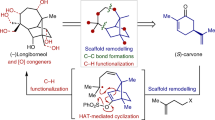
Abstract
Fusicoccane diterpenoids display intriguing biological activities, including the ability to act as modulators of 14-3-3 protein–protein interactions. However, their innate structural complexity and diverse oxygenation patterns present enormous synthetic challenges. Here we develop a modular chemoenzymatic approach that combines de novo skeletal construction and late-stage hybrid C–H oxidations to achieve the synthesis of ten complex fusicoccanes in 8–13 steps each. A convergent fragment coupling strategy allowed rapid access to a key tricyclic intermediate, which was subjected to chemical and enzymatic C–H oxidations to modularly prepare five oxidized family members. We also conceived a complementary biomimetic skeletal remodelling strategy to synthetically access five rearranged fusicoccanes with unusual bridgehead double bonds. This work may facilitate future investigation into the biological activities of the fusicoccanes and also inspire the implementation of similar hybrid strategies to provide family-level synthetic solutions to other natural product scaffolds.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Full experimental details and Supplementary Tables 1–25 are available in the Supplementary Information. Crystallographic data for compound S9 reported in this Article have been deposited at the Cambridge Crystallographic Data Centre under deposition number CCDC 2158016. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
De Boer, A. H. & de Vries-van Leeuwen, I. J. Fusicoccanes: diterpenes with surprising biological functions. Trends Plant Sci. 17, 360–368 (2012).
Ohkanda, J. Fusicoccin: a chemical modulator for 14-3-3 proteins. Chem. Lett. 50, 57–67 (2021).
Sengupta, A., Liriano, J., Bienkiewicz, E. A., Miller, B. G. & Frederich, J. H. Probing the 14-3-3 isoform-specificity profile of protein-protein interactions stabilized by fusicoccin A. ACS Omega 5, 25029–25035 (2020).
Molzan, M. et al. Stabilization of physical RAF/14-3-3 interaction by cotylenin A as treatment strategy for RAS mutant cancers. ACS Chem. Biol. 8, 1869–1875 (2013).
Zheng, D. et al. Cytotoxic fusicoccane-type diterpenoids from Streptomyces violascens isolated from Ailuropoda melanoleuca feces. J. Nat. Prod. 80, 837–844 (2017).
Kim, S., Shin, D.-S., Lee, T. & Oh, K.-B. Periconicins, two new fusicoccane diterpenes produced by an endophytic fungus Periconia sp. with antibacterial activity. J. Nat. Prod. 67, 448–450 (2004).
Stevers, L. M. et al. Modulators of 14-3-3 protein-protein interactions. J. Med. Chem. 61, 3755–3778 (2018).
Ikejiri, F., Honma, Y., Okada, T., Urano, T. & Suzumiya, J. Cotylenin A and tyrosine kinase inhibitors synergistically inhibit the growth of chronic myeloid leukemia cells. Int. J. Oncol. 52, 2061–2068 (2018).
Kasukabe, T., Okabe-Kado, J. & Honma, Y. Cotylenin A, a new differentiation inducer, and rapamycin cooperatively inhibit growth of cancer cells through induction of cyclin G2. Cancer Sci. 99, 1693–1698 (2008).
Asahi, K. et al. Cotylenin A, a plant-growth regulator, induces the differentiation in murine and human myeloid leukemia cells. Biochem. Biophys. Res. Commun. 238, 758–763 (1997).
Anders, C. et al. A semisynthetic fusicoccane stabilizes a protein-protein interaction and enhances the expression of K+ channels at the cell surface. Chem. Biol. 20, 583–593 (2013).
Hu, Z. et al. Fusicoccane-derived diterpenoids from Alternaria brassicicola: investigation of the structure–stability relationship and discovery of an IKKβ inhibitor. Org. Lett. 20, 5198–5202 (2018).
Li, F. et al. Modified fusicoccane-type diterpenoids from Alternaria brassicicola. J. Nat. Prod. 83, 1931–1938 (2020).
Tang, Y. et al. Structural revisions of a class of natural products: scaffolds of aglycon analogues of fusicoccins and cotylenins isolated from fungi. Angew. Chem. Int. Ed. 55, 4069–4073 (2016).
Ohkanda, J. et al. Structural effect of fusicoccin upon upregulation of 14-3-3 phospholigand interaction and cytotoxic activity. Chem. Eur. J. 24, 16066–16071 (2018).
Inoue, T. et al. Semisynthesis and biological evaluation of a cotylenin A mimic derived from fusicoccin A. Bioorg. Med. Chem. Lett. 28, 646–650 (2018).
Ono, Y. et al. Dioxygenases, key enzymes to determine the aglycon structures of fusicoccin and brassicicene, diterpene compounds produced by fungi. J. Am. Chem. Soc. 133, 2548–2555 (2011).
Kato, N., Okamoto, H. & Takeshita, H. Total synthesis of optically active cotylenol, a fungal metabolite having a leaf growth activity. Intramolecular ene reaction for an eight-membered ring formation. Tetrahedron 52, 3921–3932 (1996).
Williams, D. R., Robinson, L. A., Nevill, C. R. & Reddy, J. P. Strategies for the synthesis of fusicoccanes by Nazarov reactions of dolabelladienones: total synthesis of (+)-fusicoauritone. Angew. Chem. Int. Ed. 46, 915–918 (2007).
Uwamori, M., Osada, R., Sugiyama, R., Nagatani, K. & Nakada, M. Enantioselective total synthesis of cotylenin A. J. Am. Chem. Soc. 142, 5556–5561 (2020).
Chen, B. et al. A two-phase approach to fusicoccane synthesis to uncover a compound that reduces tumourigenesis in pancreatic cancer cells. Angew. Chem. Int. Ed. 61, e202117476 (2022).
Wang, Y.-Q., Xu, K., Min, L. & Li, C.-C. Asymmetric total syntheses of hypoestin A, albolic acid, and ceroplastol II. J. Am. Chem. Soc. 144, 10162–10167 (2022).
Sims, N. J., Bonnet, W. C., Lawson, D. M. & Wood, J. L. Enantioselective total synthesis if (+)-alterbrassicicene C. J. Am. Chem. Soc. 145, 37–40 (2023).
Zhang, X. et al. Divergent synthesis of complex diterpenes via a hybrid oxidative approach. Science 369, 799–806 (2020).
Tazawa, A. et al. Total biosynthesis of brassicicenes: identification of a key enzyme for skeletal diversification. Org. Lett. 20, 6178–6182 (2018).
Chakrabarty, S., Wang, Y., Perkins, J. C. & Narayan, A. R. H. Scalable biocatalytic C–H oxyfunctionalization reactions. Chem. Soc. Rev. 49, 8137–8155 (2020).
Fasan, R. Tuning P450 enzymes as oxidation catalysts. ACS Catal. 2, 647–666 (2012).
Lange, G. L., Neider, E. E., Orrom, W. J. & Wallace, D. J. Synthesis of the spirosesquiterpene (–)-acorenone and related cyclopentanoid monoterpenes. Can. J. Chem. 56, 1628–1633 (1978).
Uroos, M., Lewis, W., Blake, A. J. & Hayes, C. J. Total synthesis of (+)-cymbodiacetal: a re-evaluation of the biomimetic route. J. Org. Chem. 75, 8465–8470 (2010).
Fürstner, A. & Shi, N. Nozaki–Hiyama–Kishi reactions catalytic in chromium. J. Am. Chem. Soc. 118, 12349–12357 (1996).
Cope, A. C., Martin, M. M. & McKervey, M. A. Transannular reactions in medium-sized rings. Q. Rev. Chem. Soc. 20, 119–152 (1966).
Kilpatrick, M. & Luborsky, F. E. The conductance and vapor pressure of boron trifluoride in anhydrous hydrofuloric acid. J. Am. Chem. Soc. 76, 5865–5868 (1954).
Vedejs, E., Engler, D. A. & Telschow, J. E. Transition-metal peroxide reactions. Synthesis of α-hydroxycarbonyl compounds from enolates. J. Org. Chem. 43, 188–196 (1978).
Baek, M. et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 373, 871–876 (2021).
Zallot, R., Oberg, N. & Gerlt, J. A. The EFI web resource for genomic enzymology tools: leveraging protein, genome, and metagenome databases to discover novel enzymes and metabolic pathways. Biochemistry 58, 4169–4182 (2019).
Jiang, Y. & Renata, H. Finding superior biocatalysts via homolog screening. Chem Catal. 2, 2471–2480 (2022).
Kille, S. et al. Reducing codon redundancy and screening effort of combinatorial protein libraries created by saturation mutagenesis. ACS Synth. Biol. 2, 83–92 (2013).
Li, F., Deng, H. & Renata, H. Remote B-ring oxidation of sclareol with an engineered P450 facilitates divergent access to complex terpenoids. J. Am. Chem. Soc. 144, 7616–7621 (2022).
Li, J., Li, F., King-Smith, E. & Renata, H. Merging chemoenzymatic and radical-based retrosynthetic logic for rapid and modular synthesis of oxidized meroterpenoids. Nat. Chem. 12, 173–179 (2020).
Li, F. & Renata, H. A chiral-pool-based strategy to access trans-syn-fused drimane meroterpenoids: chemoenzymatic total syntheses of polysin, N-acetyl-polyveoline and the chrodrimanins. J. Am. Chem. Soc. 143, 18280–18286 (2021).
Li, J., Chen, F. & Renata, H. Concise chemoenzymatic synthesis of gedunin. J. Am. Chem. Soc. 144, 19238–19242 (2022).
Yu, J.-Q. & Corey, E. J. Diverse pathways for the palladium(II)-mediated oxidation of olefins by tert-butylhydroperoxide. Org. Lett. 4, 2727–2730 (2002).
Ting, C. P. & Maimone, T. J. Total synthesis of hyperforin. J. Am. Chem. Soc. 137, 10516–10519 (2015).
Ishiyama, S. & Mukaiyama, T. Novel method for the preparation of triethylsilyl peroxides from olefins by the reaction with molecular oxygen and triethylsilane catalyzed by bis(1,3-diketonato)cobalt(II). Chem. Lett. 18, 573–576 (1989).
Crossley, S. W. M., Obradors, C., Martinez, R. M. & Shenvi, R. A. Mn-, Fe-, and Co-catalyzed radical hydrofunctionalizations of olefins. Chem. Rev. 116, 8912–9000 (2016).
Isayama, S. & Mukaiyama, T. A new method for preparation of alcohols from olefins with molecular oxygen and phenylsilane by the use of bis(acetylacetonato)cobalt(II). Chem. Lett. 18, 1071–1074 (1989).
Hong, B., Luo, T. & Lei, X. Late-stage diversification of natural products. ACS Cent. Sci. 6, 622–635 (2020).
Wang, J. et al. Diversity-oriented synthesis of cyclohexenes by combining enzymatic intermolecular Diels-Alder reactions and decarboxylative functionalizations. Chem Catalysis 3, 100451 (2023).
Acknowledgements
We acknowledge funding from the National Institutes of Health grant R35GM128895 (H.R.), the Cancer Prevention and Research Institute of Texas (CPRIT) grant RR220087 (H.R.) and the Sloan Foundation (H.R.). We thank X. Yu for assistance with docking studies. We are grateful to the Shen and Bannister labs at the Wertheim UF Scripps Institute for Biomedical Innovation and Technology, and to the Nicolaou, Kürti and Xiao labs and the Shared Equipment Authority at Rice University for generous access to their reagents and instrumentation. H.R. is a CPRIT scholar in cancer research. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.J. and H.R. conceived the work. Y.J. and H.R. designed all the experiments described in the manuscript. H.R. wrote the manuscript; Y.J. assisted in writing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
Y.J. and H.R. are inventors on a patent application by The Wertheim UF Scripps Institute that covers the method to synthesize cotylenol and related fusicoccanes (patent application no. US 63/374522).
Peer review
Peer review information
Nature Chemistry thanks Jonathan George, Xiaoguang Lei and Hiroki Oguri for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–25, Fig. 1 and General experimental information, including chemical and molecular biology protocols, experimental data and characterization data.
Supplementary Data 1
Crystallographic data for compound S9; CCDC reference 2158016.
Source data
Source Data Fig. 2
Raw data for product ratios and conversions shown in Fig. 2b, and Pymol session file for the docking of compound 20 in MoBsc9, shown in Fig. 2b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Y., Renata, H. Modular chemoenzymatic synthesis of ten fusicoccane diterpenoids. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01533-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01533-w