Abstract
Ferroptosis is a distinct form of regulated cell death that is predominantly driven by the build-up of intracellular iron and lipid peroxides. Ferroptosis suppression is widely accepted to contribute to the pathogenesis of several tumours including prostate cancer. Results from some studies reported that prostate cancer cells can be highly susceptible to ferroptosis inducers, providing potential for an interesting new avenue of therapeutic intervention for advanced prostate cancer. In this Perspective, we describe novel molecular underpinnings and metabolic drivers of ferroptosis, analyse the functions and mechanisms of ferroptosis in tumours, and highlight prostate cancer-specific susceptibilities to ferroptosis by connecting ferroptosis pathways to the distinctive metabolic reprogramming of prostate cancer cells. Leveraging these novel mechanistic insights could provide innovative therapeutic opportunities in which ferroptosis induction augments the efficacy of currently available prostate cancer treatment regimens, pending the elimination of major bottlenecks for the clinical translation of these treatment combinations, such as the development of clinical-grade inhibitors of the anti-ferroptotic enzymes as well as non-invasive biomarkers of ferroptosis. These biomarkers could be exploited for diagnostic imaging and treatment decision-making.
Similar content being viewed by others
Introduction
The 5-year survival rate for localized prostate cancer is >99% but drops to 30–40% when metastasis is detected and/or with the development of castration-resistant prostate cancer (CRPC)1. Patients with advanced prostate cancer can be treated with second-generation androgen receptor (AR)-signalling inhibitors (such as enzalutamide, apalutamide, darolutamide and abiraterone), chemotherapy, radiotherapy, immunotherapy (such as sipuleucel-T), or poly(ADP-ribose) polymerase (PARP) inhibitors (for selected patients harbouring mutations in DNA-damage response genes). However, these current treatment regimens only prolong life expectancy by, on average, 2–3 years2. The limited knowledge of the underlying mechanisms that promote the initiation and development of advanced prostate cancer poses a challenge to clinicians in treating these patients.
Major advances in our understanding of the regulated cell-death pathway called ferroptosis over the past decade have the potential to transform the therapeutic landscape of cancer. Ferroptosis is an iron-dependent form of cell death that has morphological and mechanistic features distinct from those of other forms of cell death such as necrosis, apoptosis and autophagy. For instance, different from typical necrotic processes, ferroptosis is not associated with signs of organelle swelling3. Moreover, ferroptosis does not show the usual apoptosis characteristics of chromatin condensation, formation of apoptotic bodies and cytoskeletal breakdown3. Ferroptosis differs from autophagy by not forming classic double-membraned autophagosomes that enclose cellular cargo. Instead, upon ferroptosis induction, cells undergo a reduction in mitochondrial size and in mitochondrial cristae, which is accompanied by elevated membrane density3.
A notable hallmark of ferroptosis is the production of peroxides on polyunsaturated fatty acids (PUFAs)4 that are part of membrane phospholipids, as well as PUFA cholesterols5. PUFAs are particularly susceptible to lipid peroxidation because of multiple double bonds in their carbon chains, which makes this class of lipids uniquely vulnerable to hydrogen abstraction by hydroxyl, alkoxyl or hydroperoxyl radicals6. The sources of oxidants that initiate lipid peroxidation can be the mitochondria, iron overload and iron-dependent generation of reactive oxygen species (ROS), or oxidoreductases (such as NADPH-cytochrome P450 reductase, NADH-cytochrome b5 reductase and NADPH oxidases)7,8,9,10. The resultant lipid peroxidation leads to a chain reaction that causes a rapid escalation of radical initiation and corresponding radical damage, ultimately causing widespread, irreparable oxidative injury to the cell membrane and eventually, cell death11,12.
To prevent ferroptosis, cells possess an array of intrinsic systems to avert lipid peroxidation. The classical mechanism of ferroptosis defence involves glutathione peroxidase 4 (GPX4) and the glutathione (GSH) system. GPX4 uses GSH as a cofactor to promote reduction of lethal lipid hydroperoxides to their corresponding non-lethal alcohols, in turn mitigating lipid peroxidation to secure lipid bilayer membrane integrity and prevent ferroptosis13. GSH, a tripeptide consisting of glutamate, glycine and cysteine, is formed by importing cystine into cells through the system xc−, an antiporter importing cystine in exchange for glutamate14. Pharmacologically, ferroptosis can be induced by using a GPX4 inhibitor such as RAS-selective lethal 3 (RSL3), or by depleting intracellular GSH through the inhibition of system xc− through small molecules such as erastin, sulfasalazine and sorafenib15,16. Induction of ferroptosis with these agents causes an uncontrolled accumulation of ROS species, which deteriorates the cell membrane and leads to cell death17.
Beyond the canonical GPX4 system, alternative ferroptosis defence mechanisms exist. Ferroptosis suppressor protein 1 (FSP1; encoded by the AIFM2 gene) and possibly dihydroorotate dehydrogenase (DHODH) can inhibit ferroptosis in a GPX4-independent manner by reducing CoQ10 to CoQ10-H2, a free-radical-sequestering antioxidant found in the inner mitochondrial membrane that prevents lipid peroxide propagation18,19. Reduced CoQ10 is a lipid ubiquinone and acts as an antioxidant that protects lipids and proteins from oxidative damage, helping to maintain the stability and permeability of the cell membrane20. Specifically, CoQ10 acts as a mobile electron carrier within the electron transport chain, shuttling electrons between different enzyme complexes. Upon accepting electrons from NADH, CoQ10 becomes reduced (CoQ10-H2) and can donate these electrons to other molecules, including peroxide species, effectively neutralizing these molecules21. Additionally, GTP cyclohydrolase 1 (GCH1) and its metabolic derivatives tetrahydrobiopterin (BH4) and dihydrobiopterin (BH2) constitute another system that antagonizes ferroptosis independently of GPX4. GCH1 specifically shields phosphatidylcholines with two PUFA tails from oxidative damage, consequently preventing ferroptosis. GCH1 might also help to shield cells from oxidative damage by exerting a protective effect on CoQ10. GCH1 is the first rate-limiting enzyme in the metabolic pathway that produces BH4. BH4 acts as a cofactor for nitric oxide synthase, enabling the production of nitric oxide, which is a potent antioxidant. Thus, decreasing levels of BH4 can cause increased levels of superoxides, inducing ferroptosis22.
Cancer cells, which are characterized by a unique metabolic reprogramming and increased levels of ROS, often have intrinsic susceptibility to ferroptosis23. Hence, disrupting the defence mechanisms against ferroptosis in cancer cells could be a promising therapeutic approach, as these cells rely heavily on these antioxidant systems to survive under stress conditions in the harsh tumour microenvironment (TME). Results from multiple studies in preclinical models showed that targeting major ferroptosis inhibitors, such as the GPX4 enzyme or system xc−, could preferentially trigger ferroptosis in otherwise drug-resistant cancer cells, in turn enhancing the effectiveness of existing therapies24,25,26.
Mounting evidence points to the potential of exploiting ferroptosis for the treatment of advanced prostate cancer. For instance, a commonality across a variety of anti-androgen therapy-resistant, highly mesenchymal prostate cancer cells is the hypersensitivity to ferroptosis and GPX4 dependency, effects that have been linked to substantial lipid remodelling27,28. Ferroptosis inducers targeting the GPX4–GSH system have also shown anticancer activity in xenograft models across multiple prostate cancer variants including adeno-CRPC, neuroendocrine prostate cancer and double-negative prostate cancer29,30. Other ferroptosis defence pathways that function independently of GPX4, discovered as recently as 2021, remain relatively understudied in the context of prostate cancer, but might also have important roles in this disease. The therapeutic effectiveness of DHODH, FSP1 and GCH1 inhibitors in prostate cancer has been shown in a few studies. Treatment of human prostate cancer cell lines with the DHODH inhibitor leflunomide showed that the GI50 of the PTEN-mutant cells was lower than that of wild-type PTEN cells, indicating that PTEN-mutant prostate cancers show sensitivity towards DHODH inhibition31. In another study in which a ferroptosis-related prognostic gene signature was established using The Cancer Genome Atlas cohort of patients with prostate cancer, FSP1 was identified as an indicator of poor prognosis in patients with prostate cancer and a high risk of biochemical recurrence32. Notably, controversies exist in the field, which often prevent consensus regarding the importance of various pathways in ferroptosis. For example, conflicting reports exist on the role of ferroptosis defence pathways in other cancers such as renal carcinoma and fibrosarcoma19,33,34. Furthermore, scarce knowledge about the exact mechanisms of action of ferroptosis-inducing drugs and the optimal concentrations to use also confound data interpretation. For instance, results from a 2023 study showed that DHODH might not be a prevalent ferroptosis defence mechanism34, in contrast with what was previously reported19. In this study, the absence of DHODH barely sensitized cancer cells to ferroptosis compared with FSP1 deletion, whereas reported DHODH inhibitors at high concentrations were shown to sensitize cancer cells to ferroptosis by binding to and inhibiting FSP1 rather than DHODH34. Another important issue in the field is the need to delineate the comparative benefits and pitfalls of targeting distinct ferroptosis defence mechanisms in prostate cancer. For example, results from GPX4-knockout mouse models indicate that GPX4 seems to be essential for survival35, whereas FSP1-knockout mice showed normal development36, indicating that blocking FSP1 might be a less toxic therapeutic strategy than suppressing GPX4. To date, the role of GCH1–BH4 in prostate cancer has not been explored. Together, growing evidence indicates a causal role for ferroptosis in prostate cancer reduction, suggesting that the development and understanding of strategies targeting ferroptosis in prostate cancer are warranted.
In this Perspective, we describe the current understanding of metabolic mechanisms and signalling pathways that govern ferroptosis. We also highlight potential opportunities to integrate ferroptosis inducers into established standard-of-care treatments for prostate cancer, establishing a foundation for the development of rational combination therapies to enhance patient outcomes. Last, we describe methods of non-invasively monitoring ferroptosis and their utility in patient selection and as pharmacodynamic biomarkers in the context of ferroptosis-inducing cancer therapies in prostate cancer.
Metabolic processes influencing the vulnerability of prostate cancer cells to ferroptosis
In the past 10 years, progress has been made deciphering the underlying mechanisms of ferroptosis and the intricate links with cancer metabolism regulatory networks. Ferroptosis has been shown to have a crucial role at the intersection of lipid metabolism, iron control, and amino acid metabolism in the context of prostate cancer.
Lipid metabolism and ferroptosis
Ferroptosis is a lipid-dependent form of cell death. Accordingly, ferroptosis susceptibility is affected by the cell’s lipid composition and the processes involved in lipid acquisition, synthesis, storage and breakdown. Thus, understanding the unique changes in lipid metabolism that define prostate cancer cells is necessary to adequately evaluate ferroptosis sensitivity in prostate cancer. Specifically, malignant cells are often more susceptible to ferroptosis than benign cells owing to an increased demand for iron and an enhanced lipid metabolism37,38. Prostate cancer cells generate a large proportion of energy through lipid metabolism and have extensively dysregulated fatty acid metabolism39,40, making this tumour a candidate for metabolism-based therapeutics. Both early-stage and late-stage prostate cancer are characterized by the upregulation of genes linked to lipid metabolism, metabolic rewiring to oxidative phosphorylation and elevated tricarboxylic acid (TCA) cycle flux40,41,42, suggesting a resultant increase in intracellular ROS burden that could perturb iron homeostasis and encourage lipid peroxidation43. Results from proteomic studies on primary prostate tumours and bone metastases showed a strong positive correlation between the levels of enzymes involved in lipid metabolism and the onset and development of prostate cancer44. Major lipid metabolism pathways in prostate cancer that could have potential roles in ferroptosis include production of phospholipids, β-oxidation and activation of fatty acids.
The intracellular balance between MUFAs and PUFAs
The activation and inclusion of monounsaturated fatty acids (MUFAs), such as oleic acid, in membrane phospholipids is controlled by long-chain acyl-CoA synthetase 3 (ACSL3)45. MUFAs can prevent cells from undergoing ferroptosis by replacing oxidizable PUFAs from the membrane lipid bilayer4,45. Increased PUFA biosynthesis and PUFA incorporation into phospholipids are controlled by long-chain acyl-CoA synthetase 4 (ACSL4) and are essential for ferroptosis induction45,46. ACSL4 facilitates the accumulation of oxidized cellular membrane phospholipids and subsequent production of lethal lipid peroxides, in turn inducing ferroptosis47,48,49 (Fig. 1a). Thus, the balance of MUFAs and PUFAs in phospholipids greatly determines cells’ sensitivity to ferroptosis.
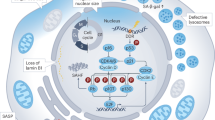
Multiple metabolic pathways contribute to the regulation of ferroptosis in prostate cancer, including iron metabolism, lipid metabolism and glutathione (GSH) synthesis pathways. a, Lipid metabolism. In de novo lipogenesis, stearoyl-CoA desaturase 1 (SCD1) catalyses the production of monounsaturated fatty acids (MUFAs) from the saturated fatty acid (SFA) palmitate. Long-chain acyl-CoA synthetase 3 (ACSL3) subsequently conjugates MUFAs with phospholipids and disrupts the synthesis of phospholipids containing polyunsaturated fatty acids (PUFAs). An increased MUFA-to-PUFA ratio can protect cells from undergoing ferroptosis. Inhibition of fatty acid synthase (FASN), another component of the de novo lipogenesis pathway, could result in ferroptosis. This effect occurs because SFA and MUFA are supplied by FASN to the Lands cycle, which is responsible for remodelling oxidized phospholipids such as phosphatidylcholines. 5’-adenosine monophosphate-activated protein kinase (AMPK)-mediated phosphorylation of acetyl-CoA carboxylase 1 (ACC), an enzyme that catalyses the carboxylation of acetyl-coenzyme A (CoA) to produce malonyl-CoA), inhibits ACC1 activity, resulting in reduced de novo lipogenesis and decreased ferroptosis in immortalized mouse embryonic fibroblasts (MEFs). Peroxidation of phospholipids containing PUFAs is the main driver of ferroptosis, with oxygenated diacyl arachidonic acid (AA)- and adrenic acid (AdA)-containing species of phosphatidylethanolamine (PE) being the most susceptible ferroptosis substrates. The ω-6 de novo PUFA synthesis pathway uses fatty acid elongase 2 and 5 (ELOVL2 and ELOVL5), and fatty acid desaturase 1 and 2 (FADS1 and FADS2) to synthesize AA and AdA from exogenously sourced linoleic acid. Carnitine palmitoyltransferase 1 (CPT1) imports acylcarnitines into the mitochondria, whereas 2,4 dienoyl-CoA reductase (DECR1) catalyses PUFA β-oxidation in the mitochondria (dashed arrow) to produce acetyl-CoA. Acetyl-CoA feeds into the tricarboxylic acid (TCA) cycle to produce hydrogen peroxide (H2O2), which reacts with Fe2+ to generate reactive oxygen species (ROS) and promote lipid peroxidation. Lipid β-oxidation can be a source of ferroptosis suppression, as this pathway diverts PUFAs away from peroxidation towards processes that support cancer-cell survival and drug resistance in castration-resistant prostate cancer (CRPC). Sterol response element-binding protein 1 (SREBP1) is a master transcriptional regulator of genes encoding proteins involved in the de novo lipogenesis, including FASN and ACACA (encoding FAS and ACC1, respectively), and is activated by the androgen receptor (AR). Additionally, the AR promotes the transcription of membrane-bound O-acyltransferase 2 (MBOAT2), which inhibits PE-AA and PE-AdA formation, preventing lipid peroxidation and ferroptosis. Furthermore, in prostate cancer, the AR has been shown to regulate the expression of long-chain acyl-CoA synthetases 3 and 4 (ACSL3 and ACSL4). Specifically, the AR promotes ACSL3 expression to promote MUFA incorporation into membranes, preventing ferroptosis. AR also transcriptionally suppresses ACSL4 expression, which prevents PUFA conjugation to CoA and, in turn, further creation of PUFA-containing phospholipids such as PE and subsequent ferroptosis. b, Iron metabolism. Iron is imported into the cell through the transferrin receptor (TfR). Fe3+ is converted to Fe2+ by the transmembrane ferrireductase six-transmembrane epithelial antigen of the prostate 3 (STEAP3) inside endosomes. Subsequently, the iron chaperone poly(rC)-binding protein 1 (PCBP1) binds and transports Fe2+ to ferritin for storage, whereas poly(rC)-binding protein 2 (PCBP2) provides molecular support and transports iron to the iron exporter ferroportin (FPN) for export. Increasing the activity of PCBP1 has been shown to suppress ferroptosis. An excess of iron can increase ROS, mainly because iron in its redox-active state (Fe2+) participates in the Fenton reaction and creates ferric iron (Fe3+) and ROS (such as hydroxyl radicals), which oxidize DNA, proteins and lipids to induce ferroptosis. In prostate cancer cells, TfR is upregulated, whereas the FPN is downregulated to preserve the labile iron pool. This process helps cancer cells to increase proliferation, movement, invasiveness and the activity of specific proteins that promote metastasis. c, Glutamate, cystine and GSH metabolism. Ferroptosis was reported to be regulated by extracellular l-glutamine levels and by enzymes involved in glutaminolysis — a glutamine-fuelled intracellular catabolic pathway. Extracellular l-glutamine enters the cell through transporters such as the alanine serine cysteine transporter 2 (ASCT2), and is converted into glutamate. Glutamate can then undergo glutaminolysis and metabolism through the TCA cycle in the mitochondria, producing ROS, which can promote ferroptosis. Glutamate also reacts with cysteine in a reaction catalysed by γ-glutamylcysteine synthetase (γ-GCS) to produce γ-glutamylcysteine. Lastly, glycine and γ-glutamylcysteine form GSH in a reaction catalysed by GSH synthetase (GS). Glutathione peroxidase 4 (GPX4) converts GSH to oxidized GSH (GSSG), using GSH as a reducing agent to reduce toxic hydroperoxides to their respective alcohols. If any step of the process is disrupted, GPX4 activity is reduced, leading to the accumulation of toxic hydroperoxides that compromise the integrity of membranes and organelles, in turn inducing ferroptosis. However, high levels of extracellular glutamine alone cannot cause ferroptosis. Straight arrows represent direct links between proteins. Dashed arrows show indirect, multiple-step links between proteins.
In prostate cancer cells, AR synchronously controls the expression of ACSL3 and ACSL4 in opposite directions to render prostate cancer cells reliant on ACSL4-mediated fatty acid metabolism50 (Fig. 1a). In samples from patients with prostate cancer, ACSL3 was shown to be moderately upregulated in early prostate cancer, but ACSL3 expression was reduced during the transition from low-grade to high-grade cancers51. Conversely, in an immunohistochemical analysis of a human prostate cancer tissue microarray derived from patients across multiple clinicopathological groups and benign prostate tissue52, the expression of ACSL4 was higher in tumour cells than in surrounding normal epithelial cells. ACSL4 expression is also higher in samples from patients with CRPC than in those from patients with hormone-naive prostate cancers52. The net incorporation of an increased amount of unsaturated fatty acids provides membrane fluidity associated with aggressive cancer cell behaviours such as increased motility and/or proliferation. Interestingly, besides AR, ACSL4 also seems to be a target of E2F. Results from a study in the prostate cancer cell lines LNCaP and PC3 showed that loss of the canonical prostate cancer tumour suppressor RB1 and the resultant E2F activation increased ACSL4 expression and elevated arachidonic acid (AA) and adrenic acid (AdA)-containing phospholipids, which act as primary substrates for lipid peroxidation53. Furthermore, in a PC3 xenograft model and a prostate epithelium-specific Pten and Rb1 double-knockout mouse model, RB1-deficient prostate tumour growth and metastasis were susceptible to ferroptosis induction53. Similar to other cancers, high ACSL4 levels were indicated as a mechanism of resistance to drugs such as chemotherapies, tamoxifen and triacsin C54,55,56,57. Together, these data indicate that in cancers such as prostate cancer, ACSL4 might be upregulated to promote disease progression, but at a cost of increased tumour susceptibility to ferroptosis inducers. This characteristic of prostate cancer can be exploited by combining AR signalling inhibitors with ferroptosis inducers, causing excessive ACSL4-dependent lipid peroxide production and ultimately, ferroptotic cell death.
De novo lipogenesis
Ferroptosis can be regulated by modulating the expression of genes involved in de novo lipogenesis, including essential lipogenic enzymes such as fatty acid synthase (FASN) and acetyl-CoA carboxylase (ACC). The irreversible carboxylation of acetyl-CoA to form malonyl-CoA is catalysed by the biotin-dependent ACC, and malonyl-CoA is subsequently converted to long-chain fatty acids by FASN58. Stearoyl-CoA desaturase 1 (SCD1) subsequently generates MUFAs from saturated fatty acids (SFAs) (Fig. 1a). Prostate cancer shows high expression of crucial proteins involved in fatty acid synthesis, including ACC and FASN, compared with other cancers. This finding comes from a comparison of 32 cancer types included in The Cancer Genome Atlas PanCan 2018 normalized analysis of primary, treatment-naive prostate cancers of ~500 men59,60. Moreover, the expression of ATP citrate lyase (ACLY), ACC and FASN are higher in prostate cancer samples from patients than in normal prostate samples, indicating an activation of de novo lipogenesis to meet the increased metabolic needs of cancer cells59. Androgens are well known to mediate the expression of these lipogenic enzymes as well as that of SCD1 to fulfil the ever-increasing lipid requirements of prostate cancer cells40,61,62. An important transcriptional controller of these lipogenic enzymes is the sterol response element-binding protein 1 (SREBP1) transcription factor. Androgens activate the SREBP1 cleavage-activating protein (SCAP), in turn leading to SREBP1 activation63,64. Active SREBP1 promotes the expression of de novo lipogenesis genes such as FASN65. FASN is a potential biomarker of prostate cancer, with FASN overexpression being associated with a malignant phenotype66,67. Results from immunohistochemistry analyses showed high expression of FASN in prostate carcinomas, particularly in metastatic cancers66,67. In fact, FASN expression can be used to predict lymph node metastases regardless of the Gleason score68. The effect of FASN and ACC in regulating ferroptosis in the context of prostate cancer is unclear. Studies conducted in other cancer types provide conflicting reports of how FASN and ACC might modulate ferroptosis in cancer. In pancreatic ductal adenocarcinoma cells, system xc− inhibition (with erastin or sulfasalazine) mediated lipid peroxidation and subsequent ferroptotic death through pyruvate oxidation-dependent fatty acid production. Indeed, the knockdown of FASN or ACC by short hairpin RNAs in these cells inhibited erastin-mediated or sulfasalazine-mediated ferroptotic death, whereas the addition of exogenous PUFAs or palmitic acid reversed this phenotype69. In another study in mouse embryonic fibroblasts, ferroptosis was inhibited by orlistat, a FASN inhibitor, indicating that ACC1–FASN-mediated lipogenesis was required for ferroptosis in this system70. Similarly, adenosine monophosphate-activated protein kinase (AMPK) has been reported to negatively regulate ferroptosis by inhibiting ACC1–FASN-mediated fatty acid synthesis in mouse embryonic fibroblasts and in the human renal cancer cell line Caki-1 (ref. 71). Conversely, in mutant-KRAS lung cancer cell lines, SFAs and MUFAs were shown to be supplied by FASN to the Lands cycle, which is responsible for remodelling oxidized phospholipids such as phosphatidylcholines72. Inhibiting the Lands cycle or FASN expression in mutant-KRAS lung cancer cell lines resulted in ferroptosis72. Thus, FASN upregulation and the resulting increase in SFA and MUFA can be a defence mechanism against lipid peroxidation. Despite these seemingly contradictory observations, the opposite effects of FASN inhibition on ferroptosis induction could be context dependent, largely relying on the available pool of peroxidation-prone PUFA or on the ability of cells to take up exogenous PUFAs upon FASN inhibition72. Specifically, a potential explanation for FASN-mediated inhibition of ferroptosis70 is that FASN inhibition might cause a reduction not only in SFA and MUFA lipogenesis but also, potentially, in PUFA biosynthesis, resulting in ferroptosis inhibition. Indeed, FASN inhibition induces adenosine monophosphate build-up, causing AMPK activation72, which could result in the suppression of downstream PUFA biosynthesis71. Hence, the net effect of these processes on PUFA levels in relation to MUFA and possibly SFA levels is likely to contribute to the regulation of ferroptosis.
AMPK can prevent the production of fatty acids through phosphorylation and the subsequent inhibition of both ACC1 and ACC2 activity under conditions of energy stress73. Inhibition of ACC by small-molecule inhibitors and by AMPK phosphorylation was found to suppress ferroptosis in mouse embryonic fibroblasts71. Moreover, deletion of the alpha catalytic subunits of AMPK was found to increase the levels of phosphatidylethanolamine (PE) species such as PE 18:0_20:4 and PE 18:0_22:4, which are drivers of ferroptosis71. Notably, AMPK is frequently activated in human prostate malignancies, and AMPK pharmacological suppression or knockdown was shown to cause prostate cancer cell death and decreased cell growth74,75,76,77,78. Thus, investigating whether inhibitors of AMPK signalling could synergize with ferroptosis inducers to impede prostate tumour growth will be an interesting area of exploration.
SCD1 produces MUFAs from SFAs by catalysing the formation of a double bond in stearoyl-CoA and palmitoyl-CoA79 (Fig. 1a). Patients with prostate cancer have a higher MUFA to SFA ratio in the blood than healthy individuals, suggesting higher SCD1 activity80,81,82. SCD1 mRNA and protein levels are higher in human prostate cancer tissue samples than in normal prostate tissue samples82. SCD1 pharmacological and genetic inhibition suppressed the proliferation of prostate cancer cells both in vitro and in xenograft models82. Moreover, SCD1-mediated synthesis of MUFAs has been shown to promote the anti-ferroptosis activity of SREBP1 (ref. 83). Results from this study also showed that PI3K–AKT–mTOR signalling activates downstream SREBP1, which subsequently activates SCD1 to increase ferroptosis-resistant MUFA synthesis and prevent lipid peroxide formation. Thus, PI3K–AKT–mTOR signalling could safeguard PTEN-defective prostate cancer cells from oxidative stress and ferroptosis through downstream SREBP1–SCD1-facilitated lipogenesis83. Similarly, in ovarian cancer cells, blocking SCD1 induced lipid oxidation and ferroptosis by reducing CoQ10, which increased the anticancer efficacy of ferroptosis inducers84.
Collectively, results from these studies indicate that cancer cells often increase de novo lipogenesis, producing ferroptosis-resistant substrates, such as SFAs and MUFAs, to simultaneously serve their metabolic needs and avoid ROS-induced ferroptotic death. Consequently, inhibitors that target enzymes involved in the de novo lipogenesis pathway could have synergistic effects with ferroptosis inducers, enhancing the efficacy of these inhibitors in inducing cancer cell death.
n-6 De novo PUFA synthesis
The PUFA biosynthesis pathway is another metabolic pathway that contributes to the balance of MUFAs and PUFAs and, ultimately, to ferroptotic sensitivity. Peroxidation of PUFAs is the main driver of ferroptosis, with oxygenated diacyl AA- and AdA-containing species of PE being the most susceptible ferroptosis substrates4,85. Specifically, exogenous addition of PE-AA-OOH but not AA-OOH to ACSL4 knockout cells increased ferroptosis-mediated cell death85, showing an essential role of the oxidative PE pathway in ferroptosis. Thus, these lipid signals are also potential new targets for biomarker and drug discovery. Typically, in the n-6 de novo PUFA synthesis pathway, fatty acid elongase 2 (ELOVL2), fatty acid elongase 5 (ELOVL5), fatty acid desaturase 1 (FADS1) and fatty acid desaturase 2 (FADS2) are used to produce the fatty acids AA and AdA from exogenously sourced linoleic acid86,87 (Fig. 1a). High expression of ELOVLs and FADS can sensitize gastric cancer cell lines to ferroptotic death, whereas low expression and activity of these proteins are associated with ferroptosis resistance88,89. In prostate cancer cells, androgen deprivation therapy was shown to amplify PUFA levels, and these levels are even higher in prostate cancer cells expressing neuroendocrine markers28,90. The essential PUFA elongation enzyme ELOVL5 is expressed at higher levels in neuroendocrine-like prostate cancer cells than in adenocarcinoma prostate cells90. Thus, PUFAs are upregulated in neuroendocrine-like prostate cancer cells. Increased PUFAs generate an increased number of lipid raft clusters in the neuroendocrine-like prostate cancer cell membrane, resulting in the induction of AKT–mTOR signalling and subsequent enzalutamide resistance. High expression of ELOVL5 increases the resistance of neuroendocrine-like prostate cancer cells to enzalutamide but can also render these cells more susceptible to ferroptosis inducers, owing to the rise in PUFAs90. These findings are consistent with those from another study in which increased AR-mediated activation of ELOVL5 was shown in prostate cancer cells, xenografts, and patients’ radical prostatectomy frozen specimens91. In this study, ELOVL5 was identified as the most commonly overexpressed ELOVL in prostate cancer compared with non-malignant prostate tissues91. These results suggest that increased ELOVL5 promotes resistance to enzalutamide, but potentially also creates a therapeutic window for ferroptosis inducers.
β-oxidation
Most studies focus on the role of AR-mediated de novo lipogenesis in prostate cancer, but mounting evidence suggests that fatty acid oxidation (FAO) is also regulated by AR signalling and facilitates the transition to CRPC. Carnitine palmitoyltransferase 1 (CPT1), specifically the CPT1A isoform, accelerates the transfer of an acyl group from coenzyme A to l-carnitine, in turn converting acyl-coenzyme As into acyl-carnitines, which can travel across membranes and enter the mitochondria92. This reaction is the rate-limiting step of FAO. High-grade neuroendocrine prostate cancer and CRPC tissue samples express higher levels of CPT1A than benign tissues93. The addition of CPT1A inhibitors such as etomoxir or ranolazine to anti-androgen treatment has been reported to have a synergistic anticancer effect in preclinical models of human prostate cancer cell lines93. In a xenograft model using CRPC cell lines, androgen withdrawal increased the susceptibility of CPT1A-knockdown tumours to enzalutamide, while enhancing tumour growth of CPT1A-overexpressing CRPC cell lines94. Another enzyme involved in FAO is 2,4 dienoyl-CoA reductase (DECR1), which catalyses the β-oxidation of PUFAs in the mitochondria. High expression levels of DECR1 were shown to increase prostate cancer cell proliferation as well as resistance to ferroptosis and to anti-androgen therapies95. Inhibition of DECR1 in human prostate cancer cell lines induced the accumulation of PUFAs in phospholipids and enhanced mitochondrial ROS, the formation of lipid peroxides and, ultimately, ferroptosis95 (Fig. 1a). Together, enzymes participating in the FAO pathway (such as CPT1A and DECR1) seem to suppress ferroptosis. One caveat is that the inhibition of β-oxidation might lower ROS production by reducing the amount of acetyl-CoA being channelled to the TCA cycle and the subsequent formation of superoxide anions generated through electron transfer. Nevertheless, ferroptosis inducers might generate enough ROS to overcome this suppression of ROS production. Prostate cancer is often characterized by increased β-oxidation; thus, a combination treatment strategy involving the inhibition of β-oxidation might further enhance the efficacy of ferroptosis inducers in some prostate cancer subtypes.
Iron metabolism and ferroptosis
Cells with elevated free (non-protein bound) iron are particularly susceptible to ferroptosis. Alterations in the transferrin receptor 1 (TfR1), poly(rC)-binding protein 1 and 2 (PCBP1 and PCBP2), and ferroportin systems have been associated with increased levels of iron accumulation within the cell (Fig. 1b). For example, when TfR1 is overexpressed, an increased amount of iron is transported into the cell, indicating that TfR1 is a positive receptor of ferroptosis96. After iron is imported through TfR1, PCBP1 and PCBP2 iron chaperones assist the labile iron to bond together and become a stored form of iron called ferritin97. PCBP2 also directly interacts with ferroportin, transferring cytoplasmic iron to ferroportin for export outside of cells98. Faulty PCBP1 and PCBP2 protein cannot form storage ferritin, leading to an increase in intracellular free iron species. Moreover, a defect in this system halts the removal of iron99. Cytosolic iron can also increase through other mechanisms100,101,102, but the aberrant expression and activity of these three proteins (PCBP1, PCBP2 and TfR1) are most commonly responsible for iron accumulation.
In prostate cancer, iron is one of the micronutrients essential for proliferation and metastasis. Many enzymes in prostate cancer cells use iron to regulate the transcriptional activity of AR, the central driver of prostate cancer, as well as to modulate other intracellular enzymatic activities that promote the production of energy, DNA synthesis and haem biosynthesis103. Accordingly, iron deficiency slows cancer cell growth, whereas iron-rich environments promote growth104,105. Consistent with a pro-cancer role for iron, iron-responsive element-binding proteins and TfRs are upregulated in various prostate cancer cell lines, and also in the sera of patients with prostate cancer, whereas the iron-exporter ferroportin is downregulated to preserve the labile iron pool106,107,108,109. The six-transmembrane epithelial antigen of the prostate 2 (STEAP2), an iron reductase enzyme that has a crucial role in reducing iron within cells, has also been shown to be overexpressed in prostate cancer cell lines and enhance cell growth, movement, and invasiveness, as well as activate pro-metastatic genes such as IL1B and MMP7 (ref. 110). However, a surplus of iron can increase ROS, which can trigger mitochondrial dysfunction, excessive lipid peroxidation, cellular damage and ferroptotic cell death111,112. Iron in its redox-active state (Fe2+) contributes directly to ROS production by participating in the Fenton reaction112 (Fig. 1b), as well as indirectly by modulating iron-dependent enzymes involved in ROS production, such as cytochrome P450 enzymes, NADPH oxidases and lipoxygenases113,114,115. Overall, this dependence on iron for metabolic needs renders prostate cancer highly susceptible to iron overload-mediated ferroptosis.
Iron overload has been identified as a potential promising treatment strategy in multiple disease models. In multiple myeloma cell lines, administering excess iron with the proteasome inhibitor bortezomib — a commonly used therapy for patients with both newly diagnosed and relapsed multiple myeloma — increased cell death, whereas lowering ferritin expression through gene silencing mitigated bortezomib resistance, indicating that iron toxicity and the concomitant excess ROS production could be used to restrict tumour growth116,117. Several prostate cancer cell lines including VCaP, LNCaP and TRAMP-C2 are particularly susceptible to iron-induced oxidative damage and ferroptosis118. A high dosage of iron in the form of iron-dextran and ferric carboxymaltose suppressed tumour growth in mice subcutaneously injected with VCaP and TRAMP-C2 cells, and synergized with bicalutamide to decrease the growth of a castration-resistant PC3 xenograft tumour mouse model, suggesting promise for targeting iron excess even in AR-indifferent prostate cancer118. Results from a study in which the human prostate cancer cell line DU145 and the TRAMP mouse model of prostate cancer were used showed that iron supplementation in the form of ferric ammonium citrate or iron dextran substantially induced RSL3-triggered lipid peroxidation, mitochondrial ROS and ferroptotic cell death119. Furthermore, enzalutamide enhanced the potency of the RSL3-plus-iron combination, resulting in an amplified anticancer effect that prevented the onset of CRPC in TRAMP mice, indicating that ferroptosis induction through iron overload can enhance the efficacy of current anti-androgen therapies119.
Iron-based nanoparticles encapsulated in hydrogen peroxide (H2O2), which are iron transporters that accumulate in tumours and catalyse Fenton reactions to generate lethal levels of hydroperoxides, are another potential method of inducing ferroptosis in cancer cells120. For instance, newly developed iron oxide nanoparticles coated with gallic acid and polyacrylic acid (IONP-GA/PAA) were shown to have an inherent cytotoxic effect against numerous glioblastoma, neuroblastoma and fibrosarcoma cell lines by inducing ferroptosis121. The ferroptotic effect of IONP-GA/PAA was reversed by conventional ferroptosis inhibitors such as deferoxamine, ciclopirox olamine and ferrostatin 1, indicating that the ROS-induced ferroptotic cell death is specifically dependent on the activity of iron derived from IONP-GA/PAA121. However, little is known about the therapeutic potential of iron oxide nanoparticles in the induction of ferroptosis in prostate cancer. To date, most studies have predominantly focused on the role of these nanoparticles in MRI and chemotherapy delivery to target sites, with little attention paid to combination therapy opportunities that could trigger ferroptosis122,123,124.
Glutamate, cystine and GSH metabolism
In addition to promoting lipid metabolism, AR signalling is essential for orchestrating the increased uptake of amino acids that regulate ferroptosis.
Glutamine, the most prevalent amino acid in the human body, is a crucial reservoir of reduced nitrogen for biosynthetic pathways, and also functions as a source of carbon to supply the TCA cycle, synthesize GSH and produce nucleotides, proteins and lipids125. Glutamine metabolism was shown to affect ferroptotic cell death and influence the vulnerability of prostate malignancies to ferroptosis (Fig. 1c). Mechanistically, glutamine is transported into the cell predominantly through the alanine serine cysteine transporter 2 (ASCT2) — a neutral amino acid transporter belonging to the solute carrier 1 family (SLC1) of proteins — and converted to glutamate, which subsequently reacts with intracellular cysteine and glycine, as mediated by the enzymes γ-glutamyl-cysteine (γ-GCS) and GSH synthase, to generate GSH and prevent lipid peroxidation14. Upon inhibition of GSH production through cystine deprivation, excess imported glutamine is channelled into glutaminolysis, a process by which cells convert glutamine into TCA cycle metabolites, instead of into GSH production, creating an additional source of ROS that promotes ferroptosis126. High levels of extracellular glutamine alone cannot cause ferroptosis. Only when l-glutamine is available and combined with cystine deprivation can ferroptosis be induced. Similarly, cysteine deprivation or blocking cystine entry alone could not cause ROS build-up, lipid peroxidation or ferroptosis under conditions of glutamine deficiency or impaired glutaminolysis96.
Glutamine metabolism could be a crucial metabolic pathway determining prostate cancer cell fate. For instance, ASCT1 and ASCT2 are upregulated by AR signalling, promoting glutamine absorption (directly and possibly indirectly through altering alternative glutamine uptake mechanisms) and metabolic assimilation127. Thus, inhibition of these transporters could slow the growth of tumours in xenograft mouse models of human prostate cancer cell lines by preventing cancer cells from absorbing glutamine, which is an essential source of carbon and nitrogen for macromolecule synthesis and anaplerosis (refilling depleted TCA cycle intermediates)127,128. Whether these pro-cancer effects of ASCT1 and ASCT2 can be attributed to the effect of these proteins on ferroptosis is unknown. Moreover, 5α-dihydrotestosterone was found to increase the levels of ASCT2 and also glutaminase in LNCaP and VCaP human prostate cancer cells127. Invasive CRPC cell lines such as DU145 and PC3 were shown to express higher basal levels of glutaminase than the less invasive cell line LNCaP129. This high dependence of prostate cancer cells on glutamine for growth can be exploited to promote ferroptosis by inhibiting GSH production with system xc− inhibitors or cystine deprivation, in turn channelling an increased amount of glutamate into the TCA cycle to accumulate excess ROS that can induce lipid peroxidation.
Cysteine has various central roles in the metabolic reprogramming of cancer cells. Following import through system xc−, cystine is converted to cysteine, which serves as a precursor for GSH production through γ-GCS (Fig. 1c). Cysteine is also a substrate for one-carbon metabolism, the TCA cycle, and fatty acid synthesis130. Cysteine metabolism has been shown to be involved in ferroptosis131,132. In this Perspective, we primarily focus on the relevance of cysteine in relation to GSH synthesis concerning prostate cancer. System xc− expression was shown to be elevated in tissue samples obtained from patients who underwent radical prostatectomy, in patient-derived xenograft models obtained from metastatic prostate cancer, as well as in the metastatic stroma of prostate cancer, and correlates positively with poor prognosis29,133. In AR-negative DU145 and PC-3 prostate cancer cells, extracellular cystine import is crucial for cell growth134. This evidence suggests that prostate cancer cells are highly dependent on cysteine metabolism and GSH production as a ROS scavenging mechanism to cope with metabolic stresses. Hence, targeting the system xc− and cysteine metabolic pathways to induce ferroptosis might be effective in the treatment of prostate cancer. Indeed, in one study, a human cystathionine-lyase termed cyst(e)inase that can reduce both cystine and cysteine levels was developed with substantial effectiveness in depleting GSH in human prostate cancer cell lines PC3, DU145 and the murine prostate cancer cell line HMVP2 (ref. 135). The use of cyst(e)inase in such cell lines resulted in G0-to-G1 arrest. This method improved survival in a chronic lymphocytic leukaemia mouse model, and also inhibited the development of prostate (AR-negative) and breast cancer xenografts, highlighting the therapeutic potential of inhibitors of the cysteine metabolic pathway135.
In another study, the direct regulation of GSH metabolism by Rb1-loss-induced E2F1 transcriptional activity was shown136. This GSH synthesis pathway has been therapeutically exploited by testing isogenic Rb1-depleted models of CRPC cell lines with the system xc− (encoded by SLC7A11) inhibitor erastin and the γ-GCS inhibitor buthionine sulfoximine136. These inhibitors were shown to have increased cytotoxicity in Rb1-knockdown compared with control prostate cancer cells, suggesting a potential novel treatment approach for RB1-deficient tumours136. Moreover, in a prostate cancer xenograft model, sulfasalazine suppressed tumour development by causing cystine deficiency and subsequent ferroptotic cell death134. These data suggest that further investigation is warranted to determine whether cystine deprivation-induced tumour regression is driven primarily by ferroptosis. Notably, sulfasalazine is an FDA-approved anti-inflammatory medication that is frequently prescribed in clinical settings to manage ulcerative colitis or rheumatoid arthritis137,138. Thus, sulfasalazine, differently from other ferroptosis inducers that have not entered clinical trials, could be rapidly repurposed for new prostate cancer trials.
Ferroptosis inducers in treatment combinations
The available treatment options for advanced prostate cancer include AR-signalling inhibition, chemotherapy, immunotherapy and radiotherapy (Fig. 2). Owing to the synthetic lethality observed between PARP inhibition and BRCA mutations, the discovery of alterations in BRCA2 and other homologous recombination repair-related genes in advanced prostate cancer has also led to the approval of PARP inhibitors for the treatment of metastatic CRPC. Crosstalk probably exists between current prostate cancer therapies and ferroptosis. Thus, combination therapies including ferroptosis inducers are anticipated to enhance the effectiveness of existing treatments (Table 1).
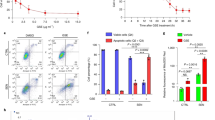
Combining ferroptosis inducers with current therapies can potentially overcome drug resistance in advanced prostate cancer. a, Ferroptosis inducers such as erastin and RAS-selective lethal 3 (RSL3) have been shown to increase the susceptibility of cancer cells to taxane-based chemotherapies in prostate cancer models30,144. Suppression of prostate cancer-associated transcript 1 (PCAT1) or upregulation of ChaC glutathione-specific γ-glutamylcyclotransferase 1 (CHAC1) could increase cell sensitivity to ferroptosis. Thus, PCAT1 and CHAC1 could be used as potential biomarkers to select patients who could receive combination therapy with docetaxel plus ferroptosis inducers to overcome docetaxel-resistant prostate cancer. b, The combination of androgen receptor (AR) signalling inhibitors with ferroptosis inducers could prevent the proliferation and metastasis of castration-resistant prostate cancer (CRPC) cell lines and xenograft mouse models28,29. Treatment with AR antagonists remodels the lipid composition of prostate cancer cells towards increased polyunsaturated fatty acid (PUFA) accumulation, rendering these cells susceptible to lipid peroxidation and increasing dependency on the lipid peroxide repair pathway to remove toxic lipid peroxides. AR, by itself, also positively regulates ferroptosis inhibitors such as SLC7A11, SLC3A2, GPX4, MBOAT2 and ACSL3, and negatively regulates ferroptosis promoters such as DECR1 and ACSL4 (refs. 50,95,157,158,159,160). Thus, inhibiting AR can further push prostate cancer cells towards a ferroptosis-prone state, justifying the rationale for combining ferroptosis inducers with AR inhibition. c, Ferroptosis induction can enhance antitumour immunity and help to overcome immunotherapy resistance. Combining ferroptosis inducers with immune checkpoint blockade has been shown to enhance antitumour immunity. Specifically, in ovarian cancer models, combining the ferroptosis inducer Cyst(e)inase with anti-PDL1 synergistically promoted T cell-mediated antitumour immunity, triggering ferroptosis in cancer cells, and slowing down tumour progression in vivo193. Moreover, CD8+ T cells activated through immunotherapy can mediate cancer cell killing through ferroptosis induction. Upon PDL1 blockade, activated CD8+ T cells release interferon-γ (IFNγ), which downregulates SLC7A11 expression in tumour cells via Janus kinase 1 (JAK1)–signal transducer and activator of transcription 1 (STAT1) signalling194. This cascade results in reduced cystine uptake, increased lipid peroxidation and subsequent ferroptosis. IFNγ released by CD8+ T cells also modifies lipid metabolism in cancer cells by activating the long-chain acyl-CoA synthetase 4 (ACSL4), in turn promoting the activation of ferroptosis-prone PUFAs and increasing tumour cell sensitivity to ferroptosis194. Last, stimulating ferroptosis also triggers the release of damage-associated molecular patterns (DAMPs) such as high mobility group box 1 (HMGB1) and ATP, and promotes the expression of cell surface-exposed calreticulin (CRT), subsequently initiating immune cell responses such as the activation of CD8+ T cells and macrophages196,197,198. Future efforts should be focused on increasing ferroptosis sensitivity only in cancer cells, sparing activated T cells. d, Little is known about radiotherapy and ferroptosis induction in advanced prostate cancer. Results from studies conducted in other cancers showed that ionizing radiation could elevate reactive oxygen species (ROS) production and, subsequently, lipid peroxidation. Radiation could also upregulate ACSL4 expression while suppressing solute carrier family 7 member 11 (SLC7A11) and glutathione (GSH) synthesis, causing a decrease in GSH and ultimately promoting ferroptosis induction214,217. A combination of radiotherapy and the poly(ADP-ribose) polymerase inhibitor (PARPi) niraparib has been shown to synergistically amplify the cytoplasmic accumulation of double-stranded DNA (dsDNA) and the subsequent activation of cyclic GMP–AMP synthase (cGAS)-mediated ferroptosis in an in vivo model of colorectal cancer219. Thus, ferroptosis inducers could enhance lipid peroxidation and ROS burden in cancer cells, in turn re-sensitizing therapy-resistant cancer cells to PARPi + radiation-induced ferroptosis. AA, arachidonic acid; APC, antigen-presenting cell; DECR1, 2,4 dienoyl-CoA reductase; GPX4, glutathione peroxidase 4; HnRNP L, heterogeneous nuclear ribonucleoprotein L; IFNR, interferon receptor; IRF1, interferon regulatory factor 1; LRP, LDL-receptor-related protein; MBOAT2, membrane-bound O-acyltransferase domain containing 2; MHC II, major histocompatibility complex class II; MUFA, monounsaturated fatty acid; PE-MUFA, phosphatidylethanolamine-containing MUFA; PE-PUFA, phosphatidylethanolamine-containing PUFA; PUFA-PL, phospholipid-containing PUFA; TCR, T cell receptor; TLR, toll-like receptor.
Ferroptosis inducers and chemotherapy
The most common chemotherapy for CRPC is docetaxel, which is a derivative of paclitaxel and, therefore, is classified as a second-generation member of the taxane family (Fig. 2a). Docetaxel works by binding and disrupting cytoskeletal microtubules during the G2 and M phases of the cell cycle, in turn impeding the normal assembly of the mitotic spindle, and preventing the completion of mitosis and proper cell proliferation, eventually resulting in cell death139. Docetaxel was also shown to inhibit the expression of the anti-apoptotic protein BCL-2, increasing the vulnerability of cancer cells to apoptosis induction140. In other studies, docetaxel was shown to prevent nuclear localization of AR in a microtubule-dependent manner141. Nevertheless, many patients inevitably acquire resistance and develop docetaxel-refractory CRPC. Thus, cabazitaxel, a distinct, third-generation taxane was authorized in 2010 by the FDA as a second-line treatment for metastatic CRPC142,143. Cabazitaxel exerts anticancer efficacy in docetaxel-resistant malignancies as well as post-docetaxel and chemotherapy-naive settings.
In a study in 22Rv1 and LNCaP95 cells, combination treatment of erastin plus docetaxel consistently outperformed the respective monotherapies in decreasing cancer cell growth30. Similarly, the ferroptosis inducers RSL3 and erastin increased docetaxel susceptibility in previously docetaxel-resistant prostate cancer cell lines144. Interestingly, the efficacy of these treatment combinations in subcutaneous xenograft tumour models of docetaxel-resistant prostate cancer cell lines was only maintained with RSL3, and not with erastin144. No mechanism for the therapeutic efficacy of this combination treatment approach was provided in either of these studies, and further research is needed to determine the effectiveness and elucidate the mechanisms of this combined approach.
Docetaxel-resistant prostate cancer cells were often shown to be insensitive to ferroptosis; some mechanisms that could explain this observation have been proposed. For instance, prostate cancer-associated transcript 1 (PCAT1), which is substantially elevated in both docetaxel-resistant clinical samples obtained from patients with prostate cancer who received radical prostatectomy and prostate cancer cell lines145, was shown to suppress ferroptosis by interacting with MYC to maintain MYC stability and promote downstream SLC7A11 expression. Deletion of PCAT1 augmented ferroptosis and the susceptibility of prostate cancer to docetaxel treatment, observed as a reduction in cell viability and colony formation upon docetaxel exposure, whereas PCAT1 overexpression reversed these effects145. In another study, the expression of ChaC glutathione-specific γ-glutamylcyclotransferase 1 (CHAC1) was shown to be lower in prostate cancer cell lines (DU145 and 22RV1) than in non-transformed human prostate epithelial RWPE-1 cells146. The activity of CHAC1 can reduce intracellular GSH levels147; thus, overexpressing CHAC1 in DU145 and 22RV1 cells resulted in increased intracellular lipid peroxides owing to the lack of the active antioxidant GSH, and sensitized prostate cancer cells not only to ferroptosis induction through erastin but also to docetaxel146. This sensitization to docetaxel was shown to be dependent on ferroptosis, as the use of a ferroptosis inhibitor abolished the docetaxel sensitivity of prostate cancer cells observed with CHAC1 overexpression146. Thus, PCAT1 and CHAC1 could be used as potential biomarkers to select the right patient population suitable for combined treatment with docetaxel and ferroptosis inducers. Patients with docetaxel-resistant prostate cancer, especially those with increased PCAT1 and suppressed CHAC1 expression, might be candidates for this combined approach. To date, combination treatments with ferroptosis inducers and cabazitaxel have not been reported.
Ferroptosis inducers and hormone therapy
Increased or sustained AR signalling remains the principal mechanism underpinning CRPC progression148,149,150. Underlying mechanisms include AR gene or enhancer amplifications, de novo androgen synthesis by cancer cells and the production of various splice variants missing the ligand-binding domain151. First-line CRPC treatments most often involve second-generation AR signalling inhibitors such as the androgen biosynthesis inhibitor abiraterone or the AR antagonists enzalutamide, apalutamide and darolutamide152,153. Co-inhibition of AR and PARP was shown to benefit genomically selected patients with prostate cancer harbouring specific mutations in homologous recombination-repair genes154,155. PARP inhibitors can downregulate the expression of SLC7A11 in a p53-dependent manner, indicating a potential role of PARP inhibitors in ferroptosis induction156. The mechanistic understanding of how these second-generation anti-androgens and PARP inhibitors could enhance the efficacy of ferroptosis inducers or vice versa in treating CRPCs is still preliminary.
AR can strengthen the oxidative resistance mechanisms of cells by directly binding to the promoter regions and promoting the expression of anti-oxidant-defence genes such as GPX4, SLC7A11, and SLC3A2 (refs. 157,158,159); thus, suppressing AR activity using anti-androgens has inherent ferroptosis-induction capability and could also synergize with ferroptosis inducers. In LNCaP cells, a substantial lipid remodelling with elevated levels of desaturation and acyl chain lengthening of membrane lipids was shown upon treatment with enzalutamide28. This enzalutamide-induced remodelling raised membrane PUFA content, increasing membrane fluidity and lipid peroxidation, which in turn led to increased susceptibility to GPX4 inhibition and ferroptosis28 (Fig. 2b). In another study in C4-2 adeno-CRPC cells, which express AR and are sensitive to anti-androgens, erastin or RSL3 in combination with either enzalutamide or abiraterone substantially decreased colony formation, migration and invasion compared with erastin or RSL3 alone29. In this study, erastin or RSL3 in combination with enzalutamide also effectively reduced tumour development in vivo in C4-2 xenografts compared with single agents, without having discernible effects on body weight29. Altogether, the combined approach using second-generation anti-androgens and ferroptosis inducers seems promising for the treatment of adeno-CRPC prostate cancer29. Last, compelling evidence supporting the use of ferroptosis inducers with second-generation anti-androgens for the treatment of CRPC was presented in a study in which ferroptosis regulation was shown to occur independently of GPX4 in prostate (and breast) cancers160. Specifically, the enzyme membrane bound O-acyltransferase domain containing 2 (MBOAT2) — which selectively transfers MUFAs to the Sn2 position of lysophosphatidylethanolamine (lyso-PE), lysophosphatidylcholine (lyso-PC) and lysophosphatidic acid (lyso-PA)161,162 — was identified as a suppressor of ferroptosis through phospholipid modification independently of GPX4 or FSP1 (ref. 160) MBOAT2 was hypothesized to compete for lyso-PE with PUFA-preferred lysophosphatidylethanolamine acyltransferase (such as LPCAT3). In this study, overexpressing MBOAT2 in the fibrosarcoma cell line HT-1080 resulted in a decrease in PE-PUFAs (particularly PE-AAs), whereas PE-MUFAs (particularly PE-OAs (18:1)) were increased. Thus, in cells or tissues with elevated MBOAT2 activity, PE remodelling could move the cells towards a state of high PE-MUFA but reduced PE-PUFA, resulting in ferroptosis resistance. Importantly, in this study, MBOAT2 was also shown to be transcriptionally upregulated by AR and its cofactor FOXA1 both in human prostate cancer samples and in human prostate cancer cell lines160 (Fig. 2b). The combination of an AR antagonist (such as enzalutamide) with ferroptosis induction (through RSL3) showed a substantial growth inhibition effect in AR-positive prostate cancer cell lines, and in prostate cancer cells resistant to single-agent hormonal therapies160. These results indicate that ferroptosis induction, either alone or in conjunction with AR-targeting agents, might constitute a promising treatment approach for advanced prostate cancer.
Ferroptosis inducers and immunotherapy
Immunotherapy has revolutionized cancer treatment since the FDA approval of the first immune checkpoint inhibitor (ICI) in 2011 (ref. 163). In prostate cancer, the launch of sipuleucel-T (Fig. 2c), an autologous active cellular immunotherapy approved for men with asymptomatic or minimally symptomatic metastatic CRPC, and ICIs for men with microsatellite instability-high disease offered additional therapeutic options for this subset of patients. However, to date, immunotherapy has had limited overall success in prostate cancer owing to the existence of a strong immunosuppressive TME preventing the infiltration of T cells, a substantial heterogeneity in genomic alterations and low expression of PDL1 (ref. 164). Nevertheless, multiple clinical trials have been carried out to investigate the efficacy of immunotherapy as a single-agent therapy and in conjunction with other non-immunotherapies. Current classes of immunotherapies being tested in advanced prostate cancer include ICIs that target CTLA4 (such as ipilimumab165,166,167) or PD1 and PDL1 (such as nivolumab168,169,170,171, pembrolizumab171,172, atezolizumab173,174, avelumab175,176 and durvalumab177); vaccine-based therapies including sipuleucel-T178, DNA vaccines179,180, PSA-TRICOM vaccine (Prostvac)181,182 and GVAX183,184,185; adoptive cell therapy such as the prostate-specific membrane antigen (PSMA) chimeric antigen receptor T cell therapy186,187,188 and tumour-infiltrating lymphocyte therapy189,190; and bispecific T cell engager antibodies that target PSMA such as AMG 212 (pasotuxizumab)191,192.
Interestingly, ferroptosis has been shown to enhance antitumour immunity and help to overcome immunotherapy resistance in melanoma, fibrosarcoma and glioma193,194,195,196,197. In one study in glioma cells, early ferroptotic cells were shown to secrete danger-associated molecular patterns (DAMPs) (such as HMGB1 and ATP), resulting in the maturation of dendritic cells and the subsequent induction of cytotoxic T cell-mediated adaptive immunity196,197,198. In the context of prostate cancer, calreticulin, which is another type of DAMP, was found to be exposed on transgenic adenocarcinoma of the mouse prostate TRAMP-C1 cancer cells after treatment with vesicles containing ascorbic acid and ferroptosis-inducing iron oxide nanocubes199. Prostate cancer is characterized by poor infiltration of CD8+ T cells200,201; thus, inducing ferroptosis in prostate cancer cells could improve cytotoxic T cell-mediated adaptive immunity and become an attractive approach to improving the efficacy of immunotherapy. Nonetheless, caution should be exerted with the use of ferroptosis inducers, as results from previous studies showed that T cells are also susceptible to ferroptosis202. For example, CD36-mediated uptake of AA from the TME by CD8+ T cells could trigger T cell ferroptosis203. Thus, efforts aimed at inducing ferroptosis in combination with immunotherapies would necessitate the discovery of novel mechanisms that selectively promote ferroptosis in cancer cells, sparing CD8+ T cells.
In other studies, immune cells were shown to induce ferroptosis in tumour cells. CD8+ T cells can induce ferroptosis in ovarian tumour cells by secreting interferon-γ (IFNγ) into the TME, suppressing the expression of SLC3A2 and SLC7A11 in tumour cells, and limiting cystine import and the ability of tumour cells to remove lipid peroxides193. Thus, combining ferroptosis inducers such as Cyst(e)inases with anti-PDL1 antibody could synergistically amplify T cell-mediated antitumour immunity193.
IFNγ and dietary AA were shown to promote ACSL4-dependent tumour ferroptosis through the JAK–STAT1–IRF1 signalling pathway in both mouse and human melanoma cell lines194. Consequently, in syngeneic mouse models bearing murine colon cancer MC38 or murine melanoma Yumm5.2 cells, combination therapy including low-dose AA and anti-PDL1 yielded maximal tumour growth inhibition compared with single-agent therapies with AA or anti-PDL1 alone194. Notably, in this study, AA alone could increase the population of IFNγ+TNF+granzyme B+CD8+ T cells in the TME, indicating that AA reduces tumour cell growth and also increases the recruitment and activity of immune cells194.
In the context of prostate cancer, deletion of the heterogeneous ribonucleoprotein L (HnRNP L) in human CRPC cell lines suppressed the expression of CD274 (encoding PDL1) by inhibiting the transcription factor YY1 (ref. 204). Thus, in the RM-1 syngeneic mouse model, knockdown of HnRNP L and the concomitant reduction in PDL1 led to CD4+ and CD8+ T cell tumour infiltration, preventing immune escape. Active T cells could in turn trigger ferroptosis in prostate cancer cells and suppress prostate cancer tumour growth. Results from this study indicate the importance of inhibiting HnRNP L to stimulate T cell-mediated ferroptosis, in turn improving the efficacy of existing immunotherapy204. In this study, the potential for combining ferroptosis inducers with anti-PDL1 blockade in prostate cancer was not further explored, but the use of ferroptosis inducers could synergistically potentiate the tumour-killing effect of anti-PDL1 blockade. Besides these studies, our knowledge of the effects of combined ferroptosis inducers with immunotherapy for advanced prostate cancer is scarce. Thus, the potential therapeutic benefits of this combination remain underexplored.
Ferroptosis inducers, radiotherapy and other DNA-damaging agents
Current systemic radiotherapy treatment regimens for advanced prostate cancer include the use of radium-223 dichloride (radium-223), an α-emitting radionuclide, to specifically target bone metastases, or molecularly targeting agents such as lutetium-177-prostate-specific membrane antigen-617 (177Lu-PSMA-617) (Fig. 2d). Radium-223 is an intravenously injected salt that mimics calcium, binding to hydroxyapatite and assimilating into bone areas where active mineralization occurs205,206. In 2022, the FDA approved 177Lu-PSMA-617 as the first PSMA-targeted radioligand therapy for the treatment of patients with PSMA-positive metastatic CRPC resistant to anti-androgen drugs and chemotherapy207,208,209. Additional PSMA-targeting radioisotopes are currently being intensely investigated for the treatment of CRPCs, such as 213Bi-PSMA-617 (ref. 210), 227Th-PSMA-IgG1 (ref. 211), and 211At-PSMA-pentanedioic acid (ref. 212). Little is known about how radiotherapy interacts with ferroptosis pathways in CRPCs, but insights into the effects of radiotherapy on ferroptosis come from studies in other cancer types.
In other cancers such as uterine sarcoma, fibrosarcoma, glioblastoma and lung carcinomas, ionizing radiations were shown to amplify ROS production, induce lipid peroxidation26,213, increase the expression of ferroptosis marker genes such as PTGS2 and ACSL4 (ref. 26), and suppress SLC7A11 expression to cause the subsequent depletion of GSH214, whereas upregulation of the ferroptosis suppressors SLC7A11 and GPX4 was shown to trigger radiation resistance26. A possible explanation for the radiation-induced repression of SLC7A11 could be that, upon exposure to radiation, p53 accumulates intracellularly to induce cell-cycle arrest and apoptosis215,216. p53 is known to transcriptionally suppress SLC7A11 expression200 by either occupying the SLC7A11 promoter region217 or removing the transcriptionally activating histone H2B on lysine 120 from the SLC7A11 promoter218. Thus, ferroptosis inducers in conjunction with radiation can further impair the anti-oxidant defences of cancer cells and show synergistic effects on tumour suppression. Moreover, cancer cells can upregulate SLC7A11 and GPX4 to build resistance against radiation26; thus, the heavy reliance on SLC7A11 and GPX4 to cope with excess ROS and toxic hydroperoxides can make these cells highly susceptible to ferroptosis inducers.
PARP inhibitors revolutionized the cancer treatment paradigm and became a mainstay of therapy with radiation in clinical trials. PARP inhibitors were shown to sensitize colon cancer cell lines to radiation-induced ferroptosis through the activation of the transcription factor 3–SLC7A11 axis219. These results suggest that a combination therapy involving radiation, PARP inhibitors and ferroptosis inducers could provide enhanced antitumour effects compared with radiation (or ferroptosis induction) alone220,221. As advanced prostate cancers (both adeno-CRPC and neuroendocrine prostate cancer) express high levels of SLC7A11, GPX4 (ref. 29), and ACSL4 (ref. 52), we hypothesize that ferroptosis inducers that suppress either SLC7A11 or GPX4 could enhance lipid peroxidation and re-sensitize therapy-resistant cancer cells to radiation-induced ferroptosis, culminating in radiosensitization.
Ferroptosis inducers and diet
Increasing interest exists in understanding how dietary changes can enhance patient well-being during cancer treatment. Results from several studies showed that dietary levels of various amino acids, MUFAs, PUFAs and glucose can influence the efficacy of ferroptosis inducers in different cancers. For example, dietary cysteine and methionine deprivation in glioma cell lines and mouse models resulted in enhanced lipid peroxidation and decreased GSH levels222. In this study, cysteine and methionine deprivation increased sensitivity to RSL3 treatment, as shown by a decrease in cell viability following treatment. Furthermore, this combination increased lipid peroxidation to levels similar to those obtained with a high dose of RSL3 alone222. Results from another study showed that intermittent methionine deprivation followed by imidazole ketone erastin treatment or cysteine deprivation enhanced ferroptosis induction though increased CHAC1 expression and GSH depletion223. These results suggest that a methionine-free and cysteine-free diet in combination with ferroptosis inducers might be an efficient therapeutic option for patients with CHAC1-deficient prostate cancer. Furthermore, co-treatment with erastin and exogenous MUFAs, specifically oleic and palmitoleic acid, resulted in suppression of ferroptosis in HT-1080 fibrosarcoma cells, A549 non-small-cell lung cancer cells, and T98G glioblastoma cells, highlighting the importance of MUFA:PUFA ratios in the TME45. Mechanistically, the anti-ferroptotic effects of oleic acid did not decrease ACSL4 or GPX4 expression, but reduced the abundance of PUFA-PLs and ROS in the plasma membrane in an ACSL3-dependent manner45. Conversely, results from another study showed that an increase in n-3 and n-6 PUFA supplementation decreased cell growth under neutral conditions but induced ferroptosis in cervical, colorectal and hypopharyngeal cancer cell lines cultured under acidic conditions, indicating that enhanced PUFA uptake by cancer cells in acidic environments increases the susceptibility of these cells to ferroptosis as a result of increased lipid peroxidation224. Moreover, a PUFA-rich diet in combination with sulfasalazine and erastin treatment was shown to increase the antitumour effect of ferroptosis inducers in HCT-116 tumour-bearing mice. In the same model, treatment with the ferroptosis inhibitor ferrostatin-1 in combination with a PUFA-rich diet, resulted in increased tumour growth and substantially reduced survival224. Together, results from these studies suggest that patients with cancer receiving treatment with ferroptosis inducers might benefit from a PUFA-rich diet compared with a MUFA-rich diet. Further investigation is needed to determine the effects of dietary fatty acid supplementation in conjunction with ferroptosis inducers in patients.
The ketogenic (keto) diet has gained attention as a therapeutic intervention for patients with end-stage cancer suffering from cancer-induced cachexia. Mouse models of colorectal and pancreatic cancers fed a keto diet showed a decrease in the GSH-to-oxidized GSH ratio in the liver, suggesting a decrease in GPX4 activity225. A decrease in cysteine, a crucial metabolite necessary for GSH synthesis, was also observed225. Moreover, tumours from keto diet-fed mice showed increased mutagenic lipid peroxides, as well as accumulation of lipid droplets and ferrous iron levels. Additionally, keto diet-fed mice showed accumulation of 4-hydroxynonenal, a product of lipid peroxidation, in the liver, suggesting increased ferroptotic cell death in these tissues225. This increase in lipid peroxidation in keto diet-fed mice was reduced following treatment with N-acetyl cysteine, an anti-oxidant that increases GSH synthesis. These results suggest that a decrease in GSH synthesis induced by keto diet yields decreased GPX4 activity, promoting ferroptosis225. Additionally, the amino acid composition of the keto diet might affect ferroptosis independently of lipid peroxidation, as the low methionine content in the keto diet was shown to largely contribute to a decrease in the expression of genes involved in FAO in the liver226. Together, results from these studies suggest that the decrease in GSH synthesis and low methionine content provided by the keto diet might further sensitize cancer cells to ferroptosis inducers. However, no studies in which treatment with ferroptosis inducers together with the keto diet was assessed in patients with cancer are currently available.
Taken together, these findings imply that diet can alter the susceptibility to ferroptosis. However, in all the currently available studies, dietary changes led to a decrease in weight compared with mice that were fed normal diets. This evidence potentially raises concerns about whether these alternative diets would be beneficial to overall patient health, despite potential therapeutic benefits. These studies are also limited to preclinical cancer models; thus, the influence of special diets on the metabolic reprogramming that regulates ferroptosis and sensitizes cancer cells to novel and existing therapies needs to be explored in clinical settings. This research will help to optimize diets that could improve the overall health of patients with cancer.
Future of ferroptosis inducers
New ferroptosis inducers such as JKE-1674 (refs. 227,228) or HSB-1216 have emerged over the past 5 years. JKE-1674, a GPX4 inhibitor, is a derivative of the selective covalent GPX4 inhibitor ML210 that is more metabolically stable and has fewer off-target effects227. Moreover, the water solubility of JKE-1674 is greatly enhanced by the α-nitroketoxime group, enabling this drug to be detected in the serum of mice227. This characteristic is an improvement over other ferroptosis inducers such as erastin and RSL3, which have low solubility and undesirable absorption, distribution, metabolism and excretion characteristics, making them unfavourable for clinical translation4,229. To date, the effect of JKE-1674 in prostate cancer was only assessed in one study. In this study, JKE-1674 suppressed prostate tumour growth and metastasis in prostate epithelium-specific Pten and Rb1 double-knockout mice53.
The development of FSP1-targeted therapeutics remains an underexplored area. iFSP1, a human FSP1-specific inhibitor, was found to sensitize glioblastoma, lung, breast and colon cancers to ferroptosis induction through GPX4 inhibition230,231. Nevertheless, iFSP1 is deemed unsuitable for use in murine models owing to its inability to bind to and inhibit mouse FSP1 (refs. 230,232). Thus, new FSP1 inhibitors with improved pharmacokinetic and pharmacodynamic properties, such as the ferroptosis sensitizer 1 (ref. 233) and NPD4928 (ref. 234), have been developed. Many of these inhibitors are yet to be evaluated in preclinical mouse models of cancer.
HSB-1216 is salinomycin encapsulated in polymeric nanoparticles made of PEG-polypropylene glycol (PPG)-PEG-modified PLA tetra-block copolymer. Salinomycin induces the sequestration of iron in lysosomes and the subsequent breakdown of lysosomal ferritin235,236,237. Lysosomal iron overload then prompts increased production of ROS and drives lysosomal membrane permeabilization, triggering a ferroptosis-like cell death pathway238. HSB-1216 was shown to suppress tumour sphere formation in a dose-dependent manner in a model of late-stage small-cell lung cancer239. In acute myeloid leukaemia cell lines, HSB-1216 also reduced clonogenic survival and increased cancer cell death240. Future studies are warranted to investigate the therapeutic promise of combining HSB-1216 with anti-androgens for the treatment of prostate cancer.
Advances in ferroptosis imaging
Considering the therapeutic potential of ferroptosis and novel approaches targeting this cell-death mechanism, using complementary diagnostic imaging and anticancer approaches will be important for understanding mechanisms of action and subsequently leveraging ferroptosis in the clinic. Ferroptosis imaging has become a major research area of interest in the past decade, both to understand the molecular mechanisms of ferroptosis — to delineate specific biological processes that might not be otherwise visualized directly — and to assess the efficacy of ferroptosis-inducing cancer therapy (for example, using imaging as a pharmacodynamic biomarker) (Box 1). Different from other types of cell death, ferroptotic cells often do not show obvious morphological changes in cell membranes, organelles, nuclei or chromatin structures, which is a challenge for the direct detection of ferroptosis241. Furthermore, several markers of ferroptosis including Fe2+ and lipid peroxides are usually transient and have relatively low expression levels, beyond detection limits of conventional assays242,243. These challenges in monitoring ferroptosis could help to explain why unique ferroptosis mechanisms were not identified as early as other types of cell death. Great efforts have been made by several groups to monitor ferroptosis-associated mechanisms both in vitro and in vivo244,245,246. Owing to the biological complexity and challenges in the detection of ferroptosis, several different approaches focusing on different aspects of the ferroptotic pathways have been used. For example, iron metabolism and lipid peroxidation are hallmarks of ferroptosis. Direct detection of these processes is difficult owing to the short lifespan of these molecules. Moreover, no overt correlations between iron levels and tumour state have been identified. Thus, products of lipid peroxidation (such as levels of malondialdehyde and 4-hydroxynonenal) are currently being investigated as stable biomarkers of ferroptosis18. However, these molecules are static and indirect markers of ferroptosis. Alternatively, novel, non-invasive imaging modalities could provide improved longitudinal and spatial insight into ferroptosis activity and tumour biology that could complement existing approaches. These technologies would provide pharmacodynamic biomarkers of ferroptosis-targeting agents and, possibly, predictive biomarkers to select the optimal patient population for this therapeutic approach. No single consensus ferroptosis pathway exists for all circumstances; thus, multiple biomarkers will probably be needed to accurately report which aspects of ferroptosis are being altered.
Fluorescence probes
Initially, imaging probes and fluorescence-based detection methods were the most widely used methodologies to monitor ferroptosis owing to the ease of detection in in vitro settings. One of the hallmarks of ferroptosis is the peroxidation of PUFAs, as uncontrolled lipid peroxidation and subsequent reactive aldehyde production leads to membrane rupture and induction of ferroptosis (Fig. 1). Several chemical approaches have been developed to capture and visualize this step in ferroptosis to identify ferroptosis susceptibility in different physiological and disease conditions247,248. In one study, a technique was developed to use photochemical activation of membrane lipid peroxidation (PALP) primarily to stratify cells and tissues based on their sensitivity to ferroptosis induction249. In studies in which lipid peroxidation was induced with a laser, fluorescence changes occurred based on the oxidation sensitivity of BODIPY-C11, enabling researchers to monitor lipid peroxidation rates and susceptibility to ferroptosis in different cell types250,251. A limitation of this technique is the requirement of tissue samples from patients, limiting the ability to assess patient sensitivity to ferroptosis induction in real time. Thus, PALP is a powerful method for identifying cell and tissue sections that are susceptible to ferroptosis-mediated cancer treatment but is not amenable to high-throughput patient screening. In another study, ferroptosis initiation was indirectly monitored by measuring H2O2 levels in live cells using fluorescent probes245. This technique provides a viable approach to measuring the cellular dynamics of redox sensitivity during ferroptosis but is currently limited to in vitro monitoring and only a handful of animal studies, primarily owing to the low signal strength and imprecise methods of specifically targeting tumour cells. These fluorescence techniques are currently used as an important tool for delineating mechanisms of action of ferroptosis. One method of measuring levels of Fe2+ and lipid peroxides are fluorescent substrates such as RhoNox-1 and BODIPY 581/591 C11 reagents. RhoNox-1 is a cell-permeable compound that can selectively detect basal and endogenous labile Fe2+ by enhancing fluorescence signal in living cells252; thus, RhoNox-1 provides advantages over conventional ferroptosis biomarkers such as malondialdehyde and 4-hydroxynonenal. Similarly, BODIPY 581/591 C11 has been used to detect cellular lipid peroxidation by fluorescence shift from red to green after reacting with lipid peroxides in living cells251.
Mass spectrometry
Mass spectrometry (MS) has been exploited to measure the levels of lipid hydroperoxides during ferroptosis253. Using the mass-to-charge ratio of lipid molecules, MS provides precise information on the molecular mass, elemental structure and the chemical structure of lipids. MS-based techniques have been used to identify biomarkers of ferroptosis85. In one study, liquid chromatography–mass spectrometry-based redox lipidomics was used to show that oxidation of specific phosphatidylethanolamines acted as death signals during ferroptosis85. In another study, matrix-assisted laser desorption/ionization-mass spectrometry was used to measure the peroxidation of long-chain PUFAs during ferroptosis in adrenal cell death254. With these MS-based approaches, unique and specific signals that trigger ferroptosis can be quantified with high precision. Substantial developments using MS-based techniques are underway for the development of reliable clinical biomarkers for ferroptosis.
PET
PET imaging enables precise, in vivo, non-invasive monitoring of ferroptosis. Some PET tracers achieve this effect by tracking labile Fe2+ ions. In one study, a novel PET radiotracer termed 18F-TRX was used to quantify the intracellular labile iron pool in mouse orthotopic human xenograft models. In this study, glioma and renal cell carcinoma cells were used owing to the high expression of the oxidoreductase STEAP3 in these cells255. This PET imaging modality offers substantial advantages over current methods based on fluorescent probes either acting by chelation or reactivity to labile ions, owing to the high sensitivity of deduction and direct translational ability in clinical settings, as PET is a widely established non-invasive approach. The safety and feasibility of this approach are currently being studied in a phase II clinical trial investigating malaria, as the structure of 18F-TRX is based on artefenomel, an antimalarial compound. The success of this trial will enable repurposing of 18F-TRX for ferroptosis detection in cancer256. If successful, 18F-TRX could also help to predict and monitor ferroptosis sensitivity during cancer treatment.
An alternative approach to detecting ferroptosis sensitivity is by monitoring transferrin uptake using the cell membrane-localized iron transporter TfR1 (ref. 257). This approach is useful in determining the iron levels and infer the ferroptosis sensitivity of cancer cells, and might also be useful for determining correlations between specific cancer types and metabolic states in personalized cancer treatment approaches. Exploiting this mechanism, human transferrin (Tf) labelled with gallium-68 (68Ga) using 2-(p-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) as a chelator (68Ga-NOTA-Tf) was developed to create PET probes that could reflect iron uptake into cancer cells258. As hypothesized, 68Ga-NOTA-Tf showed that cells expressing high TfR1 levels had higher iron levels than cells having baseline expression of TfR1. Thus, 68Ga-NOTA-Tf could be used clinically as a predictor of susceptibility to iron-dependent processes such as ferroptosis.
Another PET agent for visualizing ferroptosis is (4S)-4-(3-[18F]fluoropropyl)-l-glutamate (18F-FSPG), an 18F-labelled glutamate derivative. 18F-FSPG is transported specifically through the xCT subunit (encoded by SLC7A11) of the xc− system, which is upregulated in cancer in response to oxidative stress259. Notably, 18F-FSPG has been successfully used in rodent models to non-invasively monitor xc− activity in multiple sclerosis and has been shown to have high blood clearance and low background activity. In this study, which was primarily focused on identifying neuroimmune interactions in multiple sclerosis, 18F-FSPG uptake was substantially higher in mice with multiple sclerosis than in control mice. This effect was primarily dependent on xCT subunit activity, providing promise and proof of concept for usage of 18F-FSPG for monitoring the xc− system260. Results from these preclinical studies and ongoing clinical trials have shown 18F-FSPG to be effective in monitoring ferroptosis in patients with prostate cancer, but also in other relevant tumour models with high xc− system activity. Specifically, in a clinical evaluation study in which 18F-FSPG was used for the first time as a radiopharmaceutical for PET imaging of system xc− activity in patients with recurrent prostate cancer261, 18F-FSPG PET was able to differentiate cancerous prostate lesions from normal tissue, and 18F-FSPG uptake also correlated with pathological and IHC markers for xc− system activity. This landmark study was the first to establish the use of 18F-FSPG to monitor xc− system activity in prostate cancer. Further studies are required to establish clinical protocols of dosing, diagnostic value for different stages of cancer and comparison with other clinically established radiotracers261. Furthermore, several clinical trials are currently underway and are focused on diagnosing, predicting and monitoring of a wide variety of tumours using 18F-FSPG, such as liver, lung, breast and colorectal cancer259,261,262.
Results from these studies suggest that clinically viable methods, such as Cyst(e)inase, imidazole ketone erastin, or sulfasalazine, already exist to evaluate the efficacy of ferroptosis inducers that inhibit system xc− activity and could aid the development of ferroptosis-targeting clinical regimens.
MRI
Few methodologies have been developed for imaging ferroptosis using MRI. MRI has been used mostly for resolving soft tissue and anatomical structures in a non-invasive manner, with the use of traditional MRI acquisition being limited in imaging at the molecular scale. In 2023, this notion was challenged by a study in which an emerging MRI-based technique referred to as quantitative susceptibility mapping was used to detect ferritin levels in in vivo models263. In this study, an artemisinin-based MRI probe was developed by chelating gadolinium ions (Gd (III)) through a 1,4,7,10-tetraazacyclododecane–1,4,7,10-tetraacetic acid (DOTA) group (Art-Gd). Upon interaction with labile Fe2+ ions, Art-Gd forms carbon-centred radicals, leading to the formation of Art–Gd protein complexes, as well as the retention of these complexes in cells and tissues and enhanced longitudinal relaxation time (T1) contrast. This technique enables real-time MRI in vivo, although the feasibility and toxicity of this approach have not been confirmed in clinical settings.
Fe-based MRI contrast agents have been developed that also act as theranostic agents to induce ferroptosis. For example, nanoparticles containing the pH-responsive contrast agent DFeZd, which has a higher T1 signal in tumour sites than in normal adjacent tissues, cause reduction of Fe3+ to Fe2+ once activated in the acidic TME, promoting ferroptosis. Thus, this approach enables both detection of tumour tissues and selective induction of ferroptosis in the identified tumour sites264. In another study, a novel magnetic nanocatalyst (iRGD-PEG-ss-PEG-modified gadolinium engineering of magnetic iron oxide-loaded Dox (ipGdIO-Dox)) was developed for MRI-guided chemotherapy and ferroptosis-synergistic cancer therapies265. IpGdIO-Dox has enhanced T1- and T2-weighted MRI signals in the tumour, and simultaneously induces ferroptosis because the Fe2+ ions catalyse the production of OH radicals from H2O2. Furthermore, the ipGdIO-Dox is broken down by high GSH levels often found in advanced tumours, leading to the release of doxorubicin, which provides synergistic antitumour effects with chemotherapy. Other redox-based ferroptosis-detecting agents have also been developed and might have clinical value. For example, an Fe3O4-PLGA-Ce6 nanosystem was developed to induce ferroptosis in cancer cells, concomitantly providing efficient MRI detection properties and synergistic delivery of chemotherapeutic agents266. Many studies in which MRI-based approaches are used for ferroptosis are focused on dual detection of pH and iron metabolism, and complementary anticancer theranostic activity. This direction can be attributed to the inherent challenges of obtaining molecular-scale imaging of ferroptosis mechanisms, which lead to the need to measure multiple parameters at once (for example, pH and iron states) to obtain an accurate measure of ferroptosis. MRI detection strategies are currently limited to measurements of pH, labile Fe ions and redox levels. Thus, the development of novel MRI contrast agents that can detect additional molecules relevant to ferroptosis, such as lipid peroxides, iron transporters and GSH targets, would improve the real-time imaging of ferroptosis.
Future perspectives
Considering the potential anticancer activity of ferroptosis, great interest has been focused on developing novel methodologies and chemical tools to monitor ferroptosis in the clinical setting. Despite rapid advancements in the detection of ferroptosis, several challenges remain. Ferroptosis is a multifaceted biological process, and many detection techniques only focus on a single phenotype or marker267, and still no specific markers of ferroptosis exist241. Thus, identifying specific markers of ferroptosis is of great importance. Moving forward, developing multi-biomarker tracking techniques that enable detection of simultaneous mechanisms involved in ferroptosis such as lipid peroxidation, redox levels and Fe2+ status will be imperative. Fluorescent probes with different chemical compositions and detection strategies can also be used to observe lipid compositions within plasma membranes, serving as additional indicators in ferroptosis. However, the scope of these probes is limited by the number of channels of detection than can be used. Additionally, current fluorescent probes are limited in wavelength, as most are in the 400–500 nm range, which has low signal detection strength and autofluorescence. Thus, development of near infrared probes is warranted, as these probes would enable long-range detection and would probably increase the accuracy of measurements247.
The reliance on one-parameter detection of ferroptosis is primarily owing to the lack of clinically reliable and biologically associable ferroptosis biomarkers3. Several previously established biomarkers such as PTGS2 and CHAC1 mRNA do not have direct correlation with molecular events and do not have robust clinical relevance243. Thus, developing reliable clinical biomarkers for ferroptosis and characterizing sensitivity to ferroptosis-based treatments for cancer are of great importance. To address this challenge, advancements in redox-based phospholipidomics and the identification of specific lipids that lead to ferroptosis can enable the characterization of precise biomarkers that could be used to interrogate patient biopsy samples to robustly detect lipid peroxidation268.
Innovative approaches have been developed for detecting cancer tissues sensitive to ferroptosis-mediated anticancer therapy, but the efficacy, toxicity and timelines of these therapeutic measures in clinical settings are not fully known268,269. Predictive biomarkers of sensitivity to ferroptosis induction in tissues are under development and are largely linked to the underlying biological mechanisms of this process, such as the levels of labile Fe2+ and lipid peroxidation270. Strategies such as PALP can be applied to limited sample types, whereas real-time imaging techniques such as PET and MRI offer more tractable methods that can be readily translated to the clinic to be used as pharmacodynamic biomarkers of ferroptosis-causing agents and, possibly, to guide patient selection in the future. To date, only single-marker-based targeting approaches have been explored in preclinical settings. Thus, combining two or more markers involved in different stages of ferroptosis could provide a more accurate assessment of ferroptosis (which is a multistep process consisting of multiple regulatory nodes), yielding important breakthroughs in cancer treatment.
Current knowledge of alternative ferroptosis regulatory pathways and the role of metabolism in cancer is limited, particularly in prostate cancer271. Many open questions remain, for example, about the clear mechanistic crosstalk between ferroptosis, additional cell-death pathways and metabolic changes in tumours. An advancement in this area is the use of machine-learning tools to distinguish and classify apoptotic cell-death and ferroptosis features through the perturbation of different metabolic pathways and ferroptosis surveillance systems272. The use of machine learning is in its infancy and only tissue sections can currently be used; however, this approach has tremendous potential in analysing high-throughput data in clinical settings in combination with the establishment of clear biomarkers and robust phenotypes for ferroptosis-guided therapy.
Conclusions
Since the coining of the term ferroptosis in 2012, the number of publications investigating this mechanism has grown exponentially. In the past ~10 years, most research in the field has been dedicated to uncovering the mechanistic underpinnings and various metabolic pathways regulating lipid peroxidation and ferroptosis. Induction of ferroptosis has emerged as a promising therapeutic approach to cancer. Ferroptosis inducers, alone or in combination with existing treatments, are novel therapeutic approaches for the treatment of aggressive prostate cancer. However, additional questions remain. For instance, a gap remains in our understanding of the precise role of ferroptosis-related genes in prostate cancer cells. Reliable ferroptosis markers are urgently needed to accurately predict and monitor ferroptosis induction and its effects in cancer cells in vivo in real time. Sensitivity to ferroptosis differs across various prostate cancer cell lines. Thus, developing further strategies to overcome cellular tolerance to ferroptosis is needed. Considering the importance of AR signalling in prostate cancer progression and the emerging role of ferroptosis in cancer therapy, understanding how these pathways intersect could aid the development of improved treatment strategies. Future clinical studies are warranted to substantiate the role of ferroptosis in prostate cancer treatment. Nevertheless, rapid progress has been made towards leveraging the dependence of cancer cells on ferroptosis-related pathways as a treatment strategy. Specifically, combining ferroptosis inducers with chemotherapy, hormone therapy, radiotherapy or immunotherapy might each improve the anticancer effect of these approaches and overcome therapy resistance (Fig. 2). However, most of these findings came from preclinical studies in cell and mouse models. Currently available cell-line and patient-derived xenograft models are advantageous for studying prostate cancer biology and testing the efficacy of treatments, but the lack of heterogeneity in cell lines and the lack of appropriate in vivo models in prostate cancer research limit the translation of preclinical findings to the clinic. In particular, supraphysiological levels of nutrients in culture media can cause unpredicted effects on the phenotypic behaviour of cells and affect the metabolic fidelity of cancer-cell models, which makes the results of metabolic drug screening unreliable. Xenograft models do not fully recapitulate the human immune system, TME and different patient ethnicities, indicating the need for reliable in vivo prostate cancer models with increased fidelity to the disease. Last, numerous ferroptosis inducers have potent anticancer activity in vitro, but issues with the pharmacokinetic properties of these agents indicate that further research is needed to translate these ferroptosis-based compounds into clinical use.
Advances in ferroptosis detection approaches and biomarker identification hold promise for disease monitoring and therapy management. In this regard, new imaging methodologies for monitoring ferroptosis could be used to predict treatment efficacy of ferroptosis inducers and could also serve as pharmacodynamic biomarkers to monitor the efficacy of ferroptosis-targeting agents. A personalized approach integrating multiple biomarker-driven patient selection and subsequent tumour monitoring strategies is anticipated to help to advance the development and clinical implementation of ferroptosis-based therapies, unlocking the anticancer potential of this promising class of treatments.
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Moreira, D. M. et al. Predicting time from metastasis to overall survival in castration-resistant prostate cancer: results from SEARCH. Clin. Genitourin. Cancer 15, 60–66. e62 (2017).
Chen, X., Comish, P. B., Tang, D. & Kang, R. Characteristics and biomarkers of ferroptosis. Front. Cell Dev. Biol. 9, 637162 (2021).
Yang, W. S. et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl Acad. Sci. USA 113, E4966–E4975 (2016).
Lin, Z. et al. The lipid flippase SLC47A1 blocks metabolic vulnerability to ferroptosis. Nat. Commun. 13, 7965 (2022).
Catala, A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids 157, 1–11 (2009).
Gao, M. et al. Role of mitochondria in ferroptosis. Mol. Cell 73, 354–363 e353 (2019).
Park, M. W. et al. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 41, 101947 (2021).
Yan, B. et al. Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol. Cell 81, 355–369 e310 (2021).
Zhang, S. et al. Double-edge sword roles of iron in driving energy production versus instigating ferroptosis. Cell Death Dis. 13, 40 (2022).
von Krusenstiern, A. N. et al. Identification of essential sites of lipid peroxidation in ferroptosis. Nat. Chem. Biol. 19, 719–730 (2023).
Yang, W. S. & Stockwell, B. R. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 26, 165–176 (2016).
Conrad, M. & Friedmann Angeli, J. P. Glutathione peroxidase 4 (Gpx4) and ferroptosis: what’s so special about it? Mol. Cell Oncol. 2, e995047 (2015).
Lu, S. C. Glutathione synthesis. Biochim. Biophys. Acta 1830, 3143–3153 (2013).
Du, Y. & Guo, Z. Recent progress in ferroptosis: inducers and inhibitors. Cell Death Discov. 8, 501 (2022).
Li, Q. et al. Understanding sorafenib-induced ferroptosis and resistance mechanisms: implications for cancer therapy. Eur. J. Pharmacol. 955, 175913 (2023).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Yan, H. F. et al. Ferroptosis: mechanisms and links with diseases. Signal. Transduct. Target. Ther. 6, 49 (2021).
Mao, C. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586–590 (2021).
Cirilli, I. et al. Role of coenzyme Q10 in health and disease: an update on the last 10 years (2010–2020). Antioxidants 10, 1325 (2021).
Bentinger, M., Tekle, M. & Dallner, G. Coenzyme Q — biosynthesis and functions. Biochem. Biophys. Res. Commun. 396, 74–79 (2010).
Kraft, V. A. N. et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent. Sci. 6, 41–53 (2020).
Lei, G., Zhuang, L. & Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 22, 381–396 (2022).
Dixon, S. J. et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3, e02523 (2014).
Jeong, S. D. et al. Enhanced immunogenic cell death by apoptosis/ferroptosis hybrid pathway potentiates PD-L1 blockade cancer immunotherapy. ACS Biomater. Sci. Eng. 8, 5188–5198 (2022).
Lei, G. et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 30, 146–162 (2020).
Viswanathan, V. S. et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457 (2017).
Tousignant, K. D. et al. Therapy-induced lipid uptake and remodeling underpin ferroptosis hypersensitivity in prostate cancer. Cancer Metab. 8, 11 (2020).
Ghoochani, A. et al. Ferroptosis inducers are a novel therapeutic approach for advanced prostate cancer. Cancer Res. 81, 1583–1594 (2021).
Yang, Y. et al. Ferroptosis inducer erastin downregulates androgen receptor and its splice variants in castration-resistant prostate cancer. Oncol. Rep. 45, 25 (2021).
Mathur, D. et al. PTEN regulates glutamine flux to pyrimidine synthesis and sensitivity to dihydroorotate dehydrogenase inhibition. Cancer Discov. 7, 380–390 (2017).
Lv, Z. et al. Identifying a ferroptosis-related gene signature for predicting biochemical recurrence of prostate cancer. Front. Cell Dev. Biol. 9, 666025 (2021).
Mao, C., Liu, X., Yan, Y., Olszewski, K. & Gan, B. Reply to: DHODH inhibitors sensitize to ferroptosis by FSP1 inhibition. Nature 619, E19–E23 (2023).
Mishima, E. et al. DHODH inhibitors sensitize to ferroptosis by FSP1 inhibition. Nature 619, E9–E18 (2023).
Yoo, S. E. et al. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free. Radic. Biol. Med. 52, 1820–1827 (2012).
Mei, J., Webb, S., Zhang, B. & Shu, H. B. The p53-inducible apoptotic protein AMID is not required for normal development and tumor suppression. Oncogene 25, 849–856 (2006).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017).
Rodriguez, R., Schreiber, S. L. & Conrad, M. Persister cancer cells: iron addiction and vulnerability to ferroptosis. Mol. Cell 82, 728–740 (2022).
Scheinberg, T., Mak, B., Butler, L., Selth, L. & Horvath, L. G. Targeting lipid metabolism in metastatic prostate cancer. Ther. Adv. Med. Oncol. 15, 17588359231152839 (2023).
Mah, C. Y., Nassar, Z. D., Swinnen, J. V. & Butler, L. M. Lipogenic effects of androgen signaling in normal and malignant prostate. Asian J. Urol. 7, 258–270 (2020).
Poulose, N. et al. Genetics of lipid metabolism in prostate cancer. Nat. Genet. 50, 169–171 (2018).
Scaglia, N., Frontini-Lopez, Y. R. & Zadra, G. Prostate cancer progression: as a matter of fats. Front. Oncol. 11, 719865 (2021).
Castelli, S., De Falco, P., Ciccarone, F., Desideri, E. & Ciriolo, M. R. Lipid catabolism and ROS in cancer: a bidirectional liaison. Cancers 13, 5484 (2021).
Iglesias-Gato, D. et al. The proteome of primary prostate cancer. Eur. Urol. 69, 942–952 (2016).
Magtanong, L. et al. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem. Biol. 26, 420–432 e429 (2019).
Das, U. N. Saturated fatty acids, MUFAs and PUFAs regulate ferroptosis. Cell Chem. Biol. 26, 309–311 (2019).
Yuan, H., Li, X., Zhang, X., Kang, R. & Tang, D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 478, 1338–1343 (2016).
Yang, Y. et al. ACSL3 and ACSL4, distinct roles in ferroptosis and cancers. Cancers 14, 5896 (2022).
Doll, S. & Conrad, M. Iron and ferroptosis: a still ill-defined liaison. IUBMB Life 69, 423–434 (2017).
Ma, Y. et al. Long-chain Acyl-CoA synthetase 4-mediated fatty acid metabolism sustains androgen receptor pathway-independent prostate cancer. Mol. Cancer Res. 19, 124–135 (2021).
Marques, R. B. et al. Modulation of androgen receptor signaling in hormonal therapy-resistant prostate cancer cell lines. PLoS ONE 6, e23144 (2011).
Wu, X. et al. ACSL4 promotes prostate cancer growth, invasion and hormonal resistance. Oncotarget 6, 44849–44863 (2015).
Wang, M. E. et al. RB1-deficient prostate tumor growth and metastasis are vulnerable to ferroptosis induction via the E2F/ACSL4 axis. J. Clin. Invest. 133, e166647 (2023).
Orlando, U. D. et al. Acyl-CoA synthetase-4 is implicated in drug resistance in breast cancer cell lines involving the regulation of energy-dependent transporter expression. Biochem. Pharmacol. 159, 52–63 (2019).
Sanchez-Martinez, R., Cruz-Gil, S., Garcia-Alvarez, M. S., Reglero, G. & Ramirez de Molina, A. Complementary ACSL isoforms contribute to a non-Warburg advantageous energetic status characterizing invasive colon cancer cells. Sci. Rep. 7, 11143 (2017).
Wu, X. et al. Long chain fatty Acyl-CoA synthetase 4 is a biomarker for and mediator of hormone resistance in human breast cancer. PLoS ONE 8, e77060 (2013).
Xia, H. et al. Simultaneous silencing of ACSL4 and induction of GADD45B in hepatocellular carcinoma cells amplifies the synergistic therapeutic effect of aspirin and sorafenib. Cell Death Discov. 3, 17058 (2017).
Mashima, T., Seimiya, H. & Tsuruo, T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br. J. Cancer 100, 1369–1372 (2009).
Sena, L. A. & Denmeade, S. R. Fatty acid synthesis in prostate cancer: vulnerability or epiphenomenon? Cancer Res. 81, 4385–4393 (2021).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Swinnen, J. V., Brusselmans, K. & Verhoeven, G. Increased lipogenesis in cancer cells: new players, novel targets. Curr. Opin. Clin. Nutr. Metab. Care 9, 358–365 (2006).
Swinnen, J. V., Esquenet, M., Goossens, K., Heyns, W. & Verhoeven, G. Androgens stimulate fatty acid synthase in the human prostate cancer cell line LNCaP. Cancer Res. 57, 1086–1090 (1997).
Heemers, H. V., Verhoeven, G. & Swinnen, J. V. Androgen activation of the sterol regulatory element-binding protein pathway: current insights. Mol. Endocrinol. 20, 2265–2277 (2006).
Butler, L. M., Centenera, M. M. & Swinnen, J. V. Androgen control of lipid metabolism in prostate cancer: novel insights and future applications. Endocr. Relat. Cancer 23, R219–227, (2016).
Han, L. Q., Gao, T. Y., Yang, G. Y. & Loor, J. J. Overexpression of SREBF chaperone (SCAP) enhances nuclear SREBP1 translocation to upregulate fatty acid synthase (FASN) gene expression in bovine mammary epithelial cells. J. Dairy. Sci. 101, 6523–6531 (2018).
Rossi, S. et al. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol. Cancer Res. 1, 707–715 (2003).
Shah, U. S. et al. Fatty acid synthase gene overexpression and copy number gain in prostate adenocarcinoma. Hum. Pathol. 37, 401–409 (2006).
Epstein, J. I., Carmichael, M. & Partin, A. W. OA-519 (fatty acid synthase) as an independent predictor of pathologic state in adenocarcinoma of the prostate. Urology 45, 81–86 (1995).
Song, X. et al. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep. 34, 108767 (2021).
Li, C. et al. LKB1-AMPK axis negatively regulates ferroptosis by inhibiting fatty acid synthesis. Signal. Transduct. Target. Ther. 5, 187 (2020).
Lee, H. et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 22, 225–234 (2020).
Bartolacci, C. et al. Targeting de novo lipogenesis and the Lands cycle induces ferroptosis in KRAS-mutant lung cancer. Nat. Commun. 13, 4327 (2022).
Hardie, D. G., Ross, F. A. & Hawley, S. A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262 (2012).
Park, H. U. et al. AMP-activated protein kinase promotes human prostate cancer cell growth and survival. Mol. Cancer Ther. 8, 733–741 (2009).
Tennakoon, J. B. et al. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1ɑ-mediated metabolic switch. Oncogene 33, 5251–5261 (2014).
Lin, C. et al. Inhibition of CAMKK2 impairs autophagy and castration-resistant prostate cancer via suppression of AMPK-ULK1 signaling. Oncogene 40, 1690–1705 (2021).
Khan, A. S. & Frigo, D. E. A spatiotemporal hypothesis for the regulation, role, and targeting of AMPK in prostate cancer. Nat. Rev. Urol. 14, 164–180 (2017).
Frigo, D. E. et al. CaM kinase kinase beta-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Res. 71, 528–537 (2011).
Enoch, H. G., Catala, A. & Strittmatter, P. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J. Biol. Chem. 251, 5095–5103 (1976).
Amezaga, J. et al. Altered red blood cell membrane fatty acid profile in cancer patients. Nutrients 10, 1853 (2018).
Chavarro, J. E. et al. Blood levels of saturated and monounsaturated fatty acids as markers of de novo lipogenesis and risk of prostate cancer. Am. J. Epidemiol. 178, 1246–1255 (2013).
Fritz, V. et al. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol. Cancer Ther. 9, 1740–1754 (2010).
Yi, J., Zhu, J., Wu, J., Thompson, C. B. & Jiang, X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc. Natl Acad. Sci. USA 117, 31189–31197 (2020).
Tesfay, L. et al. Stearoyl-CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res. 79, 5355–5366 (2019).
Kagan, V. E. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017).
D’Angelo, S., Motti, M. L. & Meccariello, R. ω-3 and ω-6 polyunsaturated fatty acids, obesity and cancer. Nutrients 12, 2751 (2020).
Lee, J. M., Lee, H., Kang, S. & Park, W. J. Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients 8, 23 (2016).
Lee, J. Y. et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc. Natl Acad. Sci. USA 117, 32433–32442 (2020).
Yamane, D. et al. FADS2-dependent fatty acid desaturation dictates cellular sensitivity to ferroptosis and permissiveness for hepatitis C virus replication. Cell Chem. Biol. 29, 799–810 e794 (2022).
Xu, H. et al. ELOVL5-mediated long chain fatty acid elongation contributes to enzalutamide resistance of prostate cancer. Cancers 13, 3957 (2021).
Centenera, M. M. et al. ELOVL5 is a critical and targetable fatty acid elongase in prostate cancer. Cancer Res. 81, 1704–1718 (2021).
Schlaepfer, I. R. & Joshi, M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology 161, bqz046 (2020).
Flaig, T. W. et al. Lipid catabolism inhibition sensitizes prostate cancer cells to antiandrogen blockade. Oncotarget 8, 56051–56065 (2017).
Joshi, M. et al. CPT1A supports castration-resistant prostate cancer in androgen-deprived conditions. Cells 8, 115 (2019).
Nassar, Z. D. et al. Human DECR1 is an androgen-repressed survival factor that regulates PUFA oxidation to protect prostate tumor cells from ferroptosis. Elife 9, e54166 (2020).
Gao, M., Monian, P., Quadri, N., Ramasamy, R. & Jiang, X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 59, 298–308 (2015).
Philpott, C. C. et al. Iron-tracking strategies: chaperones capture iron in the cytosolic labile iron pool. Front. Mol. Biosci. 10, 1127690 (2023).
Yanatori, I., Richardson, D. R., Imada, K. & Kishi, F. Iron export through the transporter ferroportin 1 is modulated by the iron chaperone PCBP2. J. Biol. Chem. 291, 17303–17318 (2016).
Donovan, A. et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 1, 191–200 (2005).
Nemeth, E. & Ganz, T. The role of hepcidin in iron metabolism. Acta Haematol. 122, 78–86 (2009).
Hubert, N. & Hentze, M. W. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proc. Natl Acad. Sci. USA 99, 12345–12350 (2002).
Dai, E., Meng, L., Kang, R., Wang, X. & Tang, D. ESCRT-III-dependent membrane repair blocks ferroptosis. Biochem. Biophys. Res. Commun. 522, 415–421 (2020).
Vela, D. Iron metabolism in prostate cancer; from basic science to new therapeutic strategies. Front. Oncol. 8, 547 (2018).
Torti, S. V., Manz, D. H., Paul, B. T., Blanchette-Farra, N. & Torti, F. M. Iron and cancer. Annu. Rev. Nutr. 38, 97–125 (2018).
Brown, R. A. M. et al. Altered iron metabolism and impact in cancer biology, metastasis, and immunology. Front. Oncol. 10, 476 (2020).
Deng, Z., Manz, D. H., Torti, S. V. & Torti, F. M. Iron-responsive element-binding protein 2 plays an essential role in regulating prostate cancer cell growth. Oncotarget 8, 82231–82243 (2017).
Keer, H. N. et al. Elevated transferrin receptor content in human prostate cancer cell lines assessed in vitro and in vivo. J. Urol. 143, 381–385 (1990).
Kuvibidila, S., Gauthier, T., Warrier, R. P. & Rayford, W. Increased levels of serum transferrin receptor and serum transferrin receptor/log ferritin ratios in men with prostate cancer and the implications for body-iron stores. J. Lab. Clin. Med. 144, 176–182 (2004).
Xue, D., Zhou, C. X., Shi, Y. B., Lu, H. & He, X. Z. Decreased expression of ferroportin in prostate cancer. Oncol. Lett. 10, 913–916 (2015).
Burnell, S. E. A. et al. STEAP2 knockdown reduces the invasive potential of prostate cancer cells. Sci. Rep. 8, 6252 (2018).
Hou, W. et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12, 1425–1428 (2016).
Winterbourn, C. C. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol. Lett. 82-83, 969–974 (1995).
Dhur, A., Galan, P. & Hercberg, S. Effects of different degrees of iron deficiency on cytochrome P450 complex and pentose phosphate pathway dehydrogenases in the rat. J. Nutr. 119, 40–47 (1989).
Pistorius, E. K. & Axelrod, B. Iron, an essential component of lipoxygenase. J. Biol. Chem. 249, 3183–3186 (1974).
Yauger, Y. J. et al. Iron accentuated reactive oxygen species release by NADPH oxidase in activated microglia contributes to oxidative stress in vitro. J. Neuroinflammation 16, 41 (2019).
Bordini, J. et al. Induction of iron excess restricts malignant plasma cells expansion and potentiates bortezomib effect in models of multiple myeloma. Leukemia 31, 967–970 (2017).
Campanella, A. et al. Iron increases the susceptibility of multiple myeloma cells to bortezomib. Haematologica 98, 971–979 (2013).
Bordini, J. et al. Iron induces cell death and strengthens the efficacy of antiandrogen therapy in prostate cancer models. Clin. Cancer Res. 26, 6387–6398 (2020).
Maccarinelli, F. et al. Iron supplementation enhances RSL3-induced ferroptosis to treat naive and prevent castration-resistant prostate cancer. Cell Death Discov. 9, 81 (2023).
Liu, Y. et al. Liposomes embedded with PEGylated iron oxide nanoparticles enable ferroptosis and combination therapy in cancer. Natl Sci. Rev. 10, nwac167 (2023).
Fernandez-Acosta, R. et al. Novel iron oxide nanoparticles induce ferroptosis in a panel of cancer cell lines. Molecules 27, 3970 (2022).
Bonvin, D. et al. Tuning properties of iron oxide nanoparticles in aqueous synthesis without ligands to improve MRI relaxivity and SAR. Nanomaterials 7, 225 (2017).
Hajikarimi, Z., Khoei, S., Khoee, S. & Mahdavi, S. R. Evaluation of the cytotoxic effects of PLGA coated iron oxide nanoparticles as a carrier of 5- fluorouracil and mega-voltage X-ray radiation in DU145 prostate cancer cell line. IEEE Trans. Nanobiosci. 13, 403–408 (2014).
Kader, A. et al. Iron oxide nanoparticles for visualization of prostate cancer in MRI. Cancers 14, 2909 (2022).
Cluntun, A. A., Lukey, M. J., Cerione, R. A. & Locasale, J. W. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer 3, 169–180 (2017).
Gong, T. et al. Glutamine metabolism in cancers: targeting the oxidative homeostasis. Front. Oncol. 12, 994672 (2022).
Wang, Q. et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J. Pathol. 236, 278–289 (2015).
Wang, Q. et al. Androgen receptor and nutrient signaling pathways coordinate the demand for increased amino acid transport during prostate cancer progression. Cancer Res. 71, 7525–7536 (2011).
Cardoso, H. J. et al. Glutaminolysis is a metabolic route essential for survival and growth of prostate cancer cells and a target of 5ɑ-dihydrotestosterone regulation. Cell Oncol. 44, 385–403 (2021).
Serpa, J. Cysteine as a carbon source, a hot spot in cancer cells survival. Front. Oncol. 10, 947 (2020).
Poltorack, C. D. & Dixon, S. J. Understanding the role of cysteine in ferroptosis: progress & paradoxes. FEBS J. 289, 374–385 (2022).
Yang, J., Dai, X., Xu, H., Tang, Q. & Bi, F. Regulation of ferroptosis by amino acid metabolism in cancer. Int. J. Biol. Sci. 18, 1695–1705 (2022).
Zhong, W. et al. Extracellular redox state shift: a novel approach to target prostate cancer invasion. Free. Radic. Biol. Med. 117, 99–109 (2018).
Doxsee, D. W. et al. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 67, 162–171 (2007).
Cramer, S. L. et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 23, 120–127 (2017).
Mandigo, A. C. et al. RB/E2F1 as a master regulator of cancer cell metabolism in advanced disease. Cancer Discov. 11, 2334–2353 (2021).
Nikfar, S., Rahimi, R., Rezaie, A. & Abdollahi, M. A meta-analysis of the efficacy of sulfasalazine in comparison with 5-aminosalicylates in the induction of improvement and maintenance of remission in patients with ulcerative colitis. Dig. Dis. Sci. 54, 1157–1170 (2009).
Rains, C. P., Noble, S. & Faulds, D. Sulfasalazine. A review of its pharmacological properties and therapeutic efficacy in the treatment of rheumatoid arthritis. Drugs 50, 137–156 (1995).
Kavallaris, M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer 10, 194–204 (2010).
Ogura, T., Tanaka, Y., Tamaki, H. & Harada, M. Docetaxel induces Bcl-2- and pro-apoptotic caspase-independent death of human prostate cancer DU145 cells. Int. J. Oncol. 48, 2330–2338 (2016).
Darshan, M. S. et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 71, 6019–6029 (2011).
de Bono, J. S. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–1154 (2010).
Galsky, M. D., Dritselis, A., Kirkpatrick, P. & Oh, W. K. Cabazitaxel. Nat. Rev. Drug. Discov. 9, 677–678 (2010).
Chen, X. et al. Ferroptosis induction improves the sensitivity of docetaxel in prostate cancer. Oxid. Med. Cell. Longev. 2022, 1–16 (2022).
Jiang, X. et al. TFAP2C-mediated lncRNA PCAT1 inhibits ferroptosis in docetaxel-resistant prostate cancer through c-Myc/miR-25-3p/SLC7A11 signaling. Front. Oncol. 12, 862015 (2022).
He, S. et al. ChaC glutathione specific γ-glutamylcyclotransferase 1 inhibits cell viability and increases the sensitivity of prostate cancer cells to docetaxel by inducing endoplasmic reticulum stress and ferroptosis. Exp. Ther. Med. 22, 997 (2021).
Ogawa, T. et al. CHAC1 overexpression in human gastric parietal cells with Helicobacter pylori infection in the secretory canaliculi. Helicobacter 24, e12598 (2019).
Takeda, D. Y. et al. A somatically acquired enhancer of the androgen receptor is a noncoding driver in advanced prostate cancer. Cell 174, 422–432 e413 (2018).
Schweizer, M. T. & Yu, E. Y. Persistent androgen receptor addiction in castration-resistant prostate cancer. J. Hematol. Oncol. 8, 128 (2015).
Abida, W. et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl Acad. Sci. USA 116, 11428–11436 (2019).
Watson, P. A., Arora, V. K. & Sawyers, C. L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 15, 701–711 (2015).
Cattrini, C. et al. Apalutamide, darolutamide and enzalutamide for nonmetastatic castration-resistant prostate cancer (nmCRPC): a critical review. Cancers 14, 1492 (2022).
Desai, K., McManus, J. M. & Sharifi, N. Hormonal therapy for prostate cancer. Endocr. Rev. 42, 354–373 (2021).
Clarke, N. W. et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid. 1, EVIDoa2200043 (2022).
Chi, K. N. et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 41, 3339–3351 (2023).
Hong, T. et al. PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol. 42, 101928 (2021).
Sugiura, M. et al. Identification of AR-V7 downstream genes commonly targeted by AR/AR-V7 and specifically targeted by AR-V7 in castration resistant prostate cancer. Transl. Oncol. 14, 100915 (2021).
Yang, F. et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 35, 84–100.e8 (2023).
Sun, R. et al. Androgen receptor variants confer castration resistance in prostate cancer by counteracting antiandrogen-induced ferroptosis. Cancer Res. 83, 3192–3204 (2023).
Liang, D. et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 186, 2748–2764 e2722 (2023).
Ma, D. et al. Crystal structure of a membrane-bound O-acyltransferase. Nature 562, 286–290 (2018).
Masumoto, N. et al. Membrane bound O-acyltransferases and their inhibitors. Biochem. Soc. Trans. 43, 246–252 (2015).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Runcie, K. D. & Dallos, M. C. Prostate cancer immunotherapy — finally in from the cold? Curr. Oncol. Rep. 23, 88 (2021).
Graff, J. N. et al. Phase II study of ipilimumab in men with metastatic prostate cancer with an incomplete response to androgen deprivation therapy. Front. Oncol. 10, 1381 (2020).
Fizazi, K. et al. Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur. Urol. 78, 822–830 (2020).
Cabel, L. et al. Long-term complete remission with Ipilimumab in metastatic castrate-resistant prostate cancer: case report of two patients. J. Immunother. Cancer 5, 31 (2017).
Sharma, P. et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the checkmate 650 trial. Cancer Cell 38, 489–499 e483 (2020).
Fizazi, K. et al. Nivolumab plus rucaparib for metastatic castration-resistant prostate cancer: results from the phase 2 CheckMate 9KD trial. J. Immunother. Cancer 10, e004761 (2022).
Fizazi, K. et al. Nivolumab plus docetaxel in patients with chemotherapy-naive metastatic castration-resistant prostate cancer: results from the phase II CheckMate 9KD trial. Eur. J. Cancer 160, 61–71 (2022).
Petrylak, D. P. et al. KEYNOTE-921: phase III study of pembrolizumab plus docetaxel for metastatic castration-resistant prostate cancer. Future Oncol. 17, 3291–3299 (2021).
Antonarakis, E. S. et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J. Clin. Oncol. 38, 395–405 (2020).
Petrylak, D. P. et al. Safety and clinical activity of atezolizumab in patients with metastatic castration-resistant prostate cancer: a phase I study. Clin. Cancer Res. 27, 3360–3369 (2021).
Powles, T. et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat. Med. 28, 144–153 (2022).
Rodriguez-Vida, A. et al. Safety and efficacy of avelumab plus carboplatin in patients with metastatic castration-resistant prostate cancer in an open-label Phase Ib study. Br. J. Cancer 128, 21–29 (2023).
Kwan, E. M. et al. Avelumab combined with stereotactic ablative body radiotherapy in metastatic castration-resistant prostate cancer: the phase 2 ICE-PAC clinical trial. Eur. Urol. 81, 253–262 (2022).
Karzai, F. et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer 6, 141 (2018).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Shore, N. D. et al. CD8+ T cells impact rising PSA in biochemically relapsed cancer patients using immunotherapy targeting tumor-associated antigens. Mol. Ther. 28, 1238–1250 (2020).
Kyriakopoulos, C. E. et al. Multicenter phase I trial of a DNA vaccine encoding the androgen receptor ligand-binding domain (pTVG-AR, MVI-118) in patients with metastatic prostate cancer. Clin. Cancer Res. 26, 5162–5171 (2020).
Singh, P., Pal, S. K., Alex, A. & Agarwal, N. Development of PROSTVAC immunotherapy in prostate cancer. Future Oncol. 11, 2137–2148 (2015).
Gulley, J. L. et al. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J. Clin. Oncol. 37, 1051–1061 (2019).
van den Eertwegh, A. J. et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 13, 509–517 (2012).
Small, E. J. et al. Granulocyte macrophage colony-stimulating factor-secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin. Cancer Res. 13, 3883–3891 (2007).
Higano, C. S. et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer 113, 975–984 (2008).
Tschernia, N. P., Norberg, S. M. & Gulley, J. L. CAR T cells reach clinical milestone in prostate cancer. Nat. Med. 28, 635–636 (2022).
Perera, M. P. J. et al. Chimeric antigen receptor T-cell therapy in metastatic castrate-resistant prostate cancer. Cancers 14, 503 (2022).
Narayan, V. et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 28, 724–734 (2022).
Yunger, S. et al. Tumor-infiltrating lymphocytes from human prostate tumors reveal anti-tumor reactivity and potential for adoptive cell therapy. Oncoimmunology 8, e1672494 (2019).
Karbach, J. et al. Tumor-infiltrating lymphocytes mediate complete and durable remission in a patient with NY-ESO-1 expressing prostate cancer. J. Immunother. Cancer 11, e005847 (2023).
Kamat, N. V., Yu, E. Y. & Lee, J. K. BiTE-ing into prostate cancer with bispecific T-cell engagers. Clin. Cancer Res. 27, 2675–2677 (2021).
Hummel, H. D. et al. Pasotuxizumab, a BiTE® immune therapy for castration-resistant prostate cancer: phase I, dose-escalation study findings. Immunotherapy 13, 125–141 (2021).
Wang, W. et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569, 270–274 (2019).
Liao, P. et al. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 40, 365–378.e6 (2022).
Jiang, Z. et al. TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J. Clin. Invest. 131, e139434 (2021).
Efimova, I. et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J. Immunother. Cancer 8, e001369 (2020).
Tang, D., Kepp, O. & Kroemer, G. Ferroptosis becomes immunogenic: implications for anticancer treatments. Oncoimmunology 10, 1862949 (2020).
Wen, Q., Liu, J., Kang, R., Zhou, B. & Tang, D. The release and activity of HMGB1 in ferroptosis. Biochem. Biophys. Res. Commun. 510, 278–283 (2019).
Yu, B., Choi, B., Li, W. & Kim, D. H. Magnetic field boosted ferroptosis-like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat. Commun. 11, 3637 (2020).
Wu, Z. et al. The landscape of immune cells infiltrating in prostate cancer. Front. Oncol. 10, 517637 (2020).
Martin, A. M. et al. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 18, 325–332 (2015).
Matsushita, M. et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212, 555–568 (2015).
Ma, X. et al. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 33, 1001–1012.e5 (2021).
Zhou, X. et al. Abrogation of HnRNP L enhances anti-PD-1 therapy efficacy via diminishing PD-L1 and promoting CD8+ T cell-mediated ferroptosis in castration-resistant prostate cancer. Acta Pharm. Sin. B 12, 692–707 (2022).
Sindhu, K. K., Nehlsen, A. D. & Stock, R. G. Radium-223 for metastatic castrate-resistant prostate cancer. Pract. Radiat. Oncol. 12, 312–316 (2022).
Parker, C. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 369, 213–223 (2013).
Violet, J. et al. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J. Nucl. Med. 60, 517–523 (2019).
Sun, M., Niaz, M. O., Nelson, A., Skafida, M. & Niaz, M. J. Review of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Cureus 12, e8921 (2020).
Schuchardt, C. et al. Prostate-specific membrane antigen radioligand therapy using 177Lu-PSMA I&T and 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: comparison of safety, biodistribution, and dosimetry. J. Nucl. Med. 63, 1199–1207 (2022).
Kratochwil, C. et al. 225Ac-PSMA-617 for PSMA-targeted ɑ-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 57, 1941–1944 (2016).
Hammer, S. et al. Preclinical efficacy of a PSMA-targeted thorium-227 conjugate (PSMA-TTC), a targeted ɑ therapy for prostate cancer. Clin. Cancer Res. 26, 1985–1996 (2020).
Kiess, A. P. et al. 2S)-2-(3-(1-Carboxy-5-(4-211At-Astatobenzamido)Pentyl)Ureido)-pentanedioic acid for PSMA-targeted ɑ-particle radiopharmaceutical therapy. J. Nucl. Med. 57, 1569–1575 (2016).
Ye, L. F. et al. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem. Biol. 15, 469–484 (2020).
Lang, X. et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 9, 1673–1685 (2019).
Lowe, S. W., Schmitt, E. M., Smith, S. W., Osborne, B. A. & Jacks, T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362, 847–849 (1993).
Kastan, M. B., Onyekwere, O., Sidransky, D., Vogelstein, B. & Craig, R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51, 6304–6311 (1991).
Jiang, L. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57–62 (2015).
Wang, Y. et al. Epigenetic regulation of ferroptosis by H2B monoubiquitination and p53. EMBO Rep. 20, e47563 (2019).
Shen, D. et al. PARPi treatment enhances radiotherapy-induced ferroptosis and antitumor immune responses via the cGAS signaling pathway in colorectal cancer. Cancer Lett. 550, 215919 (2022).
Lei, G., Mao, C., Yan, Y., Zhuang, L. & Gan, B. Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell 12, 836–857 (2021).
Beretta, G. L. & Zaffaroni, N. Radiotherapy-induced ferroptosis for cancer treatment. Front. Mol. Biosci. 10, 1216733 (2023).
Upadhyayula, P. S. et al. Dietary restriction of cysteine and methionine sensitizes gliomas to ferroptosis and induces alterations in energetic metabolism. Nat. Commun. 14, 1187 (2023).
Xue, Y. et al. Intermittent dietary methionine deprivation facilitates tumoral ferroptosis and synergizes with checkpoint blockade. Nat. Commun. 14, 4758 (2023).
Dierge, E. et al. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. 33, 1701–1715 e1705 (2021).
Ferrer, M. et al. Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia. Cell Metab. 35, 1147–1162 e1147 (2023).
Pissios, P. et al. Methionine and choline regulate the metabolic phenotype of a ketogenic diet. Mol. Metab. 2, 306–313 (2013).
Eaton, J. K. et al. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat. Chem. Biol. 16, 497–506 (2020).
Eaton, J. K., Ruberto, R. A., Kramm, A., Viswanathan, V. S. & Schreiber, S. L. Diacylfuroxans are masked nitrile oxides that inhibit GPX4 covalently. J. Am. Chem. Soc. 141, 20407–20415 (2019).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Koppula, P. et al. A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat. Commun. 13, 2206 (2022).
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698 (2019).
Xavier da Silva, T. N., Schulte, C., Alves, A. N., Maric, H. M. & Friedmann Angeli, J. P. Molecular characterization of AIFM2/FSP1 inhibition by iFSP1-like molecules. Cell Death Dis. 14, 281 (2023).
Hendricks, J. M. et al. Identification of structurally diverse FSP1 inhibitors that sensitize cancer cells to ferroptosis. Cell Chem. Biol. 30, 1090–1103 e1097 (2023).
Yoshioka, H. et al. Identification of a small molecule that enhances ferroptosis via inhibition of ferroptosis suppressor protein 1 (FSP1). ACS Chem. Biol. 17, 483–491 (2022).
Antoszczak, M. & Huczynski, A. Salinomycin and its derivatives — a new class of multiple-targeted “magic bullets”. Eur. J. Med. Chem. 176, 208–227 (2019).
Miyazaki, Y., Shibuya, M., Sugawara, H., Kawaguchi, O. & Hirsoe, C. Salinomycin, a new polyether antibiotic. J. Antibiot. 27, 814–821 (1974).
Zhou, S. et al. Salinomycin: a novel anti-cancer agent with known anti-coccidial activities. Curr. Med. Chem. 20, 4095–4101 (2013).
Mai, T. T. et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat. Chem. 9, 1025–1033 (2017).
Mohammad, A. et al. Abstract 3107: HSB-1216, a novel agent for the treatment of chemotherapy-resistant ES-SCLC. Cancer Res. 81, 3107–3107 (2021).
Kharbanda, S. et al. Novel iron-mediated cell death (Ferroptosis) inducer, HSB-1216, suppress acute myeloid leukemia growth. Eur. J. Cancer https://doi.org/10.1016/s0959-8049(22)00936-4 (2022).
Li, J. et al. Ferroptosis: past, present and future. Cell Death Dis. 11, 88 (2020).
Kloditz, K. & Fadeel, B. Three cell deaths and a funeral: macrophage clearance of cells undergoing distinct modes of cell death. Cell Death Discov. 5, 65 (2019).
Zou, Y. & Schreiber, S. L. Progress in understanding ferroptosis and challenges in its targeting for therapeutic benefit. Cell Chem. Biol. 27, 463–471 (2020).
Li, Z. et al. In vivo tracking cystine/glutamate antiporter-mediated cysteine/cystine pool under ferroptosis. Anal. Chim. Acta 1125, 66–75 (2020).
Li, Z. Imaging of hydrogen peroxide (H(2)O(2)) during the ferroptosis process in living cancer cells with a practical fluorescence probe. Talanta 212, 120804 (2020).
Conrad, M. & Pratt, D. A. The chemical basis of ferroptosis. Nat. Chem. Biol. 15, 1137–1147 (2019).
Zeng, F. et al. Ferroptosis detection: from approaches to applications. Angew. Chem. Int. Ed. 62, e202300379 (2023).
Li, J., Kang, R. & Tang, D. Monitoring autophagy-dependent ferroptosis. Methods Cell Biol. 165, 163–176 (2021).
Wang, F. et al. PALP: a rapid imaging technique for stratifying ferroptosis sensitivity in normal and tumor tissues in situ. Cell Chem. Biol. 29, 157–170 e156 (2022).
Wang, F., Naowarojna, N. & Zou, Y. Stratifying ferroptosis sensitivity in cells and mouse tissues by photochemical activation of lipid peroxidation and fluorescent imaging. Star. Protoc. 3, 101189 (2022).
Drummen, G. P., van Liebergen, L. C., Op den Kamp, J. A. & Post, J. A. C11-BODIPY(581/591), an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free. Radic. Biol. Med. 33, 473–490 (2002).
Hirayama, T., Okuda, K. & Nagasawa, H. A highly selective turn-on fluorescent probe for iron(II) to visualize labile iron in living cells. Chem. Sci. 4, 1250–1256, (2013).
Kapralov, A. A. et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol. 16, 278–290 (2020).
Weigand, I. et al. Active steroid hormone synthesis renders adrenocortical cells highly susceptible to type II ferroptosis induction. Cell Death Dis. 11, 192 (2020).
Zhao, N. et al. Ferronostics: measuring tumoral ferrous iron with PET to predict sensitivity to iron-targeted cancer therapies. J. Nucl. Med. 62, 949–955 (2021).
Phyo, A. P. et al. Antimalarial activity of artefenomel (OZ439), a novel synthetic antimalarial endoperoxide, in patients with Plasmodium falciparum and Plasmodium vivax malaria: an open-label phase 2 trial. Lancet Infect. Dis. 16, 61–69 (2016).
Feng, H. et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 30, 3411–3423 e3417 (2020).
Shibata, Y., Yasui, H., Higashikawa, K. & Kuge, Y. Transferrin-based radiolabeled probe predicts the sensitivity of human renal cancer cell lines to ferroptosis inducer erastin. Biochem. Biophys. Rep. 26, 100957 (2021).
McCormick, P. N. et al. Assessment of tumor redox status through (S)-4-(3-[18F]fluoropropyl)-L-glutamic acid PET imaging of system xc− activity. Cancer Res. 79, 853–863 (2019).
Hoehne, A. et al. [18F]FSPG-PET reveals increased cystine/glutamate antiporter (xc-) activity in a mouse model of multiple sclerosis. J. Neuroinflammation 15, 55 (2018).
Park, S. Y. et al. Clinical evaluation of (4S)-4-(3-[18F]fluoropropyl)-L-glutamate (18F-FSPG) for PET/CT imaging in patients with newly diagnosed and recurrent prostate cancer. Clin. Cancer Res. 26, 5380–5387 (2020).
Park, S. Y. et al. Initial evaluation of (4S)-4-(3-[18F]fluoropropyl)-L-glutamate (FSPG) PET/CT imaging in patients with head and neck cancer, colorectal cancer, or non-Hodgkin lymphoma. EJNMMI Res. 10, 100 (2020).
Zeng, F. et al. Ferroptosis MRI for early detection of anticancer drug-induced acute cardiac/kidney injuries. Sci. Adv. 9, eadd8539 (2023).
Zhang, C. et al. Fe-based theranostic agents respond to the tumor microenvironment for MRI-guided ferroptosis-/apoptosis-inducing anticancer therapy. ACS Biomater. Sci. Eng. 8, 2610–2623 (2022).
Zhu, L. et al. Efficient magnetic nanocatalyst-induced chemo- and ferroptosis synergistic cancer therapy in combination with T1-T2 dual-mode magnetic resonance imaging through doxorubicin delivery. ACS Appl. Mater. Interfaces 14, 3621–3632 (2022).
Chen, Q. et al. Iron-based nanoparticles for MR imaging-guided ferroptosis in combination with photodynamic therapy to enhance cancer treatment. Nanoscale 13, 4855–4870 (2021).
Zheng, J. & Conrad, M. The metabolic underpinnings of ferroptosis. Cell Metab. 32, 920–937 (2020).
Bayir, H. et al. Achieving life through death: redox biology of lipid peroxidation in ferroptosis. Cell Chem. Biol. 27, 387–408 (2020).
Friedmann Angeli, J. P., Krysko, D. V. & Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 19, 405–414 (2019).
Gu, Y. et al. Targeting ferroptosis: paving new roads for drug design and discovery. Eur. J. Med. Chem. 247, 115015 (2023).
Wang, D. et al. Regulatory pathways and drugs associated with ferroptosis in tumors. Cell Death Dis. 13, 544 (2022).
Jin, J. et al. Machine learning classifies ferroptosis and apoptosis cell death modalities with TfR1 immunostaining. ACS Chem. Biol. 17, 654–660 (2022).
Shibata, Y., Yasui, H., Higashikawa, K., Miyamoto, N. & Kuge, Y. Erastin, a ferroptosis-inducing agent, sensitized cancer cells to X-ray irradiation via glutathione starvation in vitro and in vivo. PLoS ONE 14, e0225931 (2019).
Liu, W. et al. Ferroptosis inducer improves the efficacy of oncolytic virus-mediated cancer immunotherapy. Biomedicines 10, 1425 (2022).
Lu, Z. et al. Combined anti-cancer effects of platycodin D and sorafenib on androgen-independent and PTEN-deficient prostate cancer. Front. Oncol. 11, 648985 (2021).
Oh, S. J., Erb, H. H., Hobisch, A., Santer, F. R. & Culig, Z. Sorafenib decreases proliferation and induces apoptosis of prostate cancer cells by inhibition of the androgen receptor and Akt signaling pathways. Endocr. Relat. Cancer 19, 305–319 (2012).
Roh, J. L., Kim, E. H., Jang, H. & Shin, D. Aspirin plus sorafenib potentiates cisplatin cytotoxicity in resistant head and neck cancer cells through xCT inhibition. Free. Radic. Biol. Med. 104, 1–9 (2017).
Cobler, L., Zhang, H., Suri, P., Park, C. & Timmerman, L. A. xCT inhibition sensitizes tumors to gamma-radiation via glutathione reduction. Oncotarget 9, 32280–32297 (2018).
Nagane, M. et al. Sulfasalazine, an inhibitor of the cystine-glutamate antiporter, reduces DNA damage repair and enhances radiosensitivity in murine B16F10 melanoma. PLoS ONE 13, e0195151 (2018).
Shamaa, M. M. Sulfasalazine synergistically enhances the inhibitory effects of imatinib against hepatocellular carcinoma (HCC) cells by targeting NFκB, BCR/ABL, and PI3K/AKT signaling pathway-related proteins. FEBS Open. Bio 11, 588–597 (2021).
Chen, C. et al. Flubendazole plays an important anti-tumor role in different types of cancers. Int. J. Mol. Sci. 23, 519 (2022).
Zhou, X. et al. Flubendazole, FDA-approved anthelmintic, elicits valid antitumor effects by targeting P53 and promoting ferroptosis in castration-resistant prostate cancer. Pharmacol. Res. 164, 105305 (2021).
Li, M. et al. RSL3 enhances the antitumor effect of cisplatin on prostate cancer cells via causing glycolysis dysfunction. Biochem. Pharmacol. 192, 114741 (2021).
Wang, J. et al. Inhibition of phosphoglycerate dehydrogenase induces ferroptosis and overcomes enzalutamide resistance in castration-resistant prostate cancer cells. Drug Resist. Updat. 70, 100985 (2023).
Zhao, R. et al. ATF6ɑ promotes prostate cancer progression by enhancing PLA2G4A-mediated arachidonic acid metabolism and protecting tumor cells against ferroptosis. Prostate 82, 617–629 (2022).
Wang, H. et al. Discovery of ML210-Based glutathione peroxidase 4 (GPX4) degrader inducing ferroptosis of human cancer cells. Eur. J. Med. Chem. 254, 115343 (2023).
Eling, N., Reuter, L., Hazin, J., Hamacher-Brady, A. & Brady, N. R. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2, 517–532 (2015).
Luo, J. et al. Artemisinin derivative artesunate induces radiosensitivity in cervical cancer cells in vitro and in vivo. Radiat. Oncol. 9, 84 (2014).
Yin, X. et al. Artesunate suppresses the proliferation and development of estrogen receptor-alpha-positive endometrial cancer in HAND2-dependent pathway. Front. Cell Dev. Biol. 8, 606969 (2020).
Zhang, Z. Y. et al. [Artesunate combined with vinorelbine plus cisplatin in treatment of advanced non-small cell lung cancer: a randomized controlled trial]. Zhong Xi Yi Jie He Xue Bao 6, 134–138 (2008).
Sun, Y. et al. Fin56-induced ferroptosis is supported by autophagy-mediated GPX4 degradation and functions synergistically with mTOR inhibition to kill bladder cancer cells. Cell Death Dis. 12, 1028 (2021).
Khalil, R. et al. Withaferin A increases the effectiveness of immune checkpoint blocker for the treatment of non-small cell lung cancer. Cancers 15, 3089 (2023).
Kim, S. H. et al. RNA-seq reveals novel mechanistic targets of withaferin A in prostate cancer cells. Carcinogenesis 41, 778–789 (2020).
Kyakulaga, A. H., Aqil, F., Munagala, R. & Gupta, R. C. Synergistic combinations of paclitaxel and withaferin A against human non-small cell lung cancer cells. Oncotarget 11, 1399–1416 (2020).
Nishikawa, Y. et al. Withaferin A induces cell death selectively in androgen-independent prostate cancer cells but not in normal fibroblast cells. PLoS ONE 10, e0134137 (2015).
Yang, E. S., Choi, M. J., Kim, J. H., Choi, K. S. & Kwon, T. K. Combination of withaferin A and X-ray irradiation enhances apoptosis in U937 cells. Toxicol. Vitr. 25, 1803–1810 (2011).
Kristensen, G. B., Baekelandt, M., Vergote, I. B. & Trope, C. A phase II study of carboplatin and hexamethylmelamine as induction chemotherapy in advanced epithelial ovarian carcinoma. Eur. J. Cancer 31A, 1778–1780 (1995).
Malik, I. A. Altretamine is an effective palliative therapy of patients with recurrent epithelial ovarian cancer. Jpn. J. Clin. Oncol. 31, 69–73 (2001).
Woo, J. H. et al. Elucidating compound mechanism of action by network perturbation analysis. Cell 162, 441–451 (2015).
Wang, L., Chen, X. & Yan, C. Ferroptosis: an emerging therapeutic opportunity for cancer. Genes. Dis. 9, 334–346 (2022).
Qin, Z. et al. Design and synthesis of isothiocyanate-containing hybrid androgen receptor (AR) antagonist to downregulate AR and induce ferroptosis in GSH-deficient prostate cancer cells. Chem. Biol. Drug. Des. 97, 1059–1078 (2021).
Li, Q. et al. The effects of buthionine sulfoximine on the proliferation and apoptosis of biliary tract cancer cells induced by cisplatin and gemcitabine. Oncol. Lett. 11, 474–480 (2016).
Bump, E. A. & Brown, J. M. Role of glutathione in the radiation response of mammalian cells in vitro and in vivo. Pharmacol. Ther. 47, 117–136 (1990).
Guo, J. et al. Ferroptosis: a novel anti-tumor action for cisplatin. Cancer Res. Treat. 50, 445–460 (2018).
Efferth, T. Cancer combination therapies with artemisinin-type drugs. Biochem. Pharmacol. 139, 56–70 (2017).
Sundar, S. N., Marconett, C. N., Doan, V. B., Willoughby, J. A. Sr & Firestone, G. L. Artemisinin selectively decreases functional levels of estrogen receptor-ɑ and ablates estrogen-induced proliferation in human breast cancer cells. Carcinogenesis 29, 2252–2258 (2008).
Wang, T. et al. Combination treatment with artemisinin and oxaliplatin inhibits tumorigenesis in esophageal cancer EC109 cell through Wnt/β-catenin signaling pathway. Thorac. Cancer 11, 2316–2324 (2020).
Willoughby, J. A. Sr. et al. Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. J. Biol. Chem. 284, 2203–2213 (2009).
Dai, X. et al. Dihydroartemisinin: a potential natural anticancer drug. Int. J. Biol. Sci. 17, 603–622 (2021).
Han, W. et al. Co-delivery of dihydroartemisinin and pyropheophorbide-iron elicits ferroptosis to potentiate cancer immunotherapy. Biomaterials 280, 121315 (2022).
Li, Q. et al. Dihydroartemisinin as a sensitizing agent in cancer therapies. Onco Targets Ther. 14, 2563–2573 (2021).
Lin, R. et al. Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett. 381, 165–175 (2016).
Abrams, R. P., Carroll, W. L. & Woerpel, K. A. Five-membered ring peroxide selectively initiates ferroptosis in cancer cells. ACS Chem. Biol. 11, 1305–1312 (2016).
Gaschler, M. M. et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat. Chem. Biol. 14, 507–515 (2018).
Saha, A. et al. Cysteine depletion sensitizes prostate cancer cells to agents that enhance DNA damage and to immune checkpoint inhibition. J. Exp. Clin. Cancer Res. 42, 119 (2023).
Badgley, M. A. et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368, 85–89 (2020).
Acknowledgements
We are thankful to D. Chalaire, Editing Services, Research Medical Library, The UT MD Anderson Cancer Center for helping us with the editing of the manuscript. This work was supported by grants from the Department of Defense (W81XWH-19-1-0410, W81XWH-22-1-0686 and HT9425-23-1-0424 (D.E.F.)), the National Institutes of Health (R01CA184208 (D.E.F.)), an UT MD Anderson Cancer Center Quantitative Imaging Analysis Core (QIAC) Pilot Grant (D.E.F.), and a grant from the Mike Slive Foundation for Prostate Cancer Research (D.E.F.). This work was also supported by Marilyn and Frederick R. Lummis, Jr., M.D., Fellowship in Biomedical Sciences (P.H.A.C.).
Author information
Authors and Affiliations
Contributions
D.E.F., P.H.A.C., A.D., F.E.L., S.S. and E.S. researched data for the article. D.E.F., P.H.A.C., A.D., P.K.B. and E.S. contributed substantially to discussion of the content. P.H.A.C., A.D., F.E.L., S.S. and E.S. wrote the article. D.E.F., P.H.A.C., A.D., P.K.B. and E.S. reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
D.E.F. has received research funding from GTx, Inc., and has a familial relationship with Biocity Biopharmaceuticals, Hummingbird Bioscience, Bellicum Pharmaceuticals, Maia Biotechnology, Alms Therapeutics, Hinova Pharmaceuticals and Barricade Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, P.H.A., Dominic, A., Lujan, F.E. et al. Unlocking ferroptosis in prostate cancer — the road to novel therapies and imaging markers. Nat Rev Urol (2024). https://doi.org/10.1038/s41585-024-00869-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s41585-024-00869-9