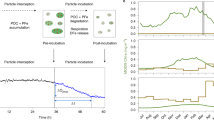
Abstract
Iron is important in regulating the ocean carbon cycle1. Although several dissolved and particulate species participate in oceanic iron cycling, current understanding emphasizes the importance of complexation by organic ligands in stabilizing oceanic dissolved iron concentrations2,3,4,5,6. However, it is difficult to reconcile this view of ligands as a primary control on dissolved iron cycling with the observed size partitioning of dissolved iron species, inefficient dissolved iron regeneration at depth or the potential importance of authigenic iron phases in particulate iron observational datasets7,8,9,10,11,12. Here we present a new dissolved iron, ligand and particulate iron seasonal dataset from the Bermuda Atlantic Time-series Study (BATS) region. We find that upper-ocean dissolved iron dynamics were decoupled from those of ligands, which necessitates a process by which dissolved iron escapes ligand stabilization to generate a reservoir of authigenic iron particles that settle to depth. When this ‘colloidal shunt’ mechanism was implemented in a global-scale biogeochemical model, it reproduced both seasonal iron-cycle dynamics observations and independent global datasets when previous models failed13,14,15. Overall, we argue that the turnover of authigenic particulate iron phases must be considered alongside biological activity and ligands in controlling ocean-dissolved iron distributions and the coupling between dissolved and particulate iron pools.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Oceanographic data collected and analysed in this study are available at https://www.bco-dmo.org/project/822807 and https://www.bco-dmo.org/dataset/888772.
Code availability
Model code is available at https://github.com/atagliab/PISCES-BAIT and output at https://doi.org/10.5281/zenodo.7378193.
References
Tagliabue, A. et al. The integral role of iron in ocean biogeochemistry. Nature 543, 51–59 (2017).
Gledhill, M. & Buck, K. N. The organic complexation of iron in the marine environment: a review. Front. Microbiol. 3, 69 (2012).
Johnson, K. S., Gordon, R. M. & Coale, K. H. What controls dissolved iron concentrations in the world ocean? Mar. Chem. 57, 137–161 (1997).
Lauderdale, J. M., Braakman, R., Forget, G., Dutkiewicz, S. & Follows, M. J. Microbial feedbacks optimize ocean iron availability. Proc. Natl Acad. Sci. 117, 4842–4849 (2020).
Parekh, P., Follows, M. J. & Boyle, E. A. Decoupling of iron and phosphate in the global ocean. Glob. Biogeochem. Cycles 19, GB2020 (2005).
Whitby, H. et al. A call for refining the role of humic-like substances in the oceanic iron cycle. Sci. Rep. 10, 6144 (2020).
Boyd, P. W., Ellwood, M. J., Tagliabue, A. & Twining, B. S. Biotic and abiotic retention, recycling and remineralization of metals in the ocean. Nat. Geosci. 10, 167–173 (2017).
Frew, R. D. et al. Particulate iron dynamics during FeCycle in subantarctic waters southeast of New Zealand. Glob. Biogeochem. Cycles 20, GB1S93 (2006).
Ohnemus, D. C., Torrie, R. & Twining, B. S. Exposing the distributions and elemental associations of scavenged particulate phases in the ocean using basin‐scale multi‐element data sets. Glob. Biogeochem. Cycles 33, 725–748 (2019).
Tagliabue, A. et al. The interplay between regeneration and scavenging fluxes drives ocean iron cycling. Nat. Commun. 10, 4960 (2019).
Cullen, J. T., Bergquist, B. A. & Moffett, J. W. Thermodynamic characterization of the partitioning of iron between soluble and colloidal species in the Atlantic Ocean. Mar. Chem. 98, 295–303 (2006).
Fitzsimmons, J. N., Bundy, R. M., Al-Subiai, S. N., Barbeau, K. A. & Boyle, E. A. The composition of dissolved iron in the dusty surface ocean: an exploration using size-fractionated iron-binding ligands. Mar. Chem. 173, 125–135 (2015).
Tagliabue, A. et al. How well do global ocean biogeochemistry models simulate dissolved iron distributions? Glob. Biogeochem. Cycles 30, 149–174 (2016).
Somes, C. J. et al. Constraining global marine iron sources and ligand‐mediated scavenging fluxes with GEOTRACES dissolved iron measurements in an ocean biogeochemical model. Glob. Biogeochem. Cycles 35, e2021GB006948 (2021).
Sedwick, P. N. et al. Dissolved iron in the Bermuda region of the subtropical North Atlantic Ocean: seasonal dynamics, mesoscale variability, and physicochemical speciation. Mar. Chem. 219, 103748 (2020).
Martinez-Garcia, A. et al. Iron fertilization of the Subantarctic Ocean during the last ice age. Science 343, 1347–1350 (2014).
Raven, J. A., Evans, M. C. W. & Korb, R. E. The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynth. Res. 60, 111–150 (1999).
Wade, J., Byrne, D. J., Ballentine, C. J. & Drakesmith, H. Temporal variation of planetary iron as a driver of evolution. Proc. Natl Acad. Sci. 118, e2109865118 (2021).
Tagliabue, A., Aumont, O. & Bopp, L. The impact of different external sources of iron on the global carbon cycle. Geophys. Res. Lett. 41, 920–926 (2014).
Buck, K. N., Sedwick, P. N., Sohst, B. & Carlson, C. A. Organic complexation of iron in the eastern tropical South Pacific: results from US GEOTRACES Eastern Pacific Zonal Transect (GEOTRACES cruise GP16). Mar. Chem. 201, 229–241 (2018).
Buck, K. N., Sohst, B. & Sedwick, P. N. The organic complexation of dissolved iron along the U.S. GEOTRACES (GA03) North Atlantic Section. Deep Sea Res. II Top. Stud. Oceanogr. 116, 152–165 (2015).
Gerringa, L. J. A., Rijkenberg, M. J. A., Schoemann, V., Laan, P. & de Baar, H. J. W. Organic complexation of iron in the West Atlantic Ocean. Mar. Chem. 177, 434–446 (2015).
Bressac, M. et al. Resupply of mesopelagic dissolved iron controlled by particulate iron composition. Nat. Geosci. 12, 995–1000 (2019).
Lamborg, C. H. et al. The flux of bio- and lithogenic material associated with sinking particles in the mesopelagic “twilight zone” of the northwest and North Central Pacific Ocean. Deep Sea Res. II Top. Stud. Oceanogr. 55, 1540–1563 (2008).
Twining, B. S. et al. Differential remineralization of major and trace elements in sinking diatoms. Limnol. Oceanogr. 59, 689–704 (2014).
Tagliabue, A. et al. Persistent uncertainties in ocean net primary production climate change projections at regional scales raise challenges for assessing impacts on ecosystem services. Front. Clim. 3, 738224 (2021).
Gunnars, A., Blomqvist, S., Johansson, P. & Andersson, C. Formation of Fe(III) oxyhydroxide colloids in freshwater and brackish seawater, with incorporation of phosphate and calcium. Geochim. Cosmochim. Acta 66, 745–758 (2002).
Feely, R. A., Trefry, J. H., Massoth, G. J. & Metz, S. A comparison of the scavenging of phosphorus and arsenic from seawater by hydrothermal iron oxyhydroxides in the Atlantic and Pacific Oceans. Deep Sea Res. A Oceanogr. Res. Pap. 38, 617–623 (1991).
Homoky, W. B. et al. Iron colloids dominate sedimentary supply to the ocean interior. Proc. Natl Acad. Sci. 118, e2016078118 (2021).
Homoky, W. B. et al. Iron and manganese diagenesis in deep sea volcanogenic sediments and the origins of pore water colloids. Geochim. Cosmochim. Acta 75, 5032–5048 (2011).
Fitzsimmons, J. N. & Boyle, E. A. Both soluble and colloidal iron phases control dissolved iron variability in the tropical North Atlantic Ocean. Geochim. Cosmochim. Acta 125, 539–550 (2014).
Kunde, K. et al. Iron distribution in the subtropical North Atlantic: the pivotal role of colloidal iron. Glob. Biogeochem. Cycles 33, 1532–1547 (2019).
Marsay, C. M., Barrett, P. M., McGillicuddy, D. J. & Sedwick, P. N. Distributions, sources, and transformations of dissolved and particulate iron on the Ross Sea continental shelf during summer. J. Geophys. Res. Oceans 122, 6371–6393 (2017).
Conway, T. M. et al. Tracing and constraining anthropogenic aerosol iron fluxes to the North Atlantic Ocean using iron isotopes. Nat. Commun. 10, 2628 (2019).
Tang, W. et al. Widespread phytoplankton blooms triggered by 2019–2020 Australian wildfires. Nature 597, 370–375 (2021).
Boyd, P. W., Mackie, D. S. & Hunter, K. A. Aerosol iron deposition to the surface ocean – modes of iron supply and biological responses. Mar. Chem. 120, 128–143 (2010).
Bowie, A. R. et al. Biogeochemical iron budgets of the Southern Ocean south of Australia: decoupling of iron and nutrient cycles in the subantarctic zone by the summertime supply. Glob. Biogeochem. Cycles 23, GB4034 (2009).
Wu, J. & Boyle, E. Iron in the Sargasso Sea: implications for the processes controlling dissolved Fe distribution in the ocean. Glob. Biogeochem. Cycles 16, 33-1–33-8 (2002).
Rijkenberg, M. J. et al. The distribution of dissolved iron in the West Atlantic Ocean. PLoS One 9, e101323 (2014).
Black, E. E. et al. Ironing out Fe residence time in the dynamic upper ocean. Glob. Biogeochem. Cycles 34, e2020GB006592 (2020).
Wagener, T., Guieu, C. & Leblond, N. Effects of dust deposition on iron cycle in the surface Mediterranean Sea: results from a mesocosm seeding experiment. Biogeosciences 7, 3769–3781 (2010).
Honeyman, B. D. & Santschi, P. H. A Brownian-pumping model for oceanic trace metal scavenging: evidence from Th isotopes. J. Mar. Res. 47, 951–992 (1989).
Wu, J., Boyle, E., Sunda, W. & Wen, L. S. Soluble and colloidal iron in the oligotrophic North Atlantic and North Pacific. Science 293, 847–849 (2001).
Völker, C. & Tagliabue, A. Modeling organic iron-binding ligands in a three-dimensional biogeochemical ocean model. Mar. Chem. 173, 67–77 (2015).
Misumi, K. et al. Slowly sinking particles underlie dissolved iron transport across the Pacific Ocean. Glob. Biogeochem. Cycles 35, e2020GB006823 (2021).
Seferian, R. et al. Tracking improvement in simulated marine biogeochemistry between CMIP5 and CMIP6. Curr. Clim. Change Rep. 6, 95–119 (2020).
Raiswell, R., Benning, L. G., Tranter, M. & Tulaczyk, S. Bioavailable iron in the Southern Ocean: the significance of the iceberg conveyor belt. Geochem. Trans. 9, 7 (2008).
von der Heyden, B. P., Roychoudhury, A. N., Mtshali, T. N., Tyliszczak, T. & Myneni, S. C. Chemically and geographically distinct solid-phase iron pools in the Southern Ocean. Science 338, 1199–1201 (2012).
Curti, L. et al. Carboxyl-richness controls organic carbon preservation during coprecipitation with iron (oxyhydr)oxides in the natural environment. Commun. Earth Environ. 2, 229 (2021).
Rauschenberg, S. & Twining, B. S. Evaluation of approaches to estimate biogenic particulate trace metals in the ocean. Mar. Chem. 171, 67–77 (2015).
Twining, B. S. et al. Taxonomic and nutrient controls on phytoplankton iron quotas in the ocean. Limnol. Oceanogr. Lett. 6, 96–106 (2021).
Rudnick, R. L. & Gao, S. in Treatise on Geochemistry, Vol. 3 (eds Holland, H. D. & Turekian, K. K.) 1–64 (Elsevier, 2003).
Shelley, R. U., Morton, P. L. & Landing, W. M. Elemental ratios and enrichment factors in aerosols from the US-GEOTRACES North Atlantic transects. Deep Sea Res. II Top. Stud. Oceanogr. 116, 262–272 (2015).
GEOTRACES Intermediate Data Product Group. The GEOTRACES Intermediate Data Product 2021 (IDP2021). https://doi.org/10.5285/cf2d9ba9-d51d-3b7c-e053-8486abc0f5fd (NERC EDS British Oceanographic Data Centre NOC, 2021).
Kwiatkowski, L., Aumont, O., Bopp, L. & Ciais, P. The impact of variable phytoplankton stoichiometry on projections of primary production, food quality, and carbon uptake in the global ocean. Glob. Biogeochem. Cycles 32, 516–528 (2018).
Ye, Y. & Völker, C. On the role of dust-deposited lithogenic particles for iron cycling in the tropical and subtropical Atlantic. Glob. Biogeochem. Cycles 31, 1543–1558 (2017).
Aumont, O., Ethé, C., Tagliabue, A., Bopp, L. & Gehlen, M. PISCES-v2: an ocean biogeochemical model for carbon and ecosystem studies. Geosci. Model Dev. 8, 2465–2513 (2015).
Hamilton, D. S. et al. Recent (1980 to 2015) trends and variability in daily‐to‐interannual soluble iron deposition from dust, fire, and anthropogenic sources. Geophys. Res. Lett. 47, e2020GL089688 (2020).
Liu, X. & Millero, F. J. The solubility of iron in seawater. Mar. Chem. 77, 43–54 (2002).
Acknowledgements
We thank the captains and crews of RV Atlantic Explorer and RV Endeavor and the BATS programme team for their invaluable assistance during the four project cruises. O. Antipova provided assistance in synchrotron data collection and analysis and S. Burns provided assistance with sampling at sea. The model simulations were undertaken on Barkla, part of the High Performance Computing facilities at the University of Liverpool, UK. A.T. and D.K. were supported by NERC award NE/S013547/1; P.S. and B.S. were supported by NSF award OCE-1829833; B.S.T., D.C.O. and L.E.S. were supported by NSF award OCE-1829819; K.N.B. and S.C. were supported by NSF award OCE-1829777; R.J. was supported by NSF award OCE-1829844. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
The overarching BAIT programme was conceptualized by P.S., K.N.B., R.J., A.T., D.C.O. and B.S.T. Field and laboratory work was conducted by K.N.B., S.C., R.J., D.C.O., L.E.S., B.S., P.S., A.T. and B.S.T. This study was designed and led by A.T., alongside K.N.B., L.E.S. and B.S.T., with further contributions from O.A., P.W.B., W.B.H. and P.S. Analysis of dissolved iron, ligands and particles was performed by P.S. and B.S., K.N.B. and S.C., and L.E.S. and B.S.T., respectively. Modelling work was undertaken by A.T. Data synthesis and model-data comparisons were conducted by A.T., D.K. and L.E.S. A.T. led the drafting of the manuscript, with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Kazuhiro Misumi, Brandy Toner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Seasonal evolution of total and stronger ligands.
Observed and modelled total (black symbols) and stronger (red symbols) ligand concentrations (nM). Black lines are model solutions at the BATS site from the PISCES-Quota model, with varying total ligands derived from DOC (using 0.09, 0.08 and 0.07 nM LT µM DOC−1). Blue lines represent model solutions from PISCES-Quota-Fe, with either prognostic stronger ligands (solid line) or DOC-derived total weaker ligands (dashed line, using 0.09 nM LT µM DOC−1).
Extended Data Fig. 2 Seasonal evolution of excess ligands.
Observed and modelled excess total (black symbols) and strong (red symbols) ligands (both in nM). Solid and dashed black lines are model solutions at the BATS site from the PISCES-Quota model, with varying total ligands derived from DOC (using 0.09, 0.08 and 0.07 nM LT (µM DOC)−1) or prognostic stronger ligands (thin black lines). Blue lines represent model solutions from PISCES-Quota-Fe, with either prognostic stronger ligands (solid line) or DOC-derived total ligands (dashed line, using 0.09 nM LT (µM DOC)−1). Values less than zero are when DFe concentrations exceed the concentrations of either L1 or LT. Only the PISCES-Quota-Fe model is able to generate the observed large excess ligand pools.
Extended Data Fig. 3 Variations in the seasonal evolution of dissolved iron.
DFe data and model solutions at the BATS site. Red crosses are DFe data for each voyage for three stations in the BATS region. All black lines are model solutions at the BATS site from the PISCES-Quota model, with total ligands derived from DOC (using 0.09 nM LT µM DOC−1) but with varying strengths of scavenging of free Fe by lithogenic particles. Blue lines represent model solutions from the new PISCES-Quota-Fe model, with either prognostic stronger ligands (solid line) or DOC-derived total ligands (dashed line, using 0.09 nM LT (µM DOC)−1). In red, we also compare the default PISCES-Quota (solid line, with total ligands derived from DOC using 0.09 nM LT µM DOC−1) and PISCES standard (dashed line) models. This demonstrates that there is little difference in the model–data mismatch in the seasonal evolution of DFe between PISCES-Quota and the standard PISCES model.
Extended Data Fig. 4 Global model–data comparison of dissolved iron.
Observed and modelled dissolved iron (nM) for ten GEOTRACES sections for PISCES-Quota-Fe and PISCES-Quota. Observations and models are binned onto the same vertical grid.
Extended Data Fig. 5 Model performance for biogeochemical metrics.
Plots showing the difference in performance between PISCES-Quota and PISCES-Quota-Fe for a suite of biogeochemical diagnostics. Average upper 100 m NO3 and PO4 are in mmol m−3, average 200–600 m O2 is in mmol m−3, total chlorophyll (T-Chl) at the surface (summed across the picophytoplankton, nanophytoplankton and diatoms) is in mg m−3 and carbon export at 100 m is in mol m−2 year−1. It can be seen that the new PISCES-Quota-Fe model does not substantially alter the biogeochemical mean state of the model.
Extended Data Fig. 6 Iron cycle fluxes in the Atlantic and Pacific oceans.
Proportional contributions of different processes to total DFe supply and removal fluxes along two example sections in the Atlantic (20° W) and Pacific (150° W) oceans from the PISCES-Quota-Fe model with prognostic strong ligands.
Supplementary information
Supplementary Table 1
Previous iron-cycle-process studies. A summary of available measurements of the ocean iron cycle from time series stations and process studies that collected temporal observations. We provide the name and broad location and year of the study, the seasonal sampling frequency, depths and whether there was concurrent sampling of DFe, PFe, total ligands (Ltot), strong and weak ligands (L1 and L2) and PFe phases (lithogenic and biogenic). The current study, in the top row, is the only one to provide such data across all seasons and iron parameters.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tagliabue, A., Buck, K.N., Sofen, L.E. et al. Authigenic mineral phases as a driver of the upper-ocean iron cycle. Nature 620, 104–109 (2023). https://doi.org/10.1038/s41586-023-06210-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06210-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.