M., Danowski, W., Wezenberg, S. J. & Feringa, B. L. Visible-light-driven tunable molecular motors based on oxindole. J. Am. Chem. Soc. 141, 7622–7627 (2019).
Huber, L. A. et al. Direct observation of hemithioindigo-motor unidirectionality. Angew. Chem. Int. Ed. 56, 14536–14539 (2017).
Franken, L. E. et al. Solvent mixing to induce molecular motor aggregation into bowl-shaped particles: underlying mechanism, particle nature, and application to control motor behavior. J. Am. Chem. Soc. 140, 7860–7868 (2018).
Chen, S. et al. Dynamic assemblies of molecular motor amphiphiles control macroscopic foam properties. J. Am. Chem. Soc. 142, 10163–10172 (2020).
Zhu, Q. et al. Multistate switching of spin selectivity in electron transport through light‐driven molecular motors. Adv. Sci. 8, 2101773 (2021).
Xu, F. et al. Dynamic control of a multistate chiral supramolecular polymer in water. J. Am. Chem. Soc. 144, 6019–6027 (2022).
Polli, D. et al. Conical intersection dynamics of the primary photoisomerization event in vision. Nature 467, 440–443 (2010).
Strambi, A., Durbeej, B., Ferré, N. & Olivucci, M. Anabaena sensory rhodopsin is a light-driven unidirectional rotor. Proc. Natl Acad. Sci. USA 107, 21322–21326 (2010).
Garcı́a, O., Nicolás, E. & Albericio, F. O-Formylation of electron-rich phenols with dichloromethyl methyl ether and TiCl4. Tetrahedron Lett. 44, 4961–4963 (2003).
Irie, M., Fukaminato, T., Matsuda, K. & Kobatake, S. Photochromism of diarylethene molecules and crystals: memories, switches, and actuators. Chem. Rev. 114, 12174–12277 (2014).
Feringa, B. L. & Browne, W. R. Molecular Switches (Wiley-VCH Verlag, 2011).
Corra, S. et al. Kinetic and energetic insights into the dissipative non-equilibrium operation of an autonomous light-powered supramolecular pump. Nat. Nanotechnol. 17, 746–751 (2022).
Sangchai, T., Al Shehimy, S., Penocchio, E. & Ragazzon, G. Artificial molecular ratchets: tools enabling endergonic processes. Angew. Chem. Int. Ed. 62, e202309501 (2023).
Kazaryan, A., Lan, Z., Schäfer, L. V., Thiel, W. & Filatov, M. Surface hopping excited-state dynamics study of the photoisomerization of a light-driven fluorene molecular rotary motor. J. Chem. Theory Comput. 7, 2189–2199 (2011).
Ryabchun, A. et al. Helix inversion controlled by molecular motors in multistate liquid crystals. Adv. Mater. 32, 2004420 (2020).
de Gennes, P.-G. & Prost, J. The Physics of Liquid Crystals (Clarendon Press, 2013).
Wang, X., Miller, D. S., Bukusoglu, E., De Pablo, J. J. & Abbott, N. L. Topological defects in liquid crystals as templates for molecular self-assembly. Nat. Mater. 15, 106–112 (2016).
Gerber, P. R. On the determination of the cholesteric screw sense by the Grandjean-Cano-method. Z. Naturforsch. 35a, 619–622 (1980).
Acknowledgements
This work was supported by the China Scholarship Council (CSC PhD fellowship no. 201808330459 to J.S.) and financial support from The Netherlands Organization for Scientific Research (NWO-CW), the Dutch Ministry of Education, Culture and Science (Gravitation programme no. 024.001.035). We acknowledge R. Sneep for mass spectral analysis and SFC training. We thank R. Toyoda for the X-ray structure analysis; Q. Zhang for fruitful discussions; and S. Wezenberg, J. de Jong and A. Faulker for work on structurally related motors. W.D. greatly acknowledges G. Ragazzon for the discussion on the kinetic asymmetry of light-driven motors. Correspondence and requests for materials should be sent to Ben Feringa b.l.feringa@rug.nl.
Author information
Authors and Affiliations
Contributions
J.S., W.D. and B.L.F. conceived the project. B.L.F. and W.D. guided and supervised the research. J.S. and W.D. synthesized the compounds. J.S. led the project and carried out all experimental studies and characterizations. S.C. conducted computations. A.S.S. and M.P.D. performed the transient absorption spectroscopy measurements and 1H NMR QY measurements. J.H., A.R. and J.S. performed the LC doping measurements. J.S., W.D., W.J.B. and B.L.F. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Stefano Corra and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–65, Tables 1–10 and Discussion.
Supplementary Data 1
Raw NMR data for Figs. 2c, 4d,g and 5a.
Supplementary Data 2
Crystallographic data for compound 4st; CCDC reference 2170238.
Supplementary Data 3
Crystallographic data for compound Z-1st; CCDC reference 2170239.
Supplementary Data 4
Crystallographic data for compound E-1st; CCDC reference 2170240.
Supplementary Data 5
Crystallographic data for compound Z-S1st; CCDC reference 2264532.
Supplementary Data 6
Crystallographic data for compound E-S1st; CCDC reference 2264533.
Source data
Source Data Fig. 2
Compilation of UV–visible spectra. Source Data Fig. 3 Compilation of UV–visible spectra, time-course photoconversion and histogram of QY along uncertainties of the fit. Source Data Fig. 4 Compilation of UV–visible spectra. Source Data Fig. 5 Compilation of CD spectra.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sheng, J., Danowski, W., Sardjan, A.S. et al. Formylation boosts the performance of light-driven overcrowded alkene-derived rotary molecular motors. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01521-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01521-0