Abstract
Bacteroidetes are abundant members of the human microbiota, utilizing a myriad of diet- and host-derived glycans in the distal gut1. Glycan uptake across the bacterial outer membrane of these bacteria is mediated by SusCD protein complexes, comprising a membrane-embedded barrel and a lipoprotein lid, which is thought to open and close to facilitate substrate binding and transport. However, surface-exposed glycan-binding proteins and glycoside hydrolases also play critical roles in the capture, processing and transport of large glycan chains. The interactions between these components in the outer membrane are poorly understood, despite being crucial for nutrient acquisition by our colonic microbiota. Here we show that for both the levan and dextran utilization systems of Bacteroides thetaiotaomicron, the additional outer membrane components assemble on the core SusCD transporter, forming stable glycan-utilizing machines that we term utilisomes. Single-particle cryogenic electron microscopy structures in the absence and presence of substrate reveal concerted conformational changes that demonstrate the mechanism of substrate capture, and rationalize the role of each component in the utilisome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding authors upon reasonable request. Cryo-EM reconstructions and corresponding coordinates have been deposited in the Electron Microscopy Data Bank and the PDB, respectively: substrate-free levan utilisome (EMD-15288 and PDB 8A9Y), levan utilisome with FOS DP 8–12 (EMD-15289 and PDB 8AA0), SusC2D2 core from the levan utilisome with FOS DP 8–12 (EMD-15290 and PDB 8AA1), inactive levan utilisome with FOS DP 15–25 (EMD-15291 and PDB 8AA2), SusC2D2 core from inactive levan utilisome with FOS DP 15–25 (EMD-1592 and PDB 8AA3), dextran utilisome consensus refinement (EMD-15293 and PDB 8AA4). Coordinates and structure factors from X-ray crystallography experiments for GHlev have been deposited in the PDB under the accession codes 7ZNR and 7ZNS. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD034863. Raw data from this study are available at the University of Leeds Data Repository: https://doi.org/10.5518/1329. Source data are provided with this paper.
References
Koropatkin, N. M., Cameron, E. A. & Martens, E. C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 10, 323–335 (2012).
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71 (2021).
Hamaker, B. R. & Tuncil, Y. E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 426, 3838–3850 (2014).
Morrison, D. J. & Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200 (2016).
Koh, A., De Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016).
Huttenhower, C. et al. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67, 593–656 (2003).
Martens, E. C., Koropatkin, N. M., Smith, T. J. & Gordon, J. I. Complex glycan catabolism by the human gut microbiota: the Bacteroidetes Sus-like paradigm. J. Biol. Chem. 284, 24673–24677 (2009).
Bolam, D. N. & van den Berg, B. TonB-dependent transport by the gut microbiota: novel aspects of an old problem. Curr. Opin. Struct. Biol. 51, 35–43 (2018).
Glenwright, A. J. et al. Structural basis for nutrient acquisition by dominant members of the human gut microbiota. Nature 541, 407–411 (2017).
Madej, M. et al. Structural and functional insights into oligopeptide acquisition by the RagAB transporter from Porphyromonas gingivalis. Nat. Microbiol. 5, 1016–1025 (2020).
Gray, D. A. et al. Insights into SusCD-mediated glycan import by a prominent gut symbiont. Nat. Commun. 12, 44 (2021).
Terrapon, N. et al. PULDB: the expanded database of polysaccharide utilization loci. Nucleic Acids Res. 46, D677–D683 (2018).
Sonnenburg, E. D. et al. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell 141, 1241–1252 (2010).
Öner, E. T., Hernández, L. & Combie, J. Review of Levan polysaccharide: from a century of past experiences to future prospects. Biotechnol. Adv. 34, 827–844 (2016).
Mardo, K. et al. A highly active endo-levanase BT1760 of a dominant mammalian gut commensal Bacteroides thetaiotaomicron cleaves not only various bacterial levans, but also levan of timothy grass. PLoS ONE 12, e0169989 (2017).
Bolam, D. N. & Sonnenburg, J. L. Mechanistic insight into polysaccharide use within the intestinal microbiota. Gut Microbes 2, 86–90 (2011).
Reeves, A. R., Wang, G. R. & Salyers, A. A. Characterization of four outer membrane proteins that play a role in utilization of starch by Bacteroides thetaiotaomicron. J. Bacteriol. 179, 643–649 (1997).
Cho, K. H. & Salyers, A. A. Biochemical analysis of interactions between outer membrane proteins that contribute to starch utilization by Bacteroides thetaiotaomicron. J. Bacteriol. 183, 7224–7230 (2001).
Karunatilaka, K. S., Cameron, E. A., Martens, E. C., Koropatkin, N. M. & Biteen, J. S. Superresolution imaging captures carbohydrate utilization dynamics in human gut symbionts. mBio 5, e02172 (2014).
Tuson, H. H., Foley, M. H., Koropatkin, N. M. & Biteen, J. S. The starch utilization system assembles around stationary starch-binding proteins. Biophys. J. 115, 242–250 (2018).
Schwanhüusser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Tamura, K. & Brumer, H. Glycan utilization systems in the human gut microbiota: a gold mine for structural discoveries. Curr. Opin. Struct. Biol. 68, 26–40 (2020).
Nilaweera, T. D., Nyenhuis, D. A. & Cafiso, D. S. Structural intermediates observed only in intact Escherichia coli indicate a mechanism for TonB-dependent transport. eLife 10, e68548 (2021).
Zmyslowski, A. M., Baxa, M. C., Gagnon, I. A. & Sosnick, T. R. HDX-MS performed on BtuB in E. coli outer membranes delineates the luminal domain’s allostery and unfolding upon B12 and TonB binding. Proc. Natl Acad. Sci. USA 119, e2119436119 (2022).
Cuyvers, S., Dornez, E., Delcour, J. A. & Courtin, C. M. Occurrence and functional significance of secondary carbohydrate binding sites in glycoside hydrolases. Crit. Rev. Biotechnol. 32, 93–107 (2011).
Ernits, K., Eek, P., Lukk, T., Visnapuu, T. & Alamäe, T. First crystal structure of an endo-levanase – the BT1760 from a human gut commensal Bacteroides thetaiotaomicron. Sci. Rep. 9, 8443 (2019).
Tamura, K. et al. Surface glycan-binding proteins are essential for cereal beta-glucan utilization by the human gut symbiont Bacteroides ovatus. Cell. Mol. Life Sci. 76, 4319–4340 (2019).
Tamura, K., Dejean, G., Van Petegem, F. & Brumer, H. Distinct protein architectures mediate species-specific beta-glucan binding and metabolism in the human gut microbiota. J. Biol. Chem. 296, 100415 (2021).
Grondin, J. M., Tamura, K., Déjean, G., Abbott, D. W. & Brumer, H. Polysaccharide utilization loci: fueling microbial communities. J. Bacteriol. 199, e00860-16 (2017).
McKee, L. S. et al. Polysaccharide degradation by the Bacteroidetes: mechanisms and nomenclature. Environ. Microbiol. Rep. 13, 559–581 (2021).
Rassam, P. et al. Supramolecular assemblies underpin turnover of outer membrane proteins in bacteria. Nature 523, 333–336 (2015).
Benn, G. et al. Phase separation in the outer membrane of Escherichia coli. Proc. Natl Acad. Sci. USA 118, e2112237118 (2021).
Briliūtė, J. et al. Complex N-glycan breakdown by gut Bacteroides involves an extensive enzymatic apparatus encoded by multiple co-regulated genetic loci. Nat. Microbiol. 4, 1571–1581 (2019).
Martens, E. C. et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 9, e1001221 (2011).
Ndeh, D. et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 544, 65–70 (2017).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Ramlaul, K., Palmer, C. M. & Aylett, C. H. S. A local agreement filtering algorithm for transmission EM reconstructions. J. Struct. Biol. 205, 30–40 (2019).
Koropatkin, N. M., Martens, E. C., Gordon, J. I. & Smith, T. J. Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices. Structure 16, 1105–1115 (2008).
Zougman, A., Selby, P. J. & Banks, R. E. Suspension trapping (STrap) sample preparation method for bottom-up proteomics analysis. Proteomics 14, 1006-1000 (2014).
HaileMariam, M. et al. S-Trap, an ultrafast sample-preparation approach for shotgun proteomics. J. Proteome Res. 17, 2917–2924 (2018).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Cox, J. et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 (2011).
Winter, G. et al. DIALS: implementation and evaluation of a new integration package. Acta Crystallogr. D 74, 85–97 (2018).
Winter, G. Xia2: an expert system for macromolecular crystallography data reduction. J. Appl. Crystallogr. 43, 186–190 (2010).
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr. D 69, 1204–1214 (2013).
Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D 62, 72–82 (2006).
Skubak, P. et al. A new MR-SAD algorithm for the automatic building of protein models from low-resolution X-ray data and a poor starting model. Int. Union of Crystallogr. J. 5, 166–171 (2018).
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010).
Krissinel, E., Uski, V., Lebedev, A., Winn, M. & Ballard, C. Distributed computing for macromolecular crystallography. Acta Crystallogr. D 74, 143–151 (2018).
Vagin, A. A. et al. REFMAC5 dictionary: organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr. D 60, 2184–2195 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 67, 235–242 (2011).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D 75, 861–877 (2019).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
Pettersen, E. F. et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Hoh, S. W., Burnley, T. & Cowtan, K. Current approaches for automated model building into cryo-EM maps using Buccaneer with CCP-EM. Acta Crystallogr. D 76, 531–541 (2020).
Burnley, T., Palmer, C. M. & Winn, M. Recent developments in the CCP-EM software suite. Acta Crystallogr. D 73, 469–477 (2017).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Acknowledgements
J.B.R.W. was supported by a Wellcome Trust 4-year PhD studentship (215064/Z/18/Z). B.v.d.B. is financially supported by a Wellcome Trust Investigator award (214222/Z/18/Z), supporting A.S. and Y.Z. M.F. is supported by a Newcastle University studentship. We acknowledge the Diamond Light Source (Didcot, UK) for beam time (proposals mx306, mx1221, mx13587 and mx18598), and thank the staff of beamlines I02, I03, I04 and I24 for support. All cryo-EM was carried out at the Astbury Biostructure Laboratory, which was financially supported by the University of Leeds and the Wellcome Trust (108466/Z/15/Z and 221524/Z/20/Z). We thank R. Thompson, E. Hesketh and D. Maskell for electron microscopy support. Protein ID mass spectrometry was carried out at the University of Leeds Mass Spectrometry Facility. We thank J. Ault and R. George for carrying out this analysis. For the purpose of open access, the authors have applied a CC BY public copyright licence to any author accepted manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
J.B.R.W. carried out cryo-EM and determined cryo-EM structures, supervised by N.A.R. A.S. purified proteins, determined X-ray crystal structures and carried out ITC, supervised by B.v.d.B. M.F. purified proteins. Y.Z. prepared OM samples for proteomics. T.H. and A.G. carried out proteomics, supervised by M.T. H.Z. purified Bt1760, supervised by D.N.B. S.F. collected X-ray crystallography data for Bt1760. A.B. solved Bt1760 crystal structures and managed the Newcastle Structural Biology Laboratory. B.v.d.B. generated B. theta strains, purified proteins and crystallized Bt1761. J.B.R.W., A.S., D.N.B., B.v.d.B. and N.A.R. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Mirjam Czjzek, Stephen Withers and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
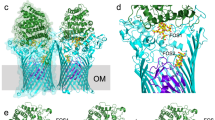
Extended Data Fig. 1 The levan and dextran utilisomes.
(a) Organisation of the levan PUL showing relative gene positions within the PUL, with functions indicated. The four OM-associated PUL components (SusClev, SusDlev, GHlev and SGBPlev) are highlighted by the grey box. An X-ray structure of GHlev (Bt1760; GH32 endo-levanase) is shown (blue; PDB-ID: 7ZNR). The AlphaFold2-predicted model for SGBPlev (Bt1761) is shown (pink) oriented such that the N-terminus is at the bottom and the proposed (C-terminal) levan binding domain is at the top. Note that the N-termini of GHlev and SGBPlev will be lipidated and associated with the outer leaflet of the OM. The cryo-EM structure of the dimeric SusCDlev complex in its open-open state is shown (SusClev is green, SusDlev is grey). (b) Organisation of the dextran PUL showing gene positions within the locus with functions labelled. OM-associated PUL components are boxed in grey. AlphaFold2-predicted models for GHdex (Bt3087; GH66 endo-dextranase), the putative SGBPdex (Bt3088) and the SusCDdex complex, are shown coloured as for the levan PUL in (a). (c) SDS-PAGE of the previously-studied sample of LDAO-purified SusCDlev10 before (asterisk) and after boiling. The boiled sample shows two weak bands in addition to those for SusClev and SusDlev, which were subsequently identified as GHlev and SGBPlev by mass spectrometry. (d) A class average obtained during 3D classification of the levan SusC2D2 core complex. The SusC and SusD components (green and grey respectively) are docked into the density. A large region of density remains unassigned (orange). (e) Isolated view of the previously unassigned density with the crystal structure of GHlev (blue cartoon) fitted into the EM density (blue) as a rigid body. The remaining density was therefore attributed to SGBPlev and is coloured magenta.
Extended Data Fig. 2 Classification of levan utilisome Data.
(a) Output of the first round of 3D classification for apo utilisome data. Yellow, purple and pink classes represent the octameric complex i.e. the complete octameric utilisome. The green class shows the additional lipoproteins associated with just one SusC unit whilst the blue class shows that a small proportion of SusC2D2 core complex was present. (b) Output of 3D classification for the levan utilisome with an active levanase in the presence of FOS DP8-12. Classes (viewed in the plane of the membrane) containing particles of the complete octameric complex were observed (blue and green) as well as hexameric complexes containing a single copy of the SGBPlev and GHlev (pink and yellow). A class containing SusCDlev in isolation is also present (purple). (c) Outputs of 3D classification for long FOS (DP15-25) showing that SGBPlev can adopt a ‘docked’ conformation proximal to both the SusD and levanase. (d) A consensus refinement of all classes containing at least one docked SGBP (yellow, pink, cyan and green in panel (c)). A mask was created around the region of interest (transparent yellow). (e) Outputs of focused classification on the masked region without alignment. A class displaying high resolution for the region of interest is marked with a red asterisk. Independent half maps were reconstructed using unmasked particles belonging to this class. (f) Sharpened reconstruction generated with the aforementioned half maps showing improved density for SGBPlev.
Extended Data Fig. 3 Conformational variability in apo and substrate-bound levan utilisomes.
(a) 3D classification of apo levan utilisome viewed from outside the cell. SusClev (green), SusDlev (grey), SGBPlev (magenta), and GHlev (blue). Classes are separated on their SusDlev lid positions. Wide-wide (WW), normal-wide (NW), and normal-normal (NN) open states (from left to right) (b) Overlay of the wide (SusD grey) and normal (SusD orange) open states of the complex. (c) Overlay of atomic models for the normal versus wide open state generated by a rigid-body fit of SusDlev into the cryoEM density. A monomer is shown for clarity and an asterisk marks the same SusDlev helix in both models. (d) A view of the utilisomes shown at high threshold in the plane of the membrane (left). Different conformations of the SGBPlev observed in 3D classification are overlaid to demonstrate the flexibility of this subunit (boxed region). The same view rotated 90° is shown (right). Disordered micelle density is shown as translucent grey. (e) Variability of the SGBPlev position in the substrate-bound utilisomes with short FOS (~DP8-12) and an active GHlev, and long FOS (DP15-25) with an inactive GHlev. A novel state is uniquely observed in the long FOS structure with one SGBPlev (orange) reaching across and contacting the SGBPlev associated with the other SusC subunit that is present in a docked state. This conformation is consistent with both SGBPlev subunits in the utilisome interacting with the same chain of substrate.
Extended Data Fig. 4 SusC extracellular loops that contribute to lipoprotein interface interactions differ in the levan and dextran utilisomes.
a, Arrangement of GHlev and SGBPlev on SusC in the levan utilisome. GHlev makes contacts with extracellular loop 1 (gold) and extracellular loop 9 (red), while SGBPlev only makes contacts with extracellular loop 1. b, Arrangement of GHdex and SGBPdex on SusC in the dextran utilisome. Here, extracellular loop 1 of SusCdex is the primary site of interaction for GHdex, while extracellular loop 9 comprises the interface with SGBPdex. For clarity, one half of the utilisome is shown in each case, and SusD components are omitted. Note that the dextran utilisome model is a composite of cryo-EM structures (SusCdex) and predicted models from AlphaFold2 (GHdex and SGBPdex).
Extended Data Fig. 5 CryoEM structure and heterogeneity of the dextran utilisome observed by cryo-EM.
Side (a) and top (b) view of the heptameric dextran utilisome map. The identical side (c) and top (d) views of a composite atomic model for dextran utilisome is shown. CryoEM data permitted refinement of SusCdex. AlphaFold2 structure predictions for SusDdex and GHdex were docked into the cryoEM map for the heptameric complex. An AlphaFold2 structure prediction for part of SGBPdex was also fit to the cryoEM map. Unambiguous density was visible only for the first two domains of SGBPdex, and the predicted model was truncated prior to the C-terminal domain. SusCdex = purple, SusDdex = pink, GHdex = cyan and SGBPdex = green. The refinement for the heptameric complex had a global resolution of 3.1 Å. (e) Refined outputs of 3D classification viewed where each map corresponds to a unique complement or arrangement of auxiliary components (as labelled). (f) Schematic of the architecture for two apo glycan utilisomes. The levan utilisome (left) is coloured as in the main text (SusClev = green, SusDlev = gray, GHlev = blue, and SGBPlev = magenta). The equivalent schematic for the substrate-free dextran utilisome is on the right. Note the different arrangement of the GH and SGBP components relative to SusD in the levan and dextran systems.
Extended Data Fig. 6 FOS binding by SusCDlev.
(a) Isolated FOS density obtained from the levan utilisome dataset with active GHlev and short FOS (DP8-12)12. Density for substrate (yellow) is shown at high (left) and low (right) thresholds. (b) Isolated FOS density obtained from the utilisome structure with inactive GHlev and long FOS (DP15-25). Levan density (orange) is shown at high (left) and low (right) thresholds. Arrows indicate missing fructose branches relative to (a). At the FOS1 site, density for the putative β2,1 decoration on Frc4 is missing. Conversely, contiguous density extends beyond the previously resolved density at FOS2, with a novel β2,1 decoration on Frc5. The substrate bound at the FOS2 site follows a similar trend with the previously modelled β2,1 linked fructose side chain being much weaker with longer FOS, while additional density attributed to another β2,6 linked monomer extends the chain towards the FOS1 site. At higher threshold levels, density connects the FOS1 and FOS2 binding sites, indicating that longer FOS (~DP15) can occupy both sites simultaneously. The connecting density is weak and indicative of multiple conformations, consistent with the absence of any contacts from SusClev to this segment. These data confirm that the transporter has considerable substrate binding promiscuity and that, as suggested previously, relatively long FOS (~15 DP) can be accommodated12. FOS models shown are from the original X-ray crystal structure of the SusCDlev complex determined in the presence of short FOS (DP6-12)12. (c) Cryo-EM structure of the inactive GHlev with FOS bound (blue) superposed with the two crystal structures (7ZNR and 7ZNS; orange, grey). (d,e) Comparison of FOS bound in the FOS3 (the active site) and FOS4 (secondary) binding sites of GHlev. The arrowheads point to breaks in the FOS chain in the crystal structures, possibly as a result of using a lower DP FOS for co-crystallization than for cryo-EM. Views in (d) and (e) are generated from a superposition.
Extended Data Fig. 7 ITC of glycan binding to recombinant SGBPlevand GHlev.
(a) Titration of 1 mM defined-length FOS into 50 μM wild type SGBPlev, suggests that ~15 fructose units are required for full affinity, which is abolished by the WAWA (W297A/W359A) mutation. (b) ITC titrations of 8 mg/ml levan, inulin or dextran 500 into 50 μM SGBPlev shows its specificity for levan. (c) ITC data from titrations of GHlev variants (all indicated residues mutated to alanine in the inactive D42A GHlev background). Levan (8 mg/ml) was titrated into 50 μM of indicated GHlev variant. Data fitting assumptions are described in the methods. (d) Surface representation of the GHlev model, with FOS shown as yellow sticks. Inset are zoomed views of the FOS3 (active site) and FOS4 (secondary) binding sites, in which atomic models in cartoon representation for FOS3 are shown with side chains for aromatic residues (Y70A, W318A). For the secondary binding site these residues are W217A, F243A, Y437A.
Extended Data Fig. 8 Conservation of the SGBPlev β2,6-FOS binding site.
a, Overview and b, Close up view of the SGBPlev FOS binding site. The aromatic and polar residues that likely interact with the FOS are shown as grey stick models. The cryo-EM structure of SGBPlev was aligned with selected homologue AlphaFold2-predicted models (c–f). c, Bacteroides sp. D2 SGBPlev (UniProt E5CCB3). d, Prevotella oralis ATCC 33269 SGBPlev (E7RM14). e, Flavobacterium commune SGBPlev (A0A1D9P8I4). f,F. cellulosilyticum SGBPlev (A0A4R5CJN9). FOS-binding residues equivalent to those in b are shown as grey stick models (if present). The FOS chain from the SGBPlev cryo-EM model is shown in b–f for reference (orange and red). The identity indicated in each panel corresponds only to the C-terminal levan-binding domain sequence compared to SGBPlev from B. theta. Although we could not confidently identify which SGBPlev residues form hydrogen bonds with FOS from the cryo-EM maps, binding site conservation analysis indicates that N295, T350, Q352 and N384 of SGBPlev are likely involved in FOS binding. The amino acid sequence alignment of the models shown here can be found in Supplementary Fig. 3.
Supplementary information
Supplementary Information
This file contains Supplementary Discussion, Figs. 1–4 and References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
White, J.B.R., Silale, A., Feasey, M. et al. Outer membrane utilisomes mediate glycan uptake in gut Bacteroidetes. Nature 618, 583–589 (2023). https://doi.org/10.1038/s41586-023-06146-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06146-w
This article is cited by
-
Particle-attached bacteria act as gatekeepers in the decomposition of complex phytoplankton polysaccharides
Microbiome (2024)
-
Capturing glycans
Nature Reviews Microbiology (2023)
-
BtuB TonB-dependent transporters and BtuG surface lipoproteins form stable complexes for vitamin B12 uptake in gut Bacteroides
Nature Communications (2023)
-
BoGH13ASus from Bacteroides ovatus represents a novel α-amylase used for Bacteroides starch breakdown in the human gut
Cellular and Molecular Life Sciences (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.