Abstract
Diets leading to caloric overload are linked to metabolic disorders and reproductive function impairment. Metabolic and hormonal abnormalities stand out as defining features of metabolic disorders, and substantially affect the functionality of the testis. Metabolic disorders induce testicular metabolic dysfunction, chronic inflammation and oxidative stress. The disruption of gastrointestinal, pancreatic, adipose tissue and testicular hormonal regulation induced by metabolic disorders can also contribute to a state of compromised fertility. In this Review, we will delve into the effects of high-fat diets and metabolic disorders on testicular metabolism and spermatogenesis, which are crucial elements for male reproductive function. Moreover, metabolic disorders have been shown to influence the epigenome of male gametes and might have a potential role in transmitting phenotype traits across generations. However, the existing evidence strongly underscores the unmet need to understand the mechanisms responsible for transgenerational paternal inheritance of male reproductive function impairment related to metabolic disorders. This knowledge could be useful for developing targeted interventions to prevent, counteract, and most of all break the perpetuation chain of male reproductive dysfunction associated with metabolic disorders across generations.
Key points
-
Diets rich in sugar and fat are associated with metabolic disorders and poor reproductive health.
-
The testis is highly sensitive to the metabolic status of the organism, as metabolic disorders promote metabolic and hormonal testicular dysregulation.
-
Metabolic disorders are associated with testicular fat accumulation, inflammation, and oxidative stress, which might trigger male reproductive dysfunction.
-
Hormonal imbalance stands out as a hallmark of obesity, with the gastrointestinal system and adipose tissue hormones having a pivotal role in testicular metabolism and function.
-
The epigenetic profile of male gametes is responsive to the metabolic state of the organism and is potentially inheritable, in turn predisposing the offspring to metabolic disorders and potentially prompting the transgenerational perpetuation of acquired metabolic disorders.
Similar content being viewed by others
Introduction
In the past few decades, remarkable technological, economic and social progress has substantially altered the lifestyle of modern society. The popularity of motor vehicles and a wide range of services has led to an increase in sedentary lifestyles. Moreover, traditional carbohydrate and fibre-rich diets have been replaced by calorie-dense foods often rich in refined sugars and fats. Regrettably, this trend has contributed to the increase of metabolic disorders such as overweight or obesity, type 2 diabetes, and cardiovascular diseases, which correlate positively with the index of social development1. According to the WHO, the prevalence of worldwide obesity nearly tripled between 1975 and 2016 (ref. 2). Moreover, as this modern lifestyle extended from high-income to middle- and low-income countries, the prevalence of obesity and other metabolic disorders tended to grow exponentially2. However, the swift rise in the incidence of metabolic disorders is not solely attributable to the lifestyle of modern society. A high-calorie diet and a sedentary lifestyle are important factors, but metabolic disorders have a multifactorial aetiology that includes genetic alterations and predisposition. In the past few decades, several genetic mutations have been linked to the development of metabolic disorders. For example, mutations in genes associated with circadian rhythm regulation have been shown to cause a wide range of metabolic abnormalities3. Another example is monogenic obesity, a condition caused by a single defective gene on the autosomes (often related to the regulation of the leptin–melanocortin pathway), which leads to a phenotype of overweight or obesity4. Additionally, various genes associated with the risk of obesity development, commonly known as obesity-related genes, have been identified. These genes encompass the fat mass and obesity gene (FTO), transmembrane protein 18 (TMEM18), glucosamine-6-phosphate isomerase 2 (GNPDA2) and melanocortin 4 receptor (MC4R)5,6.
Alarmingly, the number of children and young adults with metabolic disorders is rapidly increasing, and this escalating trend might yield substantial adverse health consequences in the near future7. Metabolic imbalance during childhood development can result in severe and enduring consequences that persist into adulthood. One often overlooked and silent comorbidity of metabolic disorders is the impairment of the endocrine and reproductive systems. Clinical and experimental evidence shows a clear negative association between obesity and healthy male reproductive function, with ~20% of male subfertility and infertility instances being directly linked to overweight and obesity8,9. The most commonly reported effects of metabolic disorders on male fertility are related to poor sperm parameters. In a study including 483 subfertile couples, overweight men (defined by a BMI >25 kg/m2) had a lower sperm count than normal-weight men10. In support of these findings, results from a study including 1,558 male military recruits reported a negative association between BMI and sperm count11. In a meta-analysis of data from 13,077 men attending fertility counselling, an increased prevalence of oligospermia or azoospermia was reported in men with overweight and obesity12. Additionally, obesity was also associated with asthenozoospermia (low sperm motility)13,14,15. Interestingly, in non-Western countries, where the prevalence of obesity is reduced, the incidence of male infertility is also reduced, providing additional epidemiological evidence of the association between obesity and healthy male reproductive function16.
In addition to the negative consequences for male reproductive health, an abnormal metabolic state can imprint metabolic signatures in male gametes. The epigenetic imprinting induced by metabolic disorders has only just begun to be unveiled, but this topic will most likely be a major area of research and a challenge in the years to come17. Estimates reported that in 2025, more than 300 million people will suffer from metabolic diseases and associated complications18. This evidence led to the disturbing yet crucial question of whether the modern Western lifestyle of fathers could be jeopardizing the health of future generations. As the molecular mechanisms of transgenerational inheritance of metabolic disorders are being unveiled, increasing evidence suggests that protecting the health of future generations will require more than a simple change in the individual lifestyle. Understanding molecular pathways through which metabolic diseases are inherited by the offspring and preventing the predisposition of individuals to develop such disorders is essential.
In this Review, we will delve into the mechanisms through which the systemic metabolic and metabolic-related hormonal status regulate testicular function and spermatogenesis. The effects of high-fat diet consumption and metabolic diseases on male fertility, which is an often overlooked comorbidity, will also be discussed. Additionally, we will discuss novel data concerning the imprinting of metabolic-related epigenetic signatures in spermatozoa, and paternal transgenerational inheritance of metabolic disorders.
Regulation of testicular metabolism
The testes are very specialized organs in which the production of spermatozoa is dependent on the metabolic state of the male individual. The somatic Sertoli cells (SCs) integrate many of these metabolic cues, which are subsequently reflected in spermatogenesis owing to SC functions, such as nutritional support and formation of the blood–testis barrier (BTB)19,20. The BTB has a crucial role in promoting a controlled and immunologically safe environment for the developing germ cells, while also selectively facilitating the passage of solutes and nutrients. SCs are responsible for forming the BTB and providing nutritional support to the developing germ cells. These cells also limit the expansion of the spermatogonia population, with each SC being able to support 30–50 germ cells21,22. In addition to the immunological and mechanical support, SC metabolism has a central role in spermatogenesis. The human germ cell line is characterized by a unique metabolism, in which lactate is used as the preferential energy source, although these cells have glucose transporters (GLUTs) and the molecular machinery for glycolysis23,24. To ensure that germ cells are supplied with the adequate lactate levels necessary for correct development, SCs developed several metabolic strategies to prioritize the production of lactate above any other metabolic pathways. SCs present a “Warburg-like” metabolism25. The high production of lactate by SCs starts with the uptake of glucose from the interstitial space by high-affinity GLUTs, mainly GLUT1 and GLUT3 (ref. 25). Glucose is then preferentially metabolized into pyruvate and subsequently into lactate. Interestingly, the unique metabolism of SCs can be compared with cancer cells, although the high glycolytic rate is not associated with rapid energy production or cellular proliferation. As a matter of fact, the population of human SCs remains roughly the same throughout an individual’s life25. The majority of lactate produced by SCs is necessary to fuel spermatogenesis and, therefore, is exported to the intratubular fluid of seminiferous tubules by the specific proton-linked monocarboxylate transporter 4 (MCT4). From this fluid, developing germ cells obtain lactate mainly through MCT2 and MCT1, which is converted to pyruvate and fuels developing germ cells’ metabolic needs26. SCs also export high amounts of acetate into the intratubular fluid. The exact role of acetate in spermatogenesis remains to be elucidated, but this metabolite has been proposed to be used by germ cells during de novo lipid synthesis, which is in accordance with the high demand for lipids in these cells25.
SCs have a crucial role in the regulation of spermatogenesis, acting as both “nurse” cells and “gatekeepers” of the seminiferous tubules. SCs integrate metabolic and hormonal cues from the human body, which are then translated into the germ cell line. Thus, a complex network is responsible for regulating SCs’ metabolism, which can be adapted based on the individual’s energy state. SCs have high metabolic plasticity, which leads to the continuous production and export of lactate through the metabolism of a wide range of energetic substrates, even under abnormal metabolic conditions25. In a shortage of glucose, SCs can use ketone bodies, fatty acids and glycogen to keep up the metabolic chain and maintain lactate production22. However, SC functions are susceptible to high concentrations of circulating fatty acids, a common feature associated with high-sugar and high-fat diets, as well as with several metabolic disorders.
Effect of high-fat diets and metabolic disorders on testicular metabolism disruption
Results from studies involving human participants have provided evidence that high-fat diets are associated with impaired male reproductive health. The association between dietary fatty acids intake and asthenozoospermia was assessed in 235 age-matched men27. In this study, a high intake of total saturated fatty acids, total trans-fatty acids, palmitic acid, and stearic acid was shown to be positively associated with the incidence of asthenozoospermia27. Interestingly, a high intake of omega-3 polyunsaturated fatty acid, such as docosahexaenoic, was regarded as protective against asthenozoospermia. These results are concordant with those from another study in which higher saturated fat intake was shown to correlate negatively with total sperm count and concentration, but omega-3 polyunsaturated fatty acids intake correlated positively with improved sperm morphology28. Although some evidence comes from human studies, most data found in the literature were obtained using animal models. Nevertheless, compelling evidence from these studies clearly shows the negative effects of high-fat diets on male reproductive health. In a study in which male C57BL/6 mice were fed a high-fat diet for 10 weeks, a decrease in total (43.85 ± 2.35% versus 53.27 ± 3.06% in mice fed a high-fat versus control diet; P < 0.05) and progressive (15.82 ± 1.19% versus 22.32 ± 1.67% in mice fed a high-fat diet versus a control diet; P < 0.05) sperm motility was observed, whereas morphological sperm defects increased (28.17 ± 1.64% versus 18.17 ± 1.74% in mice fed a high-fat diet versus a control diet; P < 0.05)29. Additionally, morphological analysis of the testes showed an atrophic seminiferous epithelium and BTB disruption. Collectively, the fertility of these mice was significantly impaired, as highlighted by low pregnancy rates obtained when these mice were mated to standard-diet-fed females (43.0 ± 3.5% versus 71.1 ± 4.4% in mice fed a high-fat diet versus a standard diet; P < 0.05)29. In support of these results, in another study, a disruption of the BTB and impaired fertility were observed in mice fed a high-fat diet for 8 weeks, which improved when mice were treated with metformin, an antidiabetic drug that reduces body weight gain and improves insulin sensitivity, preventing body weight gain30. Additionally, results from a proteomics study conducted on the testis of C57BL/6 mice fed a high-fat diet reported altered expression of proteins related to BTB integrity, oxidative stress, and lipid homeostasis compared with mice fed a standard diet31. Results from further studies highlighted that the accumulation of lipids and the subsequent lipotoxicity in the testis are associated with alterations in the BTB structure and SC function. In a study in which male C57BL/6 mice were fed a high-fat diet for 16 weeks, decreased sperm concentration was observed, although no alterations in motility were noted32. Additionally, in this study, lipid accumulation and increased reactive oxygen species (ROS) production in the testis were shown, which were associated with decreased SC number owing to increased apoptosis. Further results from in vitro experiments using the SC line TM4 and primary cultures of mouse SCs showed that treatment with high concentrations of palmitic acid (0.1–0.8 mM) induced apoptosis in a time-dependent and dose-dependent manner. Conversely, omega-3 polyunsaturated fatty acids protected SCs against palmitic acid-induced damage. These results suggested that saturated fatty acid accumulation in the testis might cause lipotoxicity to SCs, decreasing sperm quality, whereas omega-3 polyunsaturated fatty acids can be classified as protective against lipotoxicity32. In another study in which male Wistar rats were fed a high-fat diet, decreased sperm concentration and a decreased number of pups per litter were observed33. Additionally, in this study, primary cultures of rat SCs were exposed to palmitic acid (0.5 mM), which promoted lipotoxicity, intracellular lipid accumulation and increased β-oxidation compared with control conditions33. The increase in β-oxidation was associated with increased ROS production and mitochondrial dysfunction, indicated by a decreased mitochondrial maximum respiration, spare respiratory capacity and ATP-coupled respiration in palmitic acid-treated SCs; however, surprisingly, increased glycolytic rates and lactate production were also observed compared with control conditions. Taken together, these results led the authors to postulate that, despite the increased glycolytic rate and lactate production, lipid accumulation might lead to oxidative stress and disrupt SC mitochondrial respiration and ATP production, decreasing SC viability and compromising spermatogenesis. In another study, a different approach was taken to disclose the effects of high-fat diets on testis function and male fertility. A transient high-fat diet (for 60 days post-weaning) was followed by a switch to a standard diet (up to a total of 200 days post-weaning) during pubertal and early adulthood in C57BL6/J mice34. The metabolic syndrome was reversed after the diet switch, and mice lost weight; however, exposure to a high-fat diet during pubertal development resulted in permanent deterioration of sperm parameters such as viability, motility and morphology. Moreover, metabolomics analysis of the testis showed permanent alterations in testicular metabolome, which were not restored through diet switch or weight loss34. In a follow-up study from the same group and using the same model, a permanent alteration in testicular lipid composition, even after diet correction, was reported35. Specifically, saturated fatty acids were the most abundant lipids in the testicular lipidome of the standard-diet-fed mice and mice that transitioned to a standard diet after being fed a high-fat diet. Polyunsaturated fatty acids were the most abundant lipids in the testicular lipidome of mice fed a high-fat diet. Mice in the high-fat diet and transitional diet groups also showed an increased abundance of monosaturated fatty acids, including an accumulation of oleic acid, compared with mice fed a standard diet35. In this study, a strong correlation between the accumulation of pro-inflammatory omega-6 polyunsaturated fatty acids and poor sperm parameters was also reported in mice fed with a high-fat diet. Thus, high-fat diets, particularly during the developmental stages of the testis, were postulated to irreversibly change the testicular lipid content and metabolism, hampering sperm quality during adulthood.
In summary, a diet high in lipid content promotes lipid accumulation in the testis, which can negatively affect testis metabolism, mitochondrial function, and the mechanical function of supporting SCs by disrupting the BTB. Additionally, lipid accumulation is highly associated with inflammation and increased oxidative stress, both of which are known to be primary contributors to male infertility. Diet-induced alterations in sperm could be hypothesized to hinder fertilization by sperm with modified epigenomes, in turn mitigating the adverse effects of high-fat diets on the offspring, but this hypothesis is still speculative and awaiting testing. Hence, meticulous control of lipid intake through diet is imperative for sustaining healthy male reproductive function.
Testicular fat accumulation, inflammation and oxidative stress trigger male reproductive dysfunction
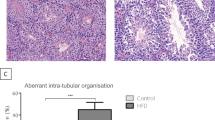
Metabolic disorders cause lipid accumulation in the testis, which can have multiple detrimental effects. An abnormal distribution of scrotal fat can cover the veins and the spermatic cord, impairing testicular thermoregulation and resulting in a rise in scrotal temperature36. This condition can negatively affect the development of germ cells, which are particularly vulnerable to heat stress37. Furthermore, the compression of cord veins by excess fat promotes testicular ischaemia, further impairing reproductive function36. Additionally, obesity and the consequent accumulation of fat in the testis have also been positively associated with altered testicular morphology, including reductions in seminiferous epithelium height, diameter and germ cell proliferation, which ultimately affect spermatogenesis38. Last, increased fat content promotes the accumulation of liposoluble toxic compounds, such as some pesticides that are absorbed through the diet and stored within fat droplets. Some of these compounds are known to be endocrine disruptors (reviewed elsewhere39).
The increased accumulation of fat in the adipose tissue is often accompanied by a state of chronic inflammation (Fig. 1). The adipose tissue is an endocrine organ and, therefore, secretes various inflammatory mediators in response to fat accumulation in adipocytes, predisposing to a pro-inflammatory state40,41. Exacerbated levels of saturated fatty acids, such as palmitic acid, can also bind to cell pattern-recognition receptors, such as the toll-like receptors (TLRs), which are involved in the innate immune system and have a crucial role in the chronic inflammatory state observed in individuals with obesity and type 2 diabetes42. Upon binding to TLRs, saturated fatty acids stimulate the JUN N-terminal kinase (JNK) and the nuclear factor-κB (NF-κB) pathway that promote the expression of inflammatory cytokines genes and macrophage infiltration43. Simultaneously, the JNK pathway is reported to directly inhibit the insulin pathway through the phosphorylation of the insulin receptor’s downstream targets at inhibitory sites44,45. Additionally, both a pro-inflammatory state and the accumulation of fat in the testis are known to promote oxidative stress. Oxidative damage is a substantial contributor to male infertility, as germ cells have limited intracellular antioxidant defences and are highly susceptible to ROS46,47. Results from clinical studies showed that 30–80% of infertile men have increased levels of ROS48,49,50. Oxidative stress is associated with disrupted spermatogenesis and alters sperm physiology, as this condition can lead to reduced sperm concentration, reduced motility, increase in abnormal shapes and DNA damage51,52,53,54. However, ROS have a dual role in spermatozoa function. During human spermatozoa capacitation, mitochondrial activity and ROS production increase substantially55. At controlled levels, ROS are essential for spermatogenesis, sperm function and fertilization, acting as a secondary messenger in several processes, including spermatozoa capacitation, sperm–oocyte fusion, acrosome reaction, and sperm head decondensation55,56,57. Thus, the regulation of ROS in the male reproductive system is currently a topic of intense research, with efforts focused on identifying novel ROS-related molecular targets and compounds that can ameliorate ROS-induced damage58. Some potential molecular targets at the intersection between metabolic disorders and male infertility have been identified, such as mitochondrial uncoupling proteins (UCPs), which have a crucial role in regulating ROS production and mitochondrial oxidative phosphorylation. Dysfunctional or altered UCP function is associated with the onset of metabolic disorders56. In a 2023 study, the expression of UCP1, UCP2 and UCP3 homologues was identified in human spermatozoa. Remarkably, results from this study showed that inhibiting UCPs using a selective inhibitor caused irreversible motility loss by impairing the glycolytic and mitochondrial metabolism in spermatozoa59. This evidence underscores the potential of targeting UCPs to treat male infertility associated with metabolic disorders.
The adipose tissue is an endocrine organ, capable of producing and releasing hormones and cytokines. Fat accumulation promotes the secretion of pro-inflammatory cytokines by adipocytes, which activate resident macrophage and predispose the adipose tissue to a chronic low-grade inflammatory state. Moreover, excessive fat accumulation also triggers the apoptosis of adipocytes, resulting in the increased release of free fatty acids (FFAs) and pro-inflammatory cytokines. Upon adipocyte apoptosis, a large influx of macrophages infiltrates the adipose tissue, exacerbating the inflammatory state and increasing oxidative stress. Increased circulating lipids are a known cause of insulin resistance, which further promotes a systemic inflammatory state and impairs testicular metabolism. With regard to the putative mechanism underlying testicular dysfunction, this phenomenon might be triggered by the increased levels of circulating FFAs, which bind to cell pattern recognition receptors, in turn activating the JUN N-terminal kinase (JNK) and the nuclear factor-κB (NF-κB) pathways. These pathways promote the expression of inflammatory cytokines, which induce macrophage activation and infiltration. Macrophages are known producers of reactive oxygen species (ROS), and macrophage infiltration is associated with oxidative stress. Simultaneously, the JNK pathway inhibits the insulin pathway through the phosphorylation of insulin receptor’s downstream targets at inhibitory sites, leading to insulin resistance and impaired glycolytic metabolism. Germ cells are highly dependent on Sertoli cells’ nutritional support owing to their high glycolytic function and lactate production. Thus, an inflammatory state can compromise the metabolic regulation of spermatogenesis. GLUT, glucose transporter; IRS1, insulin receptor substrate 1; MCT4, monocarboxylate transporter 4.
Overall, the consumption of high-fat diets and the incidence of metabolic diseases lead to an oxidative imbalance. These disorders are multifaceted and can affect every biological system, including endocrine signalling. This disruption of the endocrine system can have cascading effects on the reproductive system, affecting both testicular metabolic and hormonal functions.
Metabolic diseases, gut hormones, and testicular energy homeostasis — a vicious cycle of hormonal dysregulation
Testes are regulated not only metabolically but also hormonally through the hypothalamic–pituitary–gonadal (HPG) axis. Briefly, the gonadotropin-releasing hormone (GnRH) is synthesized and released in a pulsatile fashion by the hypothalamus, which stimulates the anterior pituitary gland to produce and release luteinizing hormone (LH) and follicle-stimulating hormone (FSH)60. Both these hormones regulate the testicular function, although through different targets — LH stimulates steroidogenesis and the release of testosterone by Leydig cells, whereas FSH modulates the function of SCs and spermatogenesis61. In turn, testosterone and inhibin produced by Leydig and SCs, respectively, regulate the activity of the hypothalamus–pituitary gland axis through a negative feedback mechanism62.
The testes respond to an individual’s metabolic status through hormonal regulation. Metabolic disorders severely affect the male reproductive function by disrupting the HPG axis and causing hormonal dysregulation (Fig. 2). This disruption leads to the development of hypogonadism, particularly secondary hypogonadism, which is characterized by low or low–normal levels of gonadotropins, and low levels of circulating testosterone63. Evidence suggests that gut-associated and adipocyte hormones can impair the release of gonadotropins by the hypothalamus–pituitary complex, particularly through metabolic diseases-associated insulin and leptin resistance64. Reduced levels of testosterone also promote increased fatty acid uptake and adiposity. Thus, male obesity and hypogonadism constitute a vicious cycle65.
The accumulation of fat induces hormonal dysregulation and chronic inflammation, which affect the testis both directly and indirectly, through the disruption of the hypothalamus-pituitary-gonads (HPG) axis. The hypothalamus releases gonadotropin-releasing hormone (GnRH), which induces the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by the pituitary gland. LH and FSH exert their function in the testis, in Leydig and Sertoli cells, respectively. In turn, Leydig cells produce testosterone and Sertoli cells release inhibin, which inhibit the hypothalamus–pituitary complex in a negative feedback fashion. Fat accumulation leads to leptin and insulin resistance, which impair the release of gonadotropins by the hypothalamus–pituitary complex and impair the hormonal regulation of the testis. Additionally, elevated levels of leptin and insulin are hypothesized to directly impair testicular function, although the molecular mechanisms are not well characterized. Conversely, fat accumulation decreases the secretion of glucagon-like peptide 1 (GLP1) and ghrelin. Both GLP1 and ghrelin directly regulate the testicular metabolic function, and decreased levels of these hormones impair the nutritional support of spermatogenesis. Overall, the testicular function is severely affected, resulting in impaired gamete production. ROS, reactive oxygen species.
Gut-associated and adipocyte hormones, which regulate food intake and energy balance, have a crucial role in this cycle. Among these hormones, ghrelin, glucagon-like protein 1 (GLP1), insulin and leptin have been shown to be particularly important for testicular hormonal and metabolic regulation. Receptors for these hormones are widely expressed in brain and testicular cells; thus, these hormones can coordinate both the metabolic state of an individual and the energetic demands of the reproductive system (Fig. 3). In a healthy individual, sex steroids, pituitary gland, gut, and adiposity signalling hormones act together to maintain the energetic homeostasis7. Imbalance of gut and adiposity signalling hormones can disrupt this energy balance in the testis, worsening the effect of metabolic disorders on male infertility.
Sertoli cells (SCs) are highly glycolytic, favouring the production of high quantities of lactate — the germ cell line preferential source of energy. For this purpose, SCs are able to orchestrate several metabolic and hormonal cues, indicating that the metabolic and hormonal status of individuals are reflected in their reproductive health. Among the energy-related hormones, gut and adiposity signal hormones are known regulators of SC metabolism, although the signalling pathways involved are not fully characterized. Among the gut hormones, glucagon-like peptide 1 (GLP1) and ghrelin are known regulators of SC function and metabolism. GLP1 functions by binding the GLP1 receptor (GLP1R), which is hypothesized to activate the phosphoinositide 3-kinases–protein kinase B (PI3K–AKT) pathway. Ghrelin acts through the growth hormone secretagogue receptor (also known as ghrelin receptor, GHSR). Ghrelin signalling is mediated by the phospholipase C–inositol triphosphate (PLC–PIP2–IP3) pathway, which stimulates the intracellular release of Ca2+ from the endoplasmic reticulum and the activation of the calcium/calmodulin-dependent protein kinase kinase-β (CaMKKβ). CaMKKβ activates the AMP-activated protein kinase (AMPK). Among the adiposity signal hormones, leptin and insulin are the most widely studied. Leptin acts through the leptin receptor (LepRb), which canonically activates the Janus kinase 2–signal transducer and activator of transcription 3 (JAK2–STAT3) pathway. The migration of STAT3 to the nucleus promotes the expression of oxidative metabolic factors, including those related to glucose and lipid metabolism. However, leptin seems to modulate SC function mainly through the insulin receptor substrate 1 (IRS1)–PI3K–AKT pathway, which is canonically activated by insulin and insulin receptor. All these pathways are hypothesized to promote the translocation of glucose transporters (GLUTs) to the plasma membrane, stimulating the glycolytic rate and controlling the supply of energy substrate for germ cell development. MCT4, monocarboxylate transporter 4; mTOR, mechanistic target of rapamycin; PIP2, phosphatidylinositol 4,5-bisphosphate; TCA, tricarboxylic acid cycle.
Gut hormones
The enteroendocrine cells found in the stomach, pancreas, and small intestine secrete a class of hormones known as gut hormones. Gut hormones control the regulation of food intake and energy balance, and are released in response to the nutritional and metabolic status of the organism66. GLP1 and ghrelin, two gut hormones, have been shown to be particularly important for testis metabolism, by regulating the reproductive system’s energy requirements.
Glucagon-like peptide 1
GLP1 is a gut peptide hormone encoded by the proglucagon gene and secreted into the bloodstream after tissue-specific post-translational processing. GLP1 has been extensively studied for its weight loss and glucose-lowering effects, as this hormone has a prominent role in regulating glucose homeostasis, gastrointestinal motility and appetite67. GLP1 is released by L-cells, a subtype of enteroendocrine cells mainly located in the small intestine, in response to the presence of nutrients, particularly sugars and fats68. For instance, glucose induces GLP1 release by interacting with electrogenic transporters, such as the sodium-glucose transporter 1 (SGLT1), leading to membrane depolarization and hormone release69. GLP1 functions through the GLP1 receptor (GLP1R), which can activate different signalling pathways, including the phosphoinositide 3-kinases (PI3K)–protein kinase B (PKB, also known as AKT), cyclic adenosine monophosphate (cAMP)–protein kinase A (PKA) and mitogen-activated protein kinase (MAPK) pathways, depending on the target cell70,71,72. Within the pancreas, GLP1 triggers insulin secretion while limiting glucagon release through the activation of the cAMP–PKA pathway73,74. Simultaneously, within the hypothalamus, GLP1 fosters the feeling of satiety, leading to a decrease in caloric consumption75. Thanks to these attributes, GLP1 analogues were formulated for treating diabetes and obesity76.
GLP1 has also been suggested to have a role in regulating the male reproductive system, although additional research is needed to fully understand the mechanisms of action. In 2019, GLP1R was first identified in primary cultures of human SCs77. In this study, exposure to GLP1 decreased glucose consumption and increased lactate production, indicating a shift in metabolic activity77. However, the alternative energetic source that fuels lactate production or the signalling pathways that mediate the action of GLP1 remain to be identified in future studies. Additionally, some evidence suggests that GLP1 might have an anti-apoptotic effect on developing germ cells77. In 2020, GLP1R was identified in human spermatozoa78. In this study, human spermatozoa were treated with a GLP1 analogue, exendin-4 (Ex-4), and progressive motility and cholesterol efflux increased after 1 h of incubation, with 300 pM of Ex-4 being the most effective treatment. The beneficial effects of Ex-4 were reversed by incubation with Ex-4 plus H89, a PKA inhibitor, suggesting that Ex-4 acts through GLP1R to regulate capacitation through a PKA-dependent signalling pathway78. Additionally, Ex-4 increased the activity of lactate dehydrogenase (LDH), glucose-6-phosphate dehydrogenase (G6PDH), lipase, and acyl-CoA dehydrogenase, whereas sperm triglyceride content was decreased, suggesting that this hormone might have a lipolytic effect in human spermatozoa78. These findings suggest that spermatozoa are a potential GLP1 target, unveiling the perspective of an additional pharmacological use for this hormone to stimulate in vitro capacitation of spermatozoa.
Ghrelin
Ghrelin, known as the hunger hormone, is a gut peptide with several physiological functions, including regulation of food intake, sleep, body weight and inflammation79. Ghrelin promotes food intake both in rodents and humans and reduces insulin secretion80. However, individuals with obesity tend to have notably lower ghrelin levels than men of normal weight. This evidence supports the idea that reduced ghrelin levels might counteract the uptake of energy81. Ghrelin acts through the growth hormone secretagogue receptor (also known as ghrelin receptor, GHSR). Two isoforms of GHSR have been identified, GHSR1a and GHSR1b, although only GHSR1a seems to be capable of transducing ghrelin signalling82. Ghrelin binding to GHSR1a mainly stimulates the phospholipase C–inositol triphosphate (PLC–IP3) pathway, which induces the intracellular cytoplasmatic release of Ca2+ from the endoplasmic reticulum83. However, the GHSR1a is also able to heterodimerize with other G protein-coupled receptors (GPCR), leading to the activation of diverse signalling pathways according to the counterpart and cell type. For instance, GHSR1a can form a heterodimer with the somatostatin receptor 5 in pancreatic β-cells and prevent insulin release through the suppression of Ca2+ influx and the inhibition of cAMP formation84,85.
Studies to assess the effects of ghrelin on male reproductive function are scarce; however, ghrelin is hypothesized to be capable of modulating testicular hormonal and metabolic function either directly or indirectly through the regulation of the HPG axis in the central nervous system. Results from in vitro studies showed that ghrelin can inhibit the testicular secretion of testosterone and suppress LH and FSH secretion by the pituitary86,87. In the human male reproductive system, ghrelin is expressed in both Leydig cells and SCs but not in germ cells88. In vitro, ghrelin was shown to inhibit the proliferative activity of Leydig cells and immature germ cells, avoiding an excess build-up of germ cells, which is crucial for spermatogonia survival89,90. In a 2016 study, the existence of GHSR in primary cultures of human SCs was confirmed, and ghrelin was shown to have a role in modulating the metabolism of these cells91. In this study, the concentration of ghrelin was chosen based on those found in healthy men (100 pM) and men with obesity (20 pM). In primary cultures of human SCs, exposure to 100 pM ghrelin decreased glucose consumption (13.0 ± 1.6 pmol/cell versus 15.9 ± 3.6 pmol/cell, P < 0.05), pyruvate consumption (19.8 ± 3.0 pmol/cell versus 26.6 ± 1.6 pmol/cell, P < 0.05), and acetate production (0.8 ± 0.1 pmol/cell versus 0.9 ± 0.1 pmol/cell, P < 0.05) compared with exposure to the 20 pM concentration. However, lactate production was not modified, owing to the increased LDH activity observed in SCs exposed to 100 pM versus 20 pM ghrelin (14.87 ± 1.57 versus 8.28 ± 1.50 nmol/min/mg of protein, P < 0.05, respectively). Based on these findings, these authors proposed that ghrelin might serve as an energy sensor for human SCs in a dose-dependent manner, but further studies are needed to fully understand the implications of these results. To date, research on the expression of GHSR or the effects of ghrelin on human spermatozoa has been scarce. Evidence suggests that ghrelin might promote capacitation in rat spermatozoa, as GHSR1a expression was reported in the acrosome region of rat spermatids and epidydimal spermatozoa92. In the same study, treatment with 1 μM ghrelin raised intracellular Ca2+ levels and enhanced progressive motility, indicating a possible involvement in sperm capacitation and acrosome reaction92. However, future research is needed to assess whether these findings in animal models can also apply to humans.
Adiposity signal hormones
Adipose signals, in a similar manner to gut hormones, are recognized regulators of energy balance and food intake. The plasma levels of insulin and leptin correlate positively with both body weight and adipose tissue mass. Thus, a consensus that these hormones meet the criteria for potential adiposity signal hormones exists93. Furthermore, leptin and insulin are crucial for the hormonal and metabolic regulation of the testes.
Insulin
Insulin is an anabolic hormone produced by the pancreatic β-cells, which is primarily responsible for regulating glucose and lipid metabolism. Insulin facilitates glucose entry into cells by promoting the translocation of GLUT4 to the plasma membrane and also promotes glycogenesis and lipogenesis94,95. The action of insulin is mediated by the insulin receptor. Insulin binding to its receptor induces a conformation change and promotes the receptor’s tyrosine kinase activity and auto-phosphorylation, which results in the tyrosine phosphorylation of the insulin receptor substrates (IRS), in turn leading to the activation of the PI3K–AKT and MAPK signalling pathways96,97.
Insulin resistance is a hallmark of metabolic diseases and is strongly associated with the development of these disorders. With regard to obesity, insulin resistance mainly occurs as a consequence of the accumulation of visceral fat and increased circulating free fatty acids, which impair the ability of insulin to stimulate insulin-sensitive tissues98,99. For example, in skeletal muscle and liver, evidence shows that lipid accumulation triggers the activation of the protein kinase C (PKC) pathway, leading to insulin resistance owing to IRS and downstream targets phosphorylation at inhibitory sites100,101,102. However, data on insulin resistance causes and effects in the male reproductive system are scarce. Men with obesity were shown to have increased serum and seminal insulin levels, which positively correlated with poor sperm parameters103. These findings led the authors to hypothesize that elevated insulin levels might be detrimental to the male reproductive system. Insulin has a crucial role in modulating the male reproductive system, both indirectly through the HPG axis and directly in the testis. In the central nervous system, insulin regulates the hypothalamus–pituitary axis in response to the metabolic and energetic state of the organism both directly, by stimulating GnRH-expressing neurons, which are located in the hypothalamus, to release GnRH into the hypophyseal portal capillaries that connect the hypothalamus to the anterior pituitary, and indirectly, by inhibiting the release of neuropeptide Y by neuropeptide Y-expressing neurons, a known inhibitor of GnRH-expressing neurons104. When insulin levels are low or insulin resistance is present, the secretion of GnRH, LH and FSH is compromised. This disruption impairs the HPG axis, substantially affecting the reproductive system’s hormonal control and, consequently, both Sertoli and Leydig cell function105,106,107. With regard to the testis, insulin has a role in testicular development and regulation of sex-determination genes expression108,109. Moreover, insulin mediates the metabolic control of spermatogenesis, both through a direct action on germ cells, and indirectly through the regulation of SCs metabolism94. In SCs, the insulin receptor was first identified in 1987, and was suggested to have specific functions in these cells’ maturation and activity110. This hormone is reported to regulate SC viability, differentiation and metabolism, particularly by stimulating the production of lactate and acetate109,111,112. Results from a study in primary cultures of human SCs cultured in the absence of insulin showed decreased transcript levels of genes associated with lactate metabolism (LDH and MCT4) and decreased glucose consumption and acetate production compared with cells cultured in the presence of insulin (10 mg/ml), suggesting that insulin is essential for the metabolic support of spermatogenesis23,112. The ability of insulin to modulate steroidogenesis has also been studied. In humans, insulin resistance correlates positively with a decrease in testosterone secretion by Leydig cells113. Data from human studies is scarce, but some evidence arises from animal models. In a study in streptozotocin-induced diabetic Wistar rats, decreased LH receptor expression was shown compared with non-diabetic rats114. In another study in which the MA-10 mouse Leydig cell line was used, high insulin concentrations (20–40 nM) inhibited the cAMP signalling pathway and decreased the expression of steroidogenic enzymes, in turn inhibiting testosterone production. These results were confirmed in mice, in which insulin injection (1 U/kg) decreased the expression of steroidogenic enzymes in the testis and impaired steroidogenesis, which was confirmed by the decreased levels of serum and testicular testosterone115. Additionally, results from a study in spontaneously diabetic Torii rats showed that hyperglycaemia correlated positively with hyperplasia of Leydig cells and increased risk of testicular tumour development, whereas insulin treatment decreased the number of hyperplasic cells and increased Leydig cell viability and testosterone production116,117. However, these results still need to be confirmed in humans. Data on the effect of insulin on germ cells in humans are scarce and conflicting, but suggest that insulin promotes human sperm capacitation, motility and acrosome reaction, which are necessary for successful oocyte fertilization118,119. Results from an in vitro study in human spermatozoa showed that the addition of insulin (0.35 ng/ml) to the culture medium improved human spermatozoa total and progressive motility and acrosome reaction119. Conversely, in another study, treatment with insulin at a higher concentration than that used in a previous study106 (10 and 100 μg/ml) did not have any effect on human spermatozoa motility120. These conflicting results indicate that further studies are needed to clarify the effects of insulin on human spermatozoa. Interestingly, some evidence of insulin production by human germ cells exists, although data are conflicting, and the function of this insulin production is poorly understood121,122. Results from a study in which human spermatozoa were observed to release insulin during in vitro capacitation suggest that insulin produced by human spermatozoa might modulate capacitation in an autocrine or paracrine manner, although further studies are required to confirm this hypothesis122.
Leptin
Leptin is a peptide hormone mainly produced by white adipocytes, whose function is to decrease food intake by modulating the feeling of satiety123. Leptin does not induce satiety by itself but through the interaction with other hypothalamic hormone pathways associated with appetite regulation123,124. The action of this hormone is modulated by the leptin receptor (LepR), also known as the obesity receptor125. Among the several isoforms of the LepR identified, the long isoform (LepRb) is considered the main signalling transductor126. This receptor is widely expressed in the human organism and has different effects depending on the target tissue and cell type. The canonical signalling pathway activated by leptin binding to LepR is the Janus kinase 2–signal transducer and activator of transcription 3 (JAK2–STAT3) pathway127.
The development of metabolic disorders is associated with leptin resistance, which results in increased levels of circulating leptin128,129. High levels of leptin in individuals with obesity are thought to be promoted by adipose tissue expansion, as adipocytes are leptin-producing cells130. Besides this regulatory role in energy intake, leptin also modulates the HPG axis both in the hypothalamus and in the testes, which makes this hormone an important mediator between energy reserves, nutritional status and the male reproductive system65. In the rat hypothalamus, leptin administration was shown to induce pulsatile GnRH release in a dose-dependent manner131. Furthermore, leptin has been shown to increase LH and FSH secretion by stimulating the growth and differentiation of pituitary cells regardless of GnRH presence or absence132. Besides the regulation of the hypothalamus-pituitary axis, leptin seems to directly modulate the male reproductive system133,134. In fact, leptin is one of the most widely studied hormones in metabolic disorders-induced male infertility, although the molecular mechanisms underlying the involvement of leptin in this process are poorly understood135,136. Results from animal studies showed that leptin is essential for the reproductive system, as an inactivating mutation of the leptin gene — observed in ob/ob mice, a mouse model of obesity — renders the animals infertile137. Leptin administration rescued the fertility of these animals, indicating causality, whereas fertility was not rescued by giving leptin to mice with LepR-inactivating mutations (db/db mice)138,139. In humans, congenital leptin deficiency is extremely rare. Results from a case report focused on a family with one adult man (22 years old) and one adult woman (34 years old) who suffered from congenital leptin deficiency showed that both male and female individuals were obese and experienced hypogonadism, puberty arrest, and infertility133. In this case, the male homozygous individual never entered puberty and had clinical features of hypogonadism, including lack of facial hair, scanty pubic and axillary hair, bilateral gynecomastia and testicular atrophy. In the same study, treatment with GnRH rescued FSH and LH levels in the man with congenital leptin deficiency, whereas human chorionic gonadotropin (hCG) rescued testosterone levels, further suggesting that leptin has a role in the hypothalamus133. In a follow-up study with the same family, leptin replacement therapy greatly improved the male individual’s body composition, metabolism, and endocrinal and gonadal function140. Interestingly, the observed reproductive effects of leptin do not seem to be tied to the JAK–STAT signalling pathway, which is canonically activated by leptin. Indeed, results from a study in mice with a LepR-specific knockout of STAT3, STAT5, or a combination of both showed that knocking out STAT3 or both STAT3 and STAT5 from LepR-expressing cells, but not STAT5 alone, led to obesity without affecting fertility141. Conversely, mice with specific deletions of PI3K catalytic subunits in LepR-expressing cells showed a lean phenotype but delayed puberty, even after receiving exogenous leptin, suggesting that the effects of leptin on gonadal function are mediated by the activation of the PI3K–AKT pathway142.
Results from clinical human studies have shown that obesity in men is associated with increased circulating levels of leptin, which in turn correlate positively with poor sperm parameters14,103. Interestingly, leptin levels in the seminal fluid are also related to poor sperm parameters. Results from a study including men with asthenozoospermia (n = 79) showed higher leptin concentration in the seminal fluid of these men than in men with normozoospermia (n = 77), although no differences were observed in serum leptin levels in this cohort143. In the same study, seminal leptin levels also correlated negatively with sperm progressive motility and serum total testosterone, suggesting that leptin might have a direct effect in the testis, which is independent of the hypothalamus–pituitary axis143. In a meta-analysis, leptin levels in both serum and seminal fluid of infertile men (n = 1,138) were higher than those observed in fertile men (n = 756). Additionally, a negative correlation between both serum and seminal leptin levels and between sperm counts and motility was reported144. Results from animal studies support these findings, as leptin administration to normal-weight mice or rats led to increased oxidative stress and poor sperm quality, suggesting that high leptin levels are associated with poor male reproductive health and impaired spermatogenesis145,146,147,148,149. Despite compelling evidence suggesting the direct regulatory role of leptin in the testis, the underlying molecular mechanisms remain unclear. Results from a study in the MA-10 mouse Leydig cell line showed that high leptin concentrations (1 μg/ml) impaired the cAMP-dependent expression of the cholesterol transporter steroidogenic acute response protein (StAR) and the limiting steroidogenic enzyme Cyp11a1, suggesting that high leptin levels might impair testosterone production150. Results from the study in which the presence of the LepR was shown for the first time in human SCs showed that 5 ng/ml leptin (the concentration usually detected in normal weight men) increased GLUT2 expression and LDH activity in SCs compared with SCs under control conditions (no leptin)151. However, this effect was not observed with leptin concentrations of 25 and 50 ng/ml, equivalent to circulating levels usually detected in men with obesity. In this study, glucose consumption and lactate production did not show consistent alterations under leptin treatment, but exposure to leptin decreased acetate production in human SCs across all concentrations tested. Further studies are required to clarify the implications of these data, but the presence of leptin seems to be able to directly modulate human SC metabolism151. Evidence from animal studies suggests that exposure to high levels of leptin disrupts the BTB. In a study in which 7-week-old male C57BL/6 mice were treated with leptin (0.1, 0.5, or 3 mg/kg), a dose-dependent disruption of the BTB was observed in SCs, and occurred through the downregulation of tight junction-related proteins145. Additionally, after leptin treatment, a dose-dependent decrease in sperm concentration and motility, and an increased percentage of morphologically abnormal sperm cells were observed, suggesting impaired spermatogenesis145. Consistent with these results, a 48-h treatment with leptin (100 nM, equivalent to 1.6 μg/ml) decreased the expression of tight junction-related proteins in the mouse SCs line TM4. The expression of these proteins was partially rescued by treatment with chemical inhibitors of PI3K and MAPK signalling pathways, and to a lesser extent by using a JAK2 chemical inhibitor, suggesting the involvement of these proteins in the signalling transduction of leptin145. However, future studies are required to further elucidate which signalling pathways mediate the effects of leptin in SCs.
Leptin and LepR were identified in human spermatozoa152. LepR was identified both in the tail of human spermatozoa and — as a soluble isoform — in the seminal fluid. Subsequently, leptin was shown to be mainly expressed in the equatorial region of the head and midpiece in non-capacitated human spermatozoa153. In this study, leptin was also reported to be released during capacitation, being responsible for both cholesterol efflux and protein tyrosine phosphorylation cascade in an autocrine or paracrine fashion. The expression pattern of the leptin receptor suggested that leptin might regulate human spermatozoa motility. However, conflicting results are reported in the literature. In one study in human spermatozoa from normozoospermic donors, leptin (20 nM, or 30.6 ng/ml) improved spermatozoa total and progressive motility, and stimulated acrosome reaction119. Conversely, results from another study in human spermatozoa from normozoospermic men showed no effects on spermatozoa motility, capacitation, or acrosome reaction upon leptin treatment (10–1,000 ng/ml)154. Interestingly, high-motility spermatozoa expressed higher levels of leptin than the low-motility fraction, suggesting that leptin might have a role during oocyte binding and fertilization. Additionally, different from other studies found in the literature, no correlation was observed between leptin or LepR seminal fluid levels and sperm parameters154.
Collectively, these findings indicate that the dysregulation of gut and adiposity signal hormones, often linked with metabolic disorders, can have adverse effects on testicular function and spermatogenesis, which could potentially be a substantial contributor to male infertility. However, data in the literature regarding the molecular mechanisms underlying these effects are conflicting, which might be ascribed to the wide range of leptin concentrations used in these studies. Thus, additional studies focused on leptin effects on the male reproductive tract are required, particularly using physiologically relevant leptin concentrations.
High-fat diets induce epigenetic changes in male gametes
High-fat diets and metabolic diseases have been hypothesized to induce epigenetic alterations in male gametes, which regulate gametes’ function, and might also passively induce the inheritance of metabolic signatures through generations.
Epigenetics refers to mitotically stable molecular mechanisms that govern genomic activity independently of the DNA sequence. Epigenetic changes include both DNA and chromatin alterations that modulate gene expression155,156. These molecular alterations include the DNA methylation of CpG dinucleotides — one of the most well-studied epigenetic markers that are generally stable and long-lasting in somatic cell–histone modifications, alterations in chromatin structure, RNA methylation and the great majority of non-coding RNAs (ncRNAs)157,158. The epigenome is characterized by plasticity, and can undergo modifications in response to environmental stimuli, including metabolic and hormonal cues. These changes can result in phenotypic and gene expression alterations without altering the genetic code159. Thus, the metabolic and hormonal changes associated with high-fat diets and metabolic diseases can be integrated and reflected in the epigenome of every cell in the body.
The mammalian germline is highly regulated by its epigenetic profile160,161,162. An increasing number of studies is highlighting the expression and regulatory function of small RNAs, such as small non-coding RNAs (sncRNAs), PIWI-interacting RNAs, tRNA-derived small RNAs and microRNAs (miRNAs) in the human germline and spermatozoa163,164,165. These small RNAs are known to be involved in the regulation (silencing) of gene expression165. Moreover, spermatozoa have specific epigenetic mechanisms such as the replacement of the majority of DNA-binding histones by protamines166. Protamination induces chromatin packaging, which greatly halts transcription in spermatozoa. As transcription is highly reduced, spermatozoa are hypothesized to rely on epigenetic regulation more than any other cell165. Results from both animal model (with a special focus on mice and rat models) and human studies have suggested that the epigenetic profile of mature spermatozoa might have a crucial role in successful fertilization and embryo development, and alterations in the epigenetic signature have been linked to infertility167,168. For instance, spermatozoa carry both the precursor and the mature form of miR-34c. Injection of a miR-34c inhibitor into zygotes was shown to inhibit DNA synthesis and substantially suppress first cleavage division169. Nevertheless, although evidence suggests that sperm miRNAs contribute to the regulation of gene expression in the early embryo, the full extent of this contribution remains to be elucidated.
Metabolic disorders can induce several epigenetic modifications that can result in impaired spermatogenesis and/or affect spermatozoa function170,171. Consequently, investigating these epigenetic changes has become a crucial area of interest in the field of reproductive biology172. Data on metabolically induced epigenetic alterations in Sertoli and Leydig cells are scarce, but increasing evidence shows that metabolic disorders and high-fat diets induce epigenetic alterations in spermatozoa, which might have implications for embryo development. Specifically, miRNAs of the miR-34 family, which are expressed in human spermatozoa and are important for embryonic development, are sensitive to the metabolic status of the organism169,170. Obesity is also related to altered methylation patterns and expression of sncRNAs in spermatozoa, although histone positioning was found to be similar in spermatozoa from men with obesity (n = 10) and men of normal weight (n = 13) aged 20–40 years old173. During protamination, several epigenetic markers previously present in histones are erased. Regardless, 5–15% of the spermatozoa’s chromatin remains bound to the nucleosome. These chromatin sites are known to be epigenetic hotspots. Interestingly, alterations in CpG hypomethylation were almost exclusively found in protamine-associated DNA, and to a lesser extent in histone-associated regions173. Results from the gene ontology (GO) analysis showed that the specific genome locations that were differently methylated were associated with the term “nervous system development”, and included 274 genes that are appetite regulators, and genes associated with obesity (MC4R, FTO, carbohydrate sulfotransferase 8 (CHST8), and SH2 binding domain-containing protein 1 (SH2B1), among others)173. In this study, bariatric surgery-induced weight loss (n = 6) was shown to lead to the remodelling of sperm DNA methylation prominently at genetic locations implicated in the central control of appetite173. Obesity-related genes, including FTO, MC4R, GNPDA2 and TMEM18, showed altered methylation patterns in spermatozoa from lean individuals compared with individuals with obesity. The same genes were also found to have altered methylation patterns in spermatozoa collected before and after bariatric surgery-induced weight loss173. In another study, FTO, MC4R and GNPDA2 transcripts were shown to be present in the mRNA pool of human spermatozoa, and the abundance of these transcripts was not associated with the BMI of individuals174. This study included a cohort of infertile couples (n = 106) undergoing medically assisted procreation at a fertility clinic. Spermatozoa GNPDA2, MC4R and FTO mRNA abundance were associated with sperm quality, embryo development and pregnancy rates. Specifically, a negative correlation between MC4R mRNA abundance and sperm vitality was reported (Pearson r = −0.3111, P = 0.0027), whereas a positive correlation was found between FTO mRNA abundance and total sperm count (Pearson r = 0.5030, P = 0.0021). A higher abundance of MC4R and GNPDA2 transcripts was also found in spermatozoa from men classified as asthenozoospermic (low sperm total motility percentage) and teratozoospermic (low percentage of morphologically normal sperm), compared with their normozoospermic counterparts. Last, the abundance of FTO mRNA in spermatozoa correlated positively with fertilization rate (Pearson r = 0.4854, P = 0.0065), and other indicators of embryonic development (such as embryo cleavage rate and high-quality embryo rate)174. FTO encodes a demethylase enzyme responsible for the demethylation of both DNA and RNA, interfering with the expression of several genes. Although the role of FTO in spermatozoa or embryo development was not investigated, the reported data suggest that the alteration of the FTO gene methylation pattern in response to obesity could be reasonably associated with a substantial risk of altering the expression of other genes owing to FTO demethylase activity175. In another study, the expression of TMEM18 and GNPDA2 transcripts in primary cultures of human SCs was shown to respond to hormonal cues associated with obesity176. Histone acetylation patterns are also altered in response to the nutritional status. High-fat diet reduced the expression of the deacetylase SIRT6 in mice spermatids, which led to increased levels of acetylated histone H3K9 (ref. 177). Interestingly, in another study in mice, some obesity-induced epigenetic alterations were shown to be partially reverted upon physical exercise intervention, and the insulin sensitivity and adiposity of the mice also improved178. However, other obesity-induced detrimental effects on the testis, such as the accumulation of pro-inflammatory omega-6 polyunsaturated fatty acids, seem to be irreversible even upon weight loss, which is probably related to the fact that sperm quality is not restored by a diet switch34,35.
Some authors argued that a direct effect of the diet on the spermatozoa epigenome would be unlikely because spermatozoa are fully differentiated cells that do not undergo further division and have a protamine-packaged high-order nuclear chromatin and highly reduced transcriptional activity165. In humans, the growth and differentiation of spermatogonia into spermatozoa require ~72–74 days, which is followed by a 2-week epididymal maturation phase179. Thus, diet-induced epigenetic modifications would occur in the developing germ cells within the testis (through enzymes that modulate the epigenome of the DNA and histones) and not mature spermatozoa in the epididymis165. This hypothesis was subsequently refuted with the discovery of epididymosomes and the study of the genetic content of these vesicles. Epididymosomes are extracellular microvesicles present in the epididymal fluid that are transferred to spermatozoa during their maturation phase180. Epididymosomes carry miRNAs that are hypothesized to be involved in diverse regulatory mechanisms not only for spermatozoa but also for epithelial cells located downstream from their release site181,182,183. Thus, the epididymis is an important site in establishing the sperm epigenome184,185, (reviewed elsewhere186). Epididymosome content seems to be influenced by environmental stimuli as well, although the role of diet-induced alterations remains to be explored182,187.
Obesity, as a complex disorder, seems to have the ability to induce substantial epigenetic alterations in spermatozoa during germ cell development, which occurs in the seminiferous tubules within the testis, and also during spermatozoa maturation in the epididymis. The epigenetic content of the male gamete is highly responsive to the metabolic health of the individual, and metabolic disorders-induced epigenetic signatures might persist even upon diet correction and weight loss. Nevertheless, data on epigenetic alterations after diet correction and weight loss is scarce in humans and must be addressed in future studies.
Paternal transgenerational inheritance of metabolic disorders
The inheritance of epigenetic signatures present in the fertilizing spermatozoon has wide-ranging implications for the offspring. These epigenetic markers can affect gene expression and contribute to the development of certain traits and diseases in the offspring188. The findings that the paternal epigenetic signatures could be transmitted to subsequent generations toppled the current theories of evolutionism; the first controversies around these findings emerged with the discovery of the demethylation process of the zygotic paternal genome189,190. The demethylation process that occurs on the paternal pronucleus in the first stages of zygotic development was thought to erase any epigenetic markers carried in the spermatozoon chromatin. Different from the paternal pronucleus, which is rapidly demethylated, methylation levels of the maternal pronucleus are largely maintained during the first stages of embryo development, being gradually demethylated during the second and third cleavage stages189. Currently, a hypomethylation state is known to be necessary for the zygote to obtain a pluripotent stem cell state during its development191. Evidence indicates that methylation levels are reduced by almost 70% during the first phase of demethylation, and are subsequently reduced to a minimum of 8% after the second demethylation cycle192,193. However, the demethylation process that occurs during mammalian embryonic development, is incomplete. Additionally, spermatozoa were hypothesized not to carry any epigenetic factors owing to the small cytoplasmatic volume and protamination. Following meiosis, histones undergo replacement with specialized protamines, a crucial step for achieving a highly compacted chromatin structure194. Thus, all epigenetic modifications were thought to be removed during protamination, ascribing the epigenetic inheritance to the mother195. Currently, ~5–15% of sperm chromatin is known to be nucleosome-bound195,196, which is enriched in imprinted gene clusters, miRNA, and other signalling factors197. Thus, a consensus that spermatozoa epigenetic imprints are transmitted to the oocyte exists198. The extent to which spermatozoa epigenetic imprints influence the metabolic condition of the progeny can be inferred from early development to birth, and even later into adulthood199. If spermatogenesis is affected during embryonic gonadal development and germline differentiation, which constitute a crucial development window, these epigenetic modifications might become permanent in the germ cell line epigenome and can be inherited by future generations200. Furthermore, the occurrence of metabolic disorders at an early age, especially during peak reproductive years, can lead to the transmission of this information to the next generation, perpetuating a chain of early-onset metabolic disorders17 (Fig. 4). In other words, the popular saying “we are what we eat” might be further extended to “we are what we eat and what our ancestors ate”. The exact molecular mechanisms are not yet fully understood, but substantial evidence indicates that the offspring of male individuals with obesity are at an increased risk of developing metabolic disorders. In a 1986 study, 540 adult Danish adoptees, along with adoptive and biological parents, were recruited. The adoptees were divided into four different weight categories (lean, normal weight, overweight and obesity). Results from this study showed that the percentage of biological parents who had overweight or obesity increased proportionally with the weight class attributed to the adoptees. This correlation was observed in both biological mothers (P < 0.001) and fathers (P < 0.04) but not in adoptive mothers. Curiously, an opposite trend was found in adoptive fathers, as the percentage of overweight adoptive fathers decreased with the increasing weight class of the adoptees201. A systematic review of studies including adoptees and twins led to the conclusion that, although family environment has an irrefutable role in the development of childhood obesity, stronger correlations exist between the weight of biological parents and their offspring, supporting the existence of genetic factors associated with obesity development202. Results from studies conducted in animal models have also shown that obesity promoted by the consumption of high-fat diets in F0 animals can severely affect the health of the offspring. The descendants (F1) of male high-fat-diet-fed rats (F0) were reported to have lower birthweight than the offspring of standard-diet-fed rats. F1 descendants of high-fat-diet-fed rats had a growth deficit and were 10% smaller at 6 months of age than F1 descendants from standard-diet-fed rats. These results were consistent with the decreased circulating levels of growth hormone and IGF-1 detected in the serum of F1 high-fat-diet-fed rats203. In 2021, a transgenerational study in mice was conducted to evaluate the effects of the continuous consumption of a high-fat diet post-weaning, and the switch to a standard diet after 60 days of post-weaning high-fat diet consumption204. Obesity in male mice, promoted by the consumption of a high-fat diet, led to considerable alterations in testicular metabolism; specifically, decreased levels of acetate (P < 0.0001) and inosine (P < 0.05) were observed in these mice compared with standard-diet-fed control mice. The diet switch also promoted alterations in the testicular metabolism compared with the standard-diet-fed mice, as shown by the increased glutamine levels (P < 0.01) and decreased levels of acetate (P < 0.0001) in these mice. Interestingly, metabolic changes were detectable in the testicular metabolome of these mice’s F1 and F2 offspring, even though these animals were fed a standard diet throughout their entire life. Specifically, the F1 offspring of the lifelong high-fat-diet-fed F0 male mice had increased testicular levels of leucine (P < 0.01), whereas F2 mice had increased testicular levels of leucine (P < 0.05) and acetate (P < 0.01) compared with the offspring of standard-diet-fed mice (F1 and F2, respectively)204. Furthermore, paternal obesity had a negative effect on sperm quality across several generations. Mice fed a high-fat diet, continuously or undergoing a diet switch, were reported to have decreased sperm motility (P < 0.01) and decreased sperm viability (P < 0.01 and P < 0.001, respectively) compared with the standard-diet-fed mice. No differences were reported between the groups regarding the sperm quality of the F1 offspring. Notably, the F2 offspring of the mice fed a high-fat diet, continuously or submitted to a diet switch, had a significant decrease in total sperm counts (P < 0.01) compared with the F2 offspring of standard-diet-fed mice204. In another study in which the same model was used205, the F2 offspring of mice fed a high-fat diet, including the diet switch group, presented changes in the hormonal reproductive axis, such as increased serum levels of FSH levels, which might be related to the deleterious effects on sperm quality previously reported in these mice. With regard to the testicular content in lipid-related metabolites, lifelong high-fat-diet-fed mice had decreased levels of choline (P < 0.0001) and 3-hydroxyl-butyrate (P < 0.01) compared with standard-diet-fed mice. In mice undergoing a diet switch, only testicular choline levels were decreased (P < 0.05) compared with standard-diet-fed mice. Increased testicular levels of choline (P < 0.05) were observed in the F1 offspring of lifelong high-fat-diet-fed mice compared with standard-diet-fed mice. The F2 offspring of mice fed a high-fat diet, including the diet switch group, were reported to have the greatest differences regarding testicular content in lipid-related metabolites, presenting increased levels of 3-HO-Butyrate (P < 0.05) compared with the F2 offspring of standard-diet-fed mice. The F2 offspring of mice fed a lifelong high-fat diet also presented a decreased testicular phosphocholine level (P < 0.05) compared with the F2 offspring of standard-diet-fed mice205. Taken together, results from both studies suggest that the offspring of high-fat diet-fed mice might have a precondition for alterations in testicular metabolism; however, the implications of these alterations, the molecular mechanisms and the rationale for the fertility impairment in F2 but not in F1 offspring of high-fat diet-fed mice remains to be disclosed in future studies. In another study from the same group using the same animal model, obesity-induced alterations were reported in the sperm miRNA content, and were also traceable in the miRNA content of descendants206. This information is not sufficient to establish that an epigenetic mechanism is responsible for the inheritability of metabolic disorders, but supports the hypothesis that the sperm epigenome has the potential to strongly influence the offspring’s health206.
High-fat diets and metabolic disorders cause hormonal and metabolic alterations in the organism, including epigenetic alterations. These epigenetic alterations also occur in the male gametes, and can arise either in the germ cell line during spermatogenesis, or later during maturation in the epididymis, through epididymosomes. Importantly, the metabolic signature on the epigenome of the father can be imprinted into the offspring, as these epigenetic markers are carried by the spermatozoon and can be imprinted in the embryo upon fertilization, potentially conditioning the health of the offspring towards the development of metabolic disorders. However, available data on this process arise from animal models, and research on the paternal inheritance of metabolic disorders in humans is still in its infancy.
In support of these findings, results from another transgenerational study in mice also led to the conclusion that sperm miRNAs are crucial mediators of the mechanism of metabolic inheritance207. In this study, F0 mice were fed a standard or high-fat diet for 10 weeks. The high-fat-diet-fed mice showed an altered transcriptional profile in the testes (specifically, the expression of 425 mRNAs) compared with the standard-diet-fed mice. Concomitantly, an altered microRNA content and a 25% reduction in global methylation of germ cell DNA were observed in the spermatozoa of high-fat-diet-fed mice207. In another study, transgenerational effects of paternal obesity and prediabetes were assessed in a mouse model208. In this study, Avy/a yellow mice descendent from an isogenic C57BL/6 colony showing an obese phenotype were mated with lean agouti congenic a/a mice. The offspring of fathers with obesity (F1) had an impaired glucose and lipid metabolism, which persisted until the third generation, although largely attenuated in the latter. Additionally, spermatozoa of F1 males showed an altered epigenetic signature, with the largest differences in abundance observed in miRNA and tRNA-derived fragments, further supporting that metabolic phenotypes might be propagated through non-genetic inheritance208. In another study in Sprague–Dawley rats, the newborn offspring of fathers fed a high-fat diet had reduced body weight and pancreatic beta cell mass209. This is not the only study in which a low birthweight of litters from high-fat-diet-fed fathers is shown203. Curiously, adult female but not male from the F1 and F2 offspring showed glucose intolerance and were resistant to high-fat-diet-induced weight gain. Additionally, the epigenome of rat spermatozoa from both fathers with obesity and F1 male offspring showed similar DNA methylation patterns and diet-induced sncRNA expression signatures209. These results provide evidence that non-genetic information can be carried from the spermatozoon to the oocyte, affecting the metabolic phenotype of the offspring. The importance of paternal sncRNAs might not only reside in the modulation of somatic tissues. In fact, ncRNAs seem to be involved in embryological and/or placental development, which could be the reason why litters from high-fat-diet-fed fathers were smaller, even though F0 females had a normal weight203,209. The F1 and F2 offspring resistance to weight gain when exposed to a high-fat diet suggests that adipose tissue plasticity is compromised in these rats. Interestingly, an increased abundance of miRNA let-7c was reported in spermatozoa from F0 fathers fed a high-fat diet and their F1 offspring (P < 0.05) compared with the F0 and F1 of standard-diet-fed rats. The expression of let-7c was also shown to be altered in the metabolic tissues (liver, extensor digitorum longus muscle and white adipose tissue) of F1 and F2 females descendent from high-fat-diet-fed F0 male rats. Collectively, these results suggest that the expression of miRNA let-7c in spermatozoa might participate in the functional remodulation of metabolic tissues. However, why this phenomenon is specific to female descendants but not males is not known209. In a 2022 study, sperm histone H3 lysine 4 tri-methylation (H3K4me3) levels were proposed as a potential metabolic sensor of paternal obesity210. In this study, obesity induced by a high-fat diet for 10–12 weeks promoted an increase in H3K4me3 in spermatozoa from mice of two different strains (C57BL/6NCrl wild type and KDM1A transgenic line). The GO analysis of the DNA regions affected by the increase in H3K4me3 in spermatozoa from F0 animals was concordant with the disturbed metabolic phenotypes found in F0–F2, which included an impaired glycaemic response and altered hepatic transcriptomes. Moreover, the GO analysis showed that sperm H3K4me3 regions affected by obesity overlapped with four-cell embryo (51.8 %) and morula embryo (44.3 %) open chromatin, which could indicate that sperm H3K4me3 might contribute to early embryo development, with a substantial enrichment of genes involved in placenta development. Based on these findings, the authors proposed that sperm H3K4me3 might constitute a component of the non-genetic mechanism linking paternal diet to offspring phenotypes210. In another study, the capacity of the RNAs extracted from the fathers to induce metabolic phenotypes in the progeny was assessed through a different methodology211. RNAs retrieved either from testis or spermatozoa of male mice fed a western-like diet were microinjected into naïve one-cell embryos, which induced the metabolic phenotype observed in the fathers. Among the 22 miRNAs identified as altered in response to the diet, the increased expression of miR19b was highlighted as a potential inducer of the observed metabolic phenotype, as the direct injection of this miRNA in one-cell embryos induced similar metabolic alterations in the progeny211.
Metabolic disorders are highly complex conditions that arise from a combination of genetic, environmental, and epigenetic factors, all of which contribute to an individual’s unique metabolic phenotype. Understanding the intricate molecular pathways involved in the development and inheritance of metabolic disorders is essential to address the alarming increase in the prevalence of these conditions and associated complications. By gaining a deeper understanding of these pathways, we can develop effective preventative measures and treatments to combat the growing prevalence of these conditions. Instances of individuals with overweight or obesity who have normal sperm quality parameters and fertility potential exist and must be considered, as this evidence highlights the complexity of the association between metabolic disorders and male infertility212,213,214.
Conclusions
The testes are highly intricate organs strongly influenced by an individual’s hormonal and metabolic status. Notably, testicular cells are highly responsive to gut hormones, meaning that the diet can have a substantial direct impact on male reproductive health. The broad effects of metabolic disorders on the testes and male reproductive function are well known, but some questions regarding the underlying molecular mechanisms and signalling pathways still remain unanswered. Metabolic disorders are linked to fat accumulation, inflammation and oxidative stress. Simultaneously, metabolic disorders induce hormonal dysregulation, particularly through energy-related hormones. Among these hormones, the gut hormones GLP1 and ghrelin, and the adiposity signal hormones insulin and leptin are important regulators of both the HPG axis — by modulating gonadotropin release by the hypothalamus–pituitary complex — and the testis — through the regulation of Sertoli, Leydig and germ cell function. Taken together, hormonal and metabolic dysregulation induced by metabolic disorders and associated with increased oxidative stress severely compromise male fertility. These processes have been mainly studied using in vitro and animal models. Human studies are scarce, but would be essential to validate the data obtained in preclinical models. For example, the mechanism of action of the energy-related hormones has been largely unexplored, with the exception of some in vitro studies. Regardless of the missing pieces, evidence suggests that metabolic disorders, specifically obesity, promote irreversible alterations of the testicular metabolome and lipidome, with a substantial effect on the metabolism of SCs. Ultimately, these alterations are reflected in a loss of sperm quality, which includes a decrease of total and progressive motility, reduced total sperm counts and the rise of morphological defects. This loss of sperm quality is followed by reduced pregnancy rates, compromising human fertility. As the field of omics continues to evolve, increased insights into these processes can be expected. Multi-omics approaches, including proteomic, lipidomic and metabolomic analyses, will offer valuable data for evaluating metabolically induced alterations in reproductive function. Omics can be used to highlight biomarkers of metabolic disease-induced dysregulation, and also to identify novel therapeutic targets for improving assisted reproductive technology outcomes. By taking advantage of these emerging platforms, an increased understanding of the effect of metabolic disorders on male fertility can be achieved, and effective strategies for managing and treating these conditions can be developed.
The transgenerational inheritance of metabolic diseases is a fairly novel and crucial area of investigation. The growing prevalence of obesity and related health issues in Western societies needs to be addressed, but understanding how these conditions can be passed to future generations is also important, as the development of metabolic disorders can be perpetuated in a never-ending chain. The well-known saying “we are what we eat” takes on new meaning when we consider that our diets can affect the health of our descendants for at least two generations and, therefore, can be extended to “we are what we eat and so will be our progeny”. However, studying the transgenerational inheritance of metabolic diseases through epigenetic alterations is a complex and challenging task. The epigenome is a highly sensitive and finely regulated network of signalling and regulatory mediators that respond to environmental stimuli. Small ncRNAs, methylation patterns and acetylation patterns are just some of the factors that constantly change in response to external factors. Identifying altered epigenetic signatures is essential, but understanding the regulatory functions of these signatures is equally important. Another challenge is that the epigenome of male gametes is modulated not only during spermatogenesis but also by extracellular microvesicles found in the seminiferous tubules, epididymis and seminal fluid. Currently, and to the best of our knowledge, no mechanistic studies have shown how the association between the metabolic and hormonal dysregulation conferred by metabolic disorders induces epigenetic alterations in male gametes. Despite these difficulties, studying the epigenome provides exciting opportunities. Through the characterization and manipulation of the epigenome, innovative assisted reproductive technology protocols could be developed in the future. This approach would enable researchers to discern and select the most suitable spermatozoa for fertilization. Moreover, delving into the epigenetic content of microvesicles provides novel avenues to tackle the escalating challenge of male infertility. In summary, studying the transgenerational inheritance of metabolic disorders through epigenetic alterations poses substantial challenges, but is a crucial area of research that provides exciting opportunities to improve human health.
References
Saklayen, M. G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 20, 12 (2018).
WHO. Obesity and Overweight (WHO, 2021).
Rudic, R. D. et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2, e377 (2004).
Huvenne, H. & Dubern, B. in: Nóbrega, C. & Rodriguez-López, R. (eds) Molecular Mechanisms Underpinning the Development of Obesity 9–21 (Springer International Publishing, 2014).
Leon-Mimila, P. et al. Contribution of common genetic variants to obesity and obesity-related traits in Mexican children and adults. PLoS ONE 8, e70640 (2013).
Hotta, K. et al. Association between obesity and polymorphisms in SEC16B, TMEM18, GNPDA2, BDNF, FAIM2 and MC4R in a Japanese population. J. Hum. Genet. 54, 727–731 (2009).
Oliveira, P. F., Sousa, M., Silva, B. M., Monteiro, M. P. & Alves, M. G. Obesity, energy balance and spermatogenesis. Reproduction 153, R173–R185 (2017).
Liu, Y. & Ding, Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction 154, R123–R131 (2017).
Du Plessis, S. S., Cabler, S., McAlister, D. A., Sabanegh, E. & Agarwal, A. The effect of obesity on sperm disorders and male infertility. Nat. Rev. Urol. 7, 153–161 (2010).
Chavarro, J. E., Toth, T. L., Wright, D. L., Meeker, J. D. & Hauser, R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil. Steril. 93, 2222–2231 (2010).
Jensen, T. K. et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil. Steril. 82, 863–870 (2004).
Sermondade, N. et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum. Reprod. Update 19, 221–231 (2013).
Hammoud, A. O. et al. Male obesity and alteration in sperm parameters. Fertil. Steril. 90, 2222–2225 (2008).
Hofny, E. R. et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil. Steril. 94, 581–584 (2010).
Wang, E. Y., Huang, Y., Du, Q. Y., Yao, G. D. & Sun, Y. P. Body mass index effects sperm quality: a retrospective study in Northern China. Asian J. Androl. 19, 234–237 (2017).
Swan, S. H., Elkin, E. P. & Fenster, L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Env. Health Perspect. 108, 961–966 (2000).
Pereira, S. C., Crisóstomo, L., Sousa, M., Oliveira, P. F. & Alves, M. G. Metabolic diseases affect male reproduction and induce signatures in gametes that may compromise the offspring health. Environ. Epigenet. 6, dvaa019 (2020).
Reuter, S. & Mrowka, R. The metabolic syndrome: the future is now. Acta Physiol. 214, 291–294 (2015).
Mruk, D. D. & Cheng, C. Y. Sertoli–Sertoli and Sertoli–germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 25, 747–806 (2004).
Mruk, D. D. & Cheng, C. Y. The mammalian blood–testis barrier: its biology and regulation. Endocr. Rev. 36, 564–591 (2015).
Weber, J. E., Russell, L. D., Wong, V. & Peterson, R. N. Three-dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli–Sertoli and Sertoli–germ-cell relationships. Am. J. Anat. 167, 163–179 (1983).
Alves, M. G. et al. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell Mol. Life Sci. 70, 777–793 (2013).
Oliveira, P. F. et al. Effect of insulin deprivation on metabolism and metabolism-associated gene transcript levels of in vitro cultured human Sertoli cells. Biochim. Biophys. Acta 1820, 84–89 (2012).
Kokk, K. et al. Immunohistochemical detection of glucose transporters class I subfamily in the mouse, rat and human testis. Medicina 40, 156–160 (2004).
Oliveira, P. F., Martins, A. D., Moreira, A. C., Cheng, C. Y. & Alves, M. G. The Warburg effect revisited — lesson from the Sertoli cell. Med. Res. Rev. 35, 126–151 (2015).
Boussouar, F. & Benahmed, M. Lactate and energy metabolism in male germ cells. Trends Endocrinol. Metab. 15, 345–350 (2004).
Eslamian, G. et al. Dietary fatty acid intakes and asthenozoospermia: a case-control study. Fertil. Steril. 103, 190–198 (2015).
Attaman, J. A. et al. Dietary fat and semen quality among men attending a fertility clinic. Hum. Reprod. 27, 1466–1474 (2012).
Fan, Y. et al. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood–testis barrier. PLoS ONE 10, e0120775 (2015).
Ye, J. et al. Metformin improves fertility in obese males by alleviating oxidative stress-induced blood–testis barrier damage. Oxid. Med. Cell Longev. 2019, 9151067 (2019).
Jarvis, S. et al. High fat diet causes distinct aberrations in the testicular proteome. Int. J. Obes. 44, 1958–1969 (2020).
Hu, X. et al. Effects of saturated palmitic acid and omega-3 polyunsaturated fatty acids on Sertoli cell apoptosis. Syst. Biol. Reprod. Med. 64, 368–380 (2018).
Luo, D. et al. High fat diet impairs spermatogenesis by regulating glucose and lipid metabolism in Sertoli cells. Life Sci. 257, 118028 (2020).
Crisostomo, L. et al. A switch from high-fat to normal diet does not restore sperm quality but prevents metabolic syndrome. Reproduction 158, 377–387 (2019).
Crisostomo, L. et al. Diet during early life defines testicular lipid content and sperm quality in adulthood. Am. J. Physiol. Endocrinol. Metab. 319, E1061–E1073 (2020).
Shafik, A. & Olfat, S. Scrotal lipomatosis. Br. J. Urol. 53, 50–54 (1981).
Zhang, M. et al. Autophagy and apoptosis act as partners to induce germ cell death after heat stress in mice. PLoS ONE 7, e41412 (2012).
Campos-Silva, P., Furriel, A., Costa, W. S., Sampaio, F. J. & Gregorio, B. M. Metabolic and testicular effects of the long-term administration of different high-fat diets in adult rats. Int. Braz. J. Urol. 41, 569–575 (2015).
Moreira, S. et al. Pesticides and male fertility: a dangerous crosstalk. Metabolites 11, 799 (2021).
Coelho, M., Oliveira, T. & Fernandes, R. Biochemistry of adipose tissue: an endocrine organ. Arch. Med. Sci. 9, 191–200 (2013).
Ellulu, M. S., Patimah, I., Khaza’ai, H., Rahmat, A. & Abed, Y. Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci. 13, 851–863 (2017).
Konner, A. C. & Bruning, J. C. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol. Metab. 22, 16–23 (2011).
Hommelberg, P. P., Plat, J., Langen, R. C., Schols, A. M. & Mensink, R. P. Fatty acid-induced NF-κB activation and insulin resistance in skeletal muscle are chain length dependent. Am. J. Physiol. Endocrinol. Metab. 296, E114–E120 (2009).
Hirosumi, J. et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002).
Lee, Y. H., Giraud, J., Davis, R. J. & White, M. F. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J. Biol. Chem. 278, 2896–2902 (2003).
Dutta, S., Sengupta, P., Slama, P. & Roychoudhury, S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int. J. Mol. Sci. 22, 10043 (2021).
Sabeti, P., Pourmasumi, S., Rahiminia, T., Akyash, F. & Talebi, A. R. Etiologies of sperm oxidative stress. Int. J. Reprod. Biomed. 14, 231–240 (2016).
Tremellen, K. Oxidative stress and male infertility — a clinical perspective. Hum. Reprod. Update 14, 243–258 (2008).
Shekarriz, M., Thomas, A. J. Jr. & Agarwal, A. Incidence and level of seminal reactive oxygen species in normal men. Urology 45, 103–107 (1995).
Iwasaki, A. & Gagnon, C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil. Steril. 57, 409–416 (1992).
Agarwal, A., Saleh, R. A. & Bedaiwy, M. A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 79, 829–843 (2003).
Said, T. M. et al. Human sperm superoxide anion generation and correlation with semen quality in patients with male infertility. Fertil. Steril. 82, 871–877 (2004).
Aziz, N. et al. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil. Steril. 81, 349–354 (2004).
Moustafa, M. H. et al. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum. Reprod. 19, 129–138 (2004).
Carrageta, D. F. et al. Mitochondrial activation and reactive oxygen-species overproduction during sperm capacitation are independent of glucose stimuli. Antioxidants 9, 750 (2020).
Monteiro, B. S., Freire-Brito, L., Carrageta, D. F., Oliveira, P. F. & Alves, M. G. Mitochondrial Uncoupling Proteins (UCPs) as key modulators of ROS homeostasis: a crosstalk between diabesity and male infertility? Antioxidants 10, 1746 (2021).
Du Plessis, S. S., Agarwal, A., Halabi, J. & Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 32, 509–520 (2015).
Martin-Hidalgo, D., Bragado, M. J., Batista, A. R., Oliveira, P. F. & Alves, M. G. Antioxidants and male fertility: from molecular studies to clinical evidence. Antioxidants 8, 89 (2019).
Carrageta, D. F. et al. Inhibition of mitochondrial uncoupling proteins arrests human spermatozoa motility without compromising viability. Antioxidants 12, 409 (2023).
Thompson, I. R. & Kaiser, U. B. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol. Cell Endocrinol. 385, 28–35 (2014).
Shah, W. et al. The molecular mechanism of sex hormones on Sertoli cell development and proliferation. Front. Endocrinol. 12, 648141 (2021).
Clavijo, R. I. & Hsiao, W. Update on male reproductive endocrinology. Transl. Androl. Urol. 7, S367–S372 (2018).
Pasquali, R. et al. European Society of Endocrinology Clinical Practice Guideline: endocrine work-up in obesity. Eur. J. Endocrinol. 182, G1–G32 (2020).
Pivonello, R. et al. Metabolic disorders and male hypogonadotropic hypogonadism. Front. Endocrinol. 10, 345 (2019).
Carrageta, D. F., Oliveira, P. F., Alves, M. G. & Monteiro, M. P. Obesity and male hypogonadism: tales of a vicious cycle. Obes. Rev. 20, 1148–1158 (2019).
Monteiro, M. P. & Batterham, R. L. The importance of the gastrointestinal tract in controlling food intake and regulating energy balance. Gastroenterology 152, 1707–1717.e2 (2017).
Muller, T. D. et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 30, 72–130 (2019).
Shah, M. & Vella, A. Effects of GLP-1 on appetite and weight. Rev. Endocr. Metab. Disord. 15, 181–187 (2014).
Reimann, F. et al. Glucose sensing in L cells: a primary cell study. Cell Metab. 8, 532–539 (2008).
Buteau, J. et al. Protein kinase Czeta activation mediates glucagon-like peptide-1-induced pancreatic β-cell proliferation. Diabetes 50, 2237–2243 (2001).
Rowlands, J., Heng, J., Newsholme, P. & Carlessi, R. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front. Endocrinol. 9, 672 (2018).
Smith, N. K., Hackett, T. A., Galli, A. & Flynn, C. R. GLP-1: molecular mechanisms and outcomes of a complex signaling system. Neurochem. Int. 128, 94–105 (2019).
Lester, L. B., Langeberg, L. K. & Scott, J. D. Anchoring of protein kinase A facilitates hormone-mediated insulin secretion. Proc. Natl Acad. Sci. USA 94, 14942–14947 (1997).
Meloni, A. R., DeYoung, M. B., Lowe, C. & Parkes, D. G. GLP-receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes. Metab. 15, 15–27 (2013).
MacDonald, P. E. et al. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 51, S434–S442 (2002).
Hinnen, D. Glucagon-Like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 30, 202–210 (2017).
Martins, A. D. et al. Metabolic dynamics of human Sertoli cells are differentially modulated by physiological and pharmacological concentrations of GLP-1. Toxicol. Appl. Pharmacol. 362, 1–8 (2019).
Rago, V. et al. Human sperm express the receptor for glucagon-like peptide-1 (GLP-1), which affects sperm function and metabolism. Endocrinology 161, bqaa031 (2020).
van der Lely, A. J., Tschop, M., Heiman, M. L. & Ghigo, E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr. Rev. 25, 426–457 (2004).
Wren, A. M. et al. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 86, 5992 (2001).
Tschop, M. et al. Circulating ghrelin levels are decreased in human obesity. Diabetes 50, 707–709 (2001).
Yanagi, S., Sato, T., Kangawa, K. & Nakazato, M. The homeostatic force of ghrelin. Cell Metab. 27, 786–804 (2018).
Hedegaard, M. A. & Holst, B. The complex signaling pathways of the ghrelin receptor. Endocrinology 161, bqaa020 (2020).
Park, S., Jiang, H., Zhang, H. & Smith, R. G. Modification of ghrelin receptor signaling by somatostatin receptor-5 regulates insulin release. Proc. Natl Acad. Sci. USA 109, 19003–19008 (2012).
Dezaki, K., Kakei, M. & Yada, T. Ghrelin uses Gɑi2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet β-cells: novel signal transduction of ghrelin. Diabetes 56, 2319–2327 (2007).
Ishikawa, T., Fujioka, H., Ishimura, T., Takenaka, A. & Fujisawa, M. Ghrelin expression in human testis and serum testosterone level. J. Androl. 28, 320–324 (2007).
Dupont, J., Maillard, V., Coyral-Castel, S., Rame, C. & Froment, P. Ghrelin in female and male reproduction. Int. J. Pept. 2010, 158102 (2010).
Gaytan, F. et al. Expression of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in normal human testis and testicular tumors. J. Clin. Endocrinol. Metab. 89, 400–409 (2004).
Barreiro, M. L. & Tena-Sempere, M. Ghrelin and reproduction: a novel signal linking energy status and fertility? Mol. Cell Endocrinol. 226, 1–9 (2004).
Kheradmand, A., Dezfoulian, O., Alirezaei, M. & Rasoulian, B. Ghrelin modulates testicular germ cells apoptosis and proliferation in adult normal rats. Biochem. Biophys. Res. Commun. 419, 299–304 (2012).
Martins, A. D. et al. Ghrelin acts as energy status sensor of male reproduction by modulating Sertoli cells glycolytic metabolism and mitochondrial bioenergetics. Mol. Cell Endocrinol. 434, 199–209 (2016).
Lukaszyk, A. et al. Expression of ghrelin receptor (GHSR-1a) in rat epididymal spermatozoa and the effects of its activation. Reprod. Biol. 12, 293–300 (2012).
Benoit, S. C., Clegg, D. J., Seeley, R. J. & Woods, S. C. Insulin and leptin as adiposity signals. Recent. Prog. Horm. Res. 59, 267–285 (2004).
Qaid, M. M., Abdelrahman, M. M. & Gonzalez-Redondo, P. Role of insulin and other related hormones in energy metabolism — a review. Cogent Food Agric. 2, 1267691 (2016).
Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2, 282–286 (2001).
White, M. F. & Kahn, C. R. Insulin action at a molecular level — 100 years of progress. Mol. Metab. 52, 101304 (2021).
Boucher, J., Kleinridders, A. & Kahn, C. R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 6, a009191 (2014).
Hardy, O. T., Czech, M. P. & Corvera, S. What causes the insulin resistance underlying obesity? Curr. Opin. Endocrinol. Diabetes Obes. 19, 81–87 (2012).
Barazzoni, R., Gortan Cappellari, G., Ragni, M. & Nisoli, E. Insulin resistance in obesity: an overview of fundamental alterations. Eat. Weight. Disord. 23, 149–157 (2018).
Bruce, C. R. et al. Overexpression of sphingosine kinase 1 prevents ceramide accumulation and ameliorates muscle insulin resistance in high-fat diet-fed mice. Diabetes 61, 3148–3155 (2012).
Szendroedi, J. et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc. Natl Acad. Sci. USA 111, 9597–9602 (2014).
Petersen, M. C. et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Invest. 126, 4361–4371 (2016).
Leisegang, K., Bouic, P. J., Menkveld, R. & Henkel, R. R. Obesity is associated with increased seminal insulin and leptin alongside reduced fertility parameters in a controlled male cohort. Reprod. Biol. Endocrinol. 12, 34 (2014).
Pralong, F. P. Insulin and NPY pathways and the control of GnRH function and puberty onset. Mol. Cell Endocrinol. 324, 82–86 (2010).
Burcelin, R., Thorens, B., Glauser, M., Gaillard, R. C. & Pralong, F. P. Gonadotropin-releasing hormone secretion from hypothalamic neurons: stimulation by insulin and potentiation by leptin. Endocrinology 144, 4484–4491 (2003).
Natah, T. M., Wtwt, M. A., Al-Saadi, H. K., Al-Saadi, A. H. & Farhood, H. F. Study the levels of adiponectin, FSH, LH, and sex hormone in type 2 diabetes (NIIDDM). J. Biol. Agric. Healthc. 3, 81 (2013).
Boukhliq, R., Miller, D. W. & Martin, G. B. Relationship between nutritional stimulation of gonadotrophin secretion and the peripheral and cerebrospinal fluid (CSF) concentrations of glucose and insulin in rams. Anim. Reprod. Sci. 41, 201–214 (1996).
Nef, S. et al. Testis determination requires insulin receptor family function in mice. Nature 426, 291–295 (2003).
Pitetti, J. L. et al. An essential role for insulin and IGF1 receptors in regulating Sertoli cell proliferation, testis size, and FSH action in mice. Mol. Endocrinol. 27, 814–827 (2013).
Oonk, R. B. & Grootegoed, J. A. Identification of insulin receptors on rat Sertoli cells. Mol. Cell Endocrinol. 49, 51–62 (1987).
Escott, G. M., Jacobus, A. P. & Loss, E. S. PI3K-dependent actions of insulin and IGF-I on seminiferous tubules from immature rats. Pflugers Arch. 465, 1497–1505 (2013).
Alves, M. G. et al. In vitro cultured human Sertoli cells secrete high amounts of acetate that is stimulated by 17β-estradiol and suppressed by insulin deprivation. Biochim. Biophys. Acta 1823, 1389–1394 (2012).
Pitteloud, N. et al. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J. Clin. Endocrinol. Metab. 90, 2636–2641 (2005).
Charreau, E. H., Calvo, J. C., Tesone, M., de Souza Valle, L. B. & Baranao, J. L. Insulin regulation of Leydig cell luteinizing hormone receptors. J. Biol. Chem. 253, 2504–2506 (1978).
Ahn, S. W. et al. Insulin directly regulates steroidogenesis via induction of the orphan nuclear receptor DAX-1 in testicular Leydig cells. J. Biol. Chem. 288, 15937–15946 (2013).
Nakane, Y. et al. Hyperglycemia contributes to the development of Leydig cell hyperplasia in male Spontaneously Diabetic Torii rats. J. Toxicol. Pathol. 33, 121–129 (2020).
Leisegang, K. & Henkel, R. The in vitro modulation of steroidogenesis by inflammatory cytokines and insulin in TM3 Leydig cells. Reprod. Biol. Endocrinol. 16, 26 (2018).
Ando, S. & Aquila, S. Arguments raised by the recent discovery that insulin and leptin are expressed in and secreted by human ejaculated spermatozoa. Mol. Cell Endocrinol. 245, 1–6 (2005).
Lampiao, F. & du Plessis, S. S. Insulin and leptin enhance human sperm motility, acrosome reaction and nitric oxide production. Asian J. Androl. 10, 799–807 (2008).
Wang, J. et al. Quantitative phosphoproteomics analysis reveals a key role of insulin growth factor 1 receptor (IGF1R) tyrosine kinase in human sperm capacitation. Mol. Cell Proteom. 14, 1104–1112 (2015).
Aitken, R. J. et al. Evidence that extrapancreatic insulin production is involved in the mediation of sperm survival. Mol. Cell Endocrinol. 526, 111193 (2021).
Aquila, S., Gentile, M., Middea, E., Catalano, S. & Ando, S. Autocrine regulation of insulin secretion in human ejaculated spermatozoa. Endocrinology 146, 552–557 (2005).
Friedman, J. M. & Halaas, J. L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998).
Rogge, G., Jones, D., Hubert, G. W., Lin, Y. & Kuhar, M. J. CART peptides: regulators of body weight, reward and other functions. Nat. Rev. Neurosci. 9, 747–758 (2008).
Tartaglia, L. A. The leptin receptor. J. Biol. Chem. 272, 6093–6096 (1997).
Lee, G. H. et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379, 632–635 (1996).
Evans, M. C., Lord, R. A. & Anderson, G. M. Multiple leptin signalling pathways in the control of metabolism and fertility: a means to different ends? Int. J. Mol. Sci. 22, 9210 (2021).
Frederich, R. C. et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1, 1311–1314 (1995).
Enriori, P. J. et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 5, 181–194 (2007).
Considine, R. V. et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334, 292–295 (1996).
Parent, A. S., Lebrethon, M. C., Gerard, A., Vandersmissen, E. & Bourguignon, J. P. Leptin effects on pulsatile gonadotropin releasing hormone secretion from the adult rat hypothalamus and interaction with cocaine and amphetamine regulated transcript peptide and neuropeptide Y. Regul. Pept. 92, 17–24 (2000).
Tezuka, M. et al. Effects of leptin on gonadotropin secretion in juvenile female rat pituitary cells. Eur. J. Endocrinol. 146, 261–266 (2002).
Strobel, A., Issad, T., Camoin, L., Ozata, M. & Strosberg, A. D. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat. Genet. 18, 213–215 (1998).
O’Rahilly, S. Life without leptin. Nature 392, 330–331 (1998).
Malik, I. A., Durairajanayagam, D. & Singh, H. J. Leptin and its actions on reproduction in males. Asian J. Androl. 21, 296–299, (2019).
Moreira, B. P., Monteiro, M. P., Sousa, M., Oliveira, P. F. & Alves, M. G. Insights into leptin signaling and male reproductive health: the missing link between overweight and subfertility? Biochem. J. 475, 3535–3560 (2018).
Barash, I. A. et al. Leptin is a metabolic signal to the reproductive system. Endocrinology 137, 3144–3147 (1996).
Mounzih, K., Lu, R. & Chehab, F. F. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology 138, 1190–1193 (1997).
Hoffmann, A. et al. Leptin within the subphysiological to physiological range dose dependently improves male reproductive function in an obesity mouse model. Endocrinology 157, 2461–2468 (2016).
Licinio, J. et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc. Natl Acad. Sci. USA 101, 4531–4536 (2004).
Singireddy, A. V., Inglis, M. A., Zuure, W. A., Kim, J. S. & Anderson, G. M. Neither signal transducer and activator of transcription 3 (STAT3) or STAT5 signaling pathways are required for leptin’s effects on fertility in mice. Endocrinology 154, 2434–2445 (2013).
Garcia-Galiano, D. et al. PI3Kɑ inactivation in leptin receptor cells increases leptin sensitivity but disrupts growth and reproduction. JCI Insight 2, e96728 (2017).
Guo, J. et al. Sperm motility inversely correlates with seminal leptin levels in idiopathic asthenozoospermia. Int. J. Clin. Exp. Med. 7, 3550–3555 (2014).
Mo, Y. et al. Leptin levels in serum or semen and its association with male infertility: a meta-analysis with 1138 cases. Int. J. Endocrinol. 2022, 9462683 (2022).
Wang, X., Zhang, X., Hu, L. & Li, H. Exogenous leptin affects sperm parameters and impairs blood testis barrier integrity in adult male mice. Reprod. Biol. Endocrinol. 16, 55 (2018).
Abbasihormozi, S. et al. Relationship of leptin administration with production of reactive oxygen species, sperm DNA fragmentation, sperm parameters and hormone profile in the adult rat. Arch. Gynecol. Obstet. 287, 1241–1249 (2013).
Almabhouh, F. A. et al. Effects of leptin on sperm count and morphology in Sprague‐Dawley rats and their reversibility following a 6‐week recovery period. Andrologia 47, 751–758 (2015).
Haron, M. N., D’Souza, U. J., Jaafar, H., Zakaria, R. & Singh, H. J. Exogenous leptin administration decreases sperm count and increases the fraction of abnormal sperm in adult rats. Fertil. Steril. 93, 322–324 (2010).
Soyupek, S. et al. Leptin expression in the testicular tissue of fertile and infertile men. Arch. Androl. 51, 239–246 (2005).
Landry, D. A., Sormany, F., Hache, J., Roumaud, P. & Martin, L. J. Steroidogenic genes expressions are repressed by high levels of leptin and the JAK/STAT signaling pathway in MA-10 Leydig cells. Mol. Cell Biochem. 433, 79–95 (2017).
Martins, A. D. et al. Leptin modulates human Sertoli cells acetate production and glycolytic profile: a novel mechanism of obesity-induced male infertility? Biochim. Biophys. Acta 1852, 1824–1832 (2015).
Jope, T., Lammert, A., Kratzsch, J., Paasch, U. & Glander, H. J. Leptin and leptin receptor in human seminal plasma and in human spermatozoa. Int. J. Androl. 26, 335–341 (2003).
Aquila, S. et al. Leptin secretion by human ejaculated spermatozoa. J. Clin. Endocrinol. Metab. 90, 4753–4761 (2005).
Li, H. W., Chiu, P. C., Cheung, M. P., Yeung, W. S. & O, W. S. Effect of leptin on motility, capacitation and acrosome reaction of human spermatozoa. Int. J. Androl. 32, 687–694 (2009).
Li, E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet. 3, 662–673 (2002).
Klose, R. J. & Bird, A. P. Genomic DNA methylation: the mark and its mediators. Trends Biochem. Sci. 31, 89–97 (2006).
Nilsson, E. E., Sadler-Riggleman, I. & Skinner, M. K. Environmentally induced epigenetic transgenerational inheritance of disease. Env. Epigenet 4, dvy016 (2018).
Skinner, M. K. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics 6, 838–842 (2011).
King, S. E. & Skinner, M. K. Epigenetic transgenerational inheritance of obesity susceptibility. Trends Endocrinol. Metab. 31, 478–494 (2020).
Lehtiniemi, T. & Kotaja, N. Germ granule-mediated RNA regulation in male germ cells. Reproduction 155, R77–R91 (2018).
Gui, Y. & Yuan, S. Epigenetic regulations in mammalian spermatogenesis: RNA-m6A modification and beyond. Cell Mol. Life Sci. 78, 4893–4905 (2021).
Fernandez-Perez, D., Brieno-Enriquez, M. A., Isoler-Alcaraz, J., Larriba, E. & Del Mazo, J. MicroRNA dynamics at the onset of primordial germ and somatic cell sex differentiation during mouse embryonic gonad development. RNA 24, 287–303 (2018).
Baxter, F. A. & Drake, A. J. Non-genetic inheritance via the male germline in mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180118 (2019).
Donkin, I. & Barres, R. Sperm epigenetics and influence of environmental factors. Mol. Metab. 14, 1–11 (2018).
Schagdarsurengin, U. & Steger, K. Epigenetics in male reproduction: effect of paternal diet on sperm quality and offspring health. Nat. Rev. Urol. 13, 584–595 (2016).
Steger, K. et al. Expression of mRNA and protein of nucleoproteins during human spermiogenesis. Mol. Hum. Reprod. 4, 939–945 (1998).
Carrell, D. T. & Hammoud, S. S. The human sperm epigenome and its potential role in embryonic development. Mol. Hum. Reprod. 16, 37–47 (2010).
Gunes, S. & Esteves, S. C. Role of genetics and epigenetics in male infertility. Andrologia 53, e13586 (2021).
Liu, W. M. et al. Sperm-borne microRNA-34c is required for the first cleavage division in mouse. Proc. Natl Acad. Sci. USA 109, 490–494 (2012).
Baldeon, R. L. et al. Type 2 diabetes monocyte MicroRNA and mRNA expression: dyslipidemia associates with increased differentiation-related genes but not inflammatory activation. PLoS ONE 10, e0129421 (2015).
Mahmoud, A. M. An overview of epigenetics in obesity: the role of lifestyle and therapeutic interventions. Int. J. Mol. Sci. 23, 1341 (2022).
Guerra-Carvalho, B. et al. Molecular mechanisms regulating spermatogenesis in vertebrates: environmental, metabolic, and epigenetic factor effects. Anim. Reprod. Sci. 246, 106896 (2022).
Donkin, I. et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 23, 369–378 (2016).
Pereira, S. C. et al. Expression of obesity-related genes in human spermatozoa affects the outcomes of reproductive treatments. F. S Sci. 2, 164–175 (2021).
Relier, S., Rivals, E. & David, A. The multifaceted functions of the fat mass and obesity-associated protein (FTO) in normal and cancer cells. RNA Biol. 19, 132–142 (2022).
Pereira, S. C. et al. Obesity-related genes are expressed in human Sertoli cells and modulated by energy homeostasis regulating hormones. J. Cell Physiol. 236, 5265–5277 (2021).
Palmer, N. O., Fullston, T., Mitchell, M., Setchell, B. P. & Lane, M. SIRT6 in mouse spermatogenesis is modulated by diet-induced obesity. Reprod. Fertil. Dev. 23, 929–939 (2011).
McPherson, N. O., Owens, J. A., Fullston, T. & Lane, M. Preconception diet or exercise intervention in obese fathers normalizes sperm microRNA profile and metabolic syndrome in female offspring. Am. J. Physiol. Endocrinol. Metab. 308, E805–E821 (2015).
Heller, C. G. & Clermont, Y. Spermatogenesis in man: an estimate of its duration. Science 140, 184–186 (1963).
Sullivan, R. Epididymosomes: a heterogeneous population of microvesicles with multiple functions in sperm maturation and storage. Asian J. Androl. 17, 726–729 (2015).
Belleannee, C. Extracellular microRNAs from the epididymis as potential mediators of cell-to-cell communication. Asian J. Androl. 17, 730–736 (2015).
Reilly, J. N. et al. Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci. Rep. 6, 31794 (2016).
Ding, Y. et al. MicroRNA-222 transferred from semen extracellular vesicles inhibits sperm apoptosis by targeting BCL2L11. Front. Cell Dev. Biol. 9, 736864 (2021).
Nixon, B. et al. The microRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biol. Reprod. 93, 91 (2015).
Trigg, N. A., Eamens, A. L. & Nixon, B. The contribution of epididymosomes to the sperm small RNA profile. Reproduction 157, R209–R223 (2019).
Chen, H., Alves, M. B. R. & Belleannee, C. Contribution of epididymal epithelial cell functions to sperm epigenetic changes and the health of progeny. Hum. Reprod. Update 28, 51–66 (2021).
Alshanbayeva, A., Tanwar, D. K., Roszkowski, M., Manuella, F. & Mansuy, I. M. Early life stress affects the miRNA cargo of epididymal extracellular vesicles in mousedagger. Biol. Reprod. 105, 593–602 (2021).
Gapp, K. et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 17, 667–669 (2014).
Mayer, W., Niveleau, A., Walter, J., Fundele, R. & Haaf, T. Demethylation of the zygotic paternal genome. Nature 403, 501–502 (2000).
Noble, D., Jablonka, E., Joyner, M. J., Muller, G. B. & Omholt, S. W. Evolution evolves: physiology returns to centre stage. J. Physiol. 592, 2237–2244 (2014).
Paranjpe, S. S. & Veenstra, G. J. Establishing pluripotency in early development. Biochim. Biophys. Acta 1849, 626–636 (2015).
Tang, W. W., Kobayashi, T., Irie, N., Dietmann, S. & Surani, M. A. Specification and epigenetic programming of the human germ line. Nat. Rev. Genet. 17, 585–600 (2016).
Gkountela, S. et al. DNA demethylation dynamics in the human prenatal germline. Cell 161, 1425–1436 (2015).
Braun, R. E. Packaging paternal chromosomes with protamine. Nat. Genet. 28, 10–12 (2001).
Wykes, S. M. & Krawetz, S. A. The structural organization of sperm chromatin. J. Biol. Chem. 278, 29471–29477 (2003).
van der Heijden, G. W. et al. Sperm-derived histones contribute to zygotic chromatin in humans. BMC Dev. Biol. 8, 34 (2008).
Hammoud, S. S. et al. Distinctive chromatin in human sperm packages genes for embryo development. Nature 460, 473–478 (2009).
Miller, D. Ensuring continuity of the paternal genome: potential roles for spermatozoal RNA in mammalian embryogenesis. Soc. Reprod. Fertil. Suppl. 65, 373–389 (2007).
Perez, M. F. & Lehner, B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol. 21, 143–151 (2019).
Ding, G. L. et al. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J. Androl. 17, 948–953 (2015).
Stunkard, A. J. et al. An adoption study of human obesity. N. Engl. J. Med. 314, 193–198 (1986).
Silventoinen, K., Rokholm, B., Kaprio, J. & Sorensen, T. I. The genetic and environmental influences on childhood obesity: a systematic review of twin and adoption studies. Int. J. Obes. 34, 29–40 (2010).
Lecomte, V., Maloney, C. A., Wang, K. W. & Morris, M. J. Effects of paternal obesity on growth and adiposity of male rat offspring. Am. J. Physiol. Endocrinol. Metab. 312, E117–E125 (2017).
Crisostomo, L. et al. Inheritable testicular metabolic memory of high-fat diet causes transgenerational sperm defects in mice. Sci. Rep. 11, 9444 (2021).
Crisostomo, L. et al. Inherited metabolic memory of high-fat diet impairs testicular fatty acid content and sperm parameters. Mol. Nutr. Food Res. 66, e2100680 (2022).
Crisostomo, L. et al. Testicular “Inherited Metabolic Memory” of ancestral high-fat diet is associated with sperm sncRNA content. Biomedicines 10, 909 (2022).
Fullston, T. et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 27, 4226–4243 (2013).
Cropley, J. E. et al. Male-lineage transmission of an acquired metabolic phenotype induced by grand-paternal obesity. Mol. Metab. 5, 699–708 (2016).
de Castro Barbosa, T. et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol. Metab. 5, 184–197 (2016).
Pepin, A. S., Lafleur, C., Lambrot, R., Dumeaux, V. & Kimmins, S. Sperm histone H3 lysine 4 tri-methylation serves as a metabolic sensor of paternal obesity and is associated with the inheritance of metabolic dysfunction. Mol. Metab. 59, 101463 (2022).
Grandjean, V. et al. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci. Rep. 5, 18193 (2015).
Wegner, C. C., Clifford, A. L., Jilbert, P. M., Henry, M. A. & Gentry, W. L. Abnormally high body mass index and tobacco use are associated with poor sperm quality as revealed by reduced sperm binding to hyaluronan-coated slides. Fertil. Steril. 93, 332–334 (2010).
Rybar, R., Kopecka, V., Prinosilova, P., Markova, P. & Rubes, J. Male obesity and age in relationship to semen parameters and sperm chromatin integrity. Andrologia 43, 286–291 (2011).
Al-Ali, B. M. et al. Body mass index has no impact on sperm quality but on reproductive hormones levels. Andrologia 46, 106–111 (2014).
Acknowledgements
This work was supported by the Portuguese Foundation for Science and Technology: D.F. Carrageta (SFRH/BD/136779/2018 and COVID/BD/153209/2023), S.C. Pereira (2021.05487.BD), UMIB (UIDB/00215/2020 and UIDP/00215/2020), ITR (LA/P/0064/2020), IBiMED (UIDP/04501/2020 and UIDB/04501/2020) and LAQV/REQUIMTE (UIDB/50006/2020), co-funded by FEDER funds through the COMPETE/QREN, FSE/POPH, and POCI — COMPETE 2020 (POCI-01-0145-FEDER- 007491) funds; co-funded by the Portuguese Society of Diabetology (Sociedade Portuguesa de Diabetologia) through the Charneco da Costa Grant (2021).
Author information
Authors and Affiliations
Contributions
M.G.A., D.F.C. and S.C.P. researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Adam Watkins, Jorma Toppari and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carrageta, D.F., Pereira, S.C., Ferreira, R. et al. Signatures of metabolic diseases on spermatogenesis and testicular metabolism. Nat Rev Urol (2024). https://doi.org/10.1038/s41585-024-00866-y
Accepted:
Published:
DOI: https://doi.org/10.1038/s41585-024-00866-y