Abstract
Cancer-specific TCF1+ stem-like CD8+ T cells can drive protective anticancer immunity through expansion and effector cell differentiation1,2,3,4; however, this response is dysfunctional in tumours. Current cancer immunotherapies2,5,6,7,8,9 can promote anticancer responses through TCF1+ stem-like CD8+ T cells in some but not all patients. This variation points towards currently ill-defined mechanisms that limit TCF1+CD8+ T cell-mediated anticancer immunity. Here we demonstrate that tumour-derived prostaglandin E2 (PGE2) restricts the proliferative expansion and effector differentiation of TCF1+CD8+ T cells within tumours, which promotes cancer immune escape. PGE2 does not affect the priming of TCF1+CD8+ T cells in draining lymph nodes. PGE2 acts through EP2 and EP4 (EP2/EP4) receptor signalling in CD8+ T cells to limit the intratumoural generation of early and late effector T cell populations that originate from TCF1+ tumour-infiltrating CD8+ T lymphocytes (TILs). Ablation of EP2/EP4 signalling in cancer-specific CD8+ T cells rescues their expansion and effector differentiation within tumours and leads to tumour elimination in multiple mouse cancer models. Mechanistically, suppression of the interleukin-2 (IL-2) signalling pathway underlies the PGE2-mediated inhibition of TCF1+ TIL responses. Altogether, we uncover a key mechanism that restricts the IL-2 responsiveness of TCF1+ TILs and prevents anticancer T cell responses that originate from these cells. This study identifies the PGE2–EP2/EP4 axis as a molecular target to restore IL-2 responsiveness in anticancer TILs to achieve cancer immune control.
Similar content being viewed by others
Main
Increased production of the bioactive lipid PGE2 downstream of aberrant cyclooxygenase 1 (COX1; encoded by Ptgs1) and COX2 (encoded by Ptgs2) activity is observed in many human tumours and is associated with cancer progression and poor patient survival10,11,12,13. Studies using preclinical mouse cancer models have demonstrated that tumour-derived PGE2 has an important role in tumour escape from anticancer immunity14,15. PGE2 signalling is mediated by four G protein-coupled receptors that are broadly expressed on various immune cell populations, EP1, EP2, EP3 and EP4 (encoded by PTGER1, PTGER2, PTGER3 and PTGER4, respectively), of which signalling through EP2/EP4 can suppress immune cell function16. Previous studies have implicated a role for PGE2 in the regulation of T cell biology and function17,18,19,20; however, the impact of PGE2 on TCF1+CD8+ T cells and their ability to mount protective anticancer responses remains unclear.
The PGE2–EP2/EP4 axis controls anticancer CD8+ T cell responses
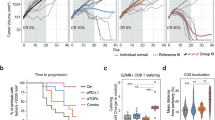
To determine the role of PGE2 in tumour escape from anticancer T cell responses, we generated Cd4crePtger2−/−Ptger4fl/fl mice in which Cre recombinase activity induces the deletion of EP4 in CD4+ and CD8+ T cells on a global EP2-deficient background. We also generated additional control mice that lack only EP2 (Ptger2−/−Ptger4fl/fl mice), which enabled the testing of possible effects of global EP2 deficiency. T cell profiling in Cd4crePtger2−/−Ptger4fl/fl mice and Ptger2−/−Ptger4fl/fl mice compared with C57BL/6 wild-type (WT) mice revealed normal CD4+ and CD8+ T cell abundance and subset composition in lymphoid organs (Extended Data Fig. 1a–g). Unaltered T cell composition was similarly observed in GzmbcrePtger2−/−Ptger4fl/fl mice, which lack EP2 and EP4 in CD8+ T cells expressing granzyme B (GZMB) (Extended Data Fig. 1a–g). Notably, after tumour challenge, Cd4crePtger2−/−Ptger4fl/fl mice exhibited improved tumour immune control, and fully rejected tumours formed by immune-evasive, PGE2-producing (control) BRAFV600E melanoma cells (Fig. 1a and Extended Data Fig. 2a). This was not the case for Ptger2−/−Ptger4fl/fl mice and WT mice, in which control BRAFV600E tumours progressively grew (Fig. 1a and Extended Data Fig. 2a). We further validated that BRAFV600E melanoma depended on tumour-derived PGE2 to evade anticancer immunity by demonstrating that COX-deficient Ptgs1/Ptgs2−/− BRAFV600E melanoma, which lacks PGE2 production, failed to escape immune control (Fig. 1a and Extended Data Fig. 2a). We also confirmed that this effect is mediated by CD8+ T cells14 (Extended Data Fig. 2b). Extending our analysis to other mouse tumour models, tumours formed by Panc02 pancreatic cancer cells similarly exhibited complete regression in Cd4crePtger2−/−Ptger4fl/fl mice but not in Ptger2−/−Ptger4fl/fl or WT mice (Fig. 1b and Extended Data Fig. 2c). Similar results were observed for tumours derived from MC38 colorectal cancer cells (Extended Data Fig. 2d,e).
a, Tumour growth profiles of 2 × 105 Ptgs1/Ptgs2−/− or control BRAFV600E melanoma cells transplanted into WT mice, Ptger2−/−Ptger4fl/fl mice and Cd4crePtger2−/−Ptger4fl/fl mice (n = 10 each). b, Growth profiles of 2 × 106 Panc02 cells transplanted into WT mice, Ptger2−/−Ptger4fl/fl mice and Cd4crePtger2−/−Ptger4fl/fl mice (n = 8 each). c–e, WT mice, Ptger2−/−Ptger4fl/fl mice and Cd4crePtger2−/−Ptger4fl/fl mice were subcutaneously (s.c.) injected with 2 × 106 control or Ptgs1/Ptgs2−/− BRAFV600E cells and TILs were analysed 11 days later by flow cytometry. c, Plots showing the frequencies of CD8+ and CD4+ TILs among CD45+ immune cells and expression of the activation marker CD44. d, Quantification of TIL numbers (CD8+ TILs: Ptgs1/Ptgs2−/− into WT, n = 9; control into WT, n = 10; control into Ptger2−/−Ptger4fl/fl, n = 7; control into Cd4crePtger2−/−Ptger4fl/fl, n = 10; CD4+ TILs: Ptgs1/Ptgs2−/− into WT, n = 8; control into WT, n = 10; control into Ptger2−/−Ptger4fl/fl; n = 7; control into Cd4crePtger2−/−Ptger4fl/fl, n = 8). e, TIL frequencies (Ptgs1/Ptgs2−/− into WT, n = 8; control into WT, n = 8; control into Ptger2−/−Ptger4fl/fl, n = 7; control into Cd4crePtger2−/−Ptger4fl/fl, n = 8). f, Effect of CD8+ and CD4+ T cell depletion on control BRAFV600E tumour growth in Cd4crePtger2−/−Ptger4fl/fl mice (Cd4crePtger2−/−Ptger4fl/fl, n = 8; WT, n = 9). Data in a, b and d–f are pooled from two (b,f) or three (a,d,e) independent experiments and depicted as the mean ± s.e.m. Plots in c show data for 1 tumour representative of n = 7 tumours from 2 independent experiments. P values are from two-way analysis of variance (ANOVA) with Bonferroni’s correction for multiple testing (a,b,f) or one-way ANOVA with Dunnett’s multiple-comparison test (d,e). NS, not significant (P ≥ 0.05).
Immune control of control BRAFV600E melanoma tumours in Cd4crePtger2−/−Ptger4fl/fl mice was linked to markedly increased CD8+ TIL accumulation (Fig. 1c–e). By contrast, no substantial differences were observed for CD4+ TILs (Fig. 1c–e). This result suggests that although PGE2–EP2/EP4 signalling may affect CD4+ T cell function, these cells, at least in BRAFV600E tumours, do not have a major role in immune escape. Consistently, antibody-mediated T cell depletion confirmed the relevance of CD8+ but not CD4+ T cells for immune control of PGE2-producing BRAFV600E tumours in Cd4crePtger2−/−Ptger4fl/fl mice (Fig. 1f). Taken together, these data suggest that EP2/EP4 signalling controls the accumulation of CD8+ TILs in PGE2-producing tumours and that this is important for cancer immune evasion.
PGE2 does not affect CD8+ T cell priming
Priming of anticancer CD8+ T cells in tumour-draining lymph nodes (tdLNs) by type 1 conventional dendritic cells (cDC1s) that transport tumour antigens to tdLNs is thought to underlie anticancer CD8+ T cell responses21,22. To test whether PGE2 impairs cDC1-mediated CD8+ T cell priming, we injected WT mice with PGE2-producing control or PGE2-deficient Ptgs1/Ptgs2−/− BRAFV600E melanoma cells engineered to express the model antigen ovalbumin (OVA). We then determined the presence of migratory CD103+ cDC1 cross-presenting OVA-derived peptides on major histocompatibility complex (MHC) class I molecules in tdLNs (Extended Data Fig. 3a). Migratory cDC1s in both models cross-presented tumour-derived OVA protein with similar efficiency, as determined by staining for OVA(257–264) (SIINFEKL) peptide loading of the MHC class I molecule H-2Kb (Extended Data Fig. 3b,c). To further examine T cell priming, we adoptively transferred naive CD8+ OT-I T cells (Extended Data Fig. 3d), which express a transgenic T cell receptor (TCR) specific for OVA, into mice subsequently transplanted with BRAFV600E-OVA tumours. Naive (CD44low) OT-I T cells efficiently expanded into CD44+TCF1+ OT-I T cells within tdLNs in both groups (Extended Data Fig. 3e,f). This result demonstrates that T cell priming is unaffected. Consistent with these data, we did not detect substantial PGE2 levels in tdLNs from control BRAFV600E tumours or in other distant organs (Extended Data Fig. 3g). Moreover, progressive outgrowth of control BRAFV600E tumours and efficient immune control of Ptgs1/Ptgs2−/− BRAFV600E tumours was unchanged following tumour transplantation to the same lymph drainage site (Extended Data Fig. 3h). These findings imply that an anticancer CD8+ T cell response initiated in the shared tdLN achieves effective elimination of the PGE2-deficient tumour but nevertheless fails in the co-transplanted PGE2-producing tumour. Taken together, these data demonstrate that PGE2 controls anticancer CD8+ T cell responses locally within tumour tissue, which raises the question of how it affects CD8+ TILs.
PGE2 controls CD8+ TIL effector expansion
CD8+ TILs are heterogenous and comprise at least two phenotypically and functionally distinct populations: (1) proliferation and differentiation competent TCF1+ cells that lack cytotoxic effector functions (often referred to as ‘stem-like’ or ‘precursor of exhausted’ T cells); and (2) TIM-3+(TCF1−) cells that encompass more differentiated effector and terminally differentiated or exhausted T cells. TCF1+CD8+ T cells fulfil an essential role in anticancer immunity by giving rise to TIM-3+ progeny through proliferative expansion and effector differentiation2,5,8,9. This process is pivotal for anticancer immunity that at least in part occurs locally within tumour tissue1,2,3.
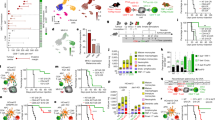
Our results raised the question of whether interference with effector differentiation of TCF1+CD8+ TILs underlies the PGE2-mediated impairment of anticancer immunity. To address this issue across the single-cell landscape of CD8+ TILs, we performed parallel single-cell RNA sequencing (scRNA-seq) and single-cell TCR sequencing (scTCR-seq) of CD8+ TILs sorted from BRAFV600E tumours at day 11 after tumour transplantation into Cd4crePtger2−/−Ptger4fl/fl mice and Ptger2−/−Ptger4fl/fl mice (as control) (Fig. 2a and Extended Data Fig. 4a). We also included GzmbcrePtger2−/−Ptger4fl/fl mice (Fig. 2a), reasoning that this would enable us to determine the impact of EP2/EP4-mediated PGE2 signalling on those CD8+ T cells undergoing effector differentiation within tumour tissue. We further included four biological replicates in each group to ensure that heterogeneity among individual tumours is reflected in our analysis. scRNA-seq analysis revealed eight TIL clusters (Fig. 2b) that all expressed Pdcd1 (which encodes PD-1), the activation marker Cd44 and the transcription factor (TF) Tox (Extended Data Fig. 4b), a result consistent with their activation history. Of note, CD8+ TILs displayed equally high protein expression of CD44, TOX and PD-1 that did not differ among cells isolated from tumours in Ptger2−/−Ptger4fl/fl mice, Cd4crePtger2−/−Ptger4fl/fl mice and GzmbcrePtger2−/−Ptger4fl/fl mice (Extended Data Fig. 4c).
a–g, scRNA-seq analyses of CD8+ TILs in control BRAFV600E tumours from Ptger2−/−Ptger4fl/fl mice, Cd4crePtger2−/−Ptger4fl/fl mice and GzmbcePtger2−/−Ptger4fl/fl mice (n = 4 each). a, Experimental design. b, Uniform manifold approximation and projection (UMAP) plot of 12,516 CD8+ TILs coloured according to cluster classification. c, Visualization of Tcf7 and Havcr2 transcript levels. d, PCPT plot showing expression levels of selected genes. e, Developmental trajectory prediction by unsupervised slingshot analysis. f,g, Comparison of CD8+ TIL clusters among Ptger2−/−Ptger4fl/fl mice, Cd4crePtger2−/−Ptger4fl/fl mice and GzmbcrePtger2−/−Ptger4fl/fl mice. f, Density analysis. g, Quantification relative to cluster 1 (n = 4 each). h,i, scTCR-seq analyses of CD8+ TILs from n = 3 tumours for each group. h, UMAP visualizations of T cell clonotype distribution. i, Quantification of T cell clonotype frequency. j–n, TIM-3+ effector CD8+ T cell differentiation in tumour tissue. Cd4crePtger2−/−Ptger4fl/fl mice bearing control BRAFV600E tumours were injected with FTY720 or NaCl as control. j, Experimental design. k, Representative flow cytometry plots showing TCF1 and TIM-3 expression among CD44+CD8+ TILs. l, Average percentages of CD8+ TIL populations across n = 6 tumours. m, Quantification of CD8+ TIL numbers (n = 6). n, Analysis of tumour mass (n = 10). Anti-CD8β, antibody-mediated CD8+ T cell depletion in the absence of FTY720 treatment. Data in a–h are from one experiment. Data in g are depicted as box plots extending from the 25th to 75th percentiles with the median as the centre and the whiskers corresponding to the minimum and maximum values. Data in k–n are pooled from two (k,l,m) or three (n) independent experiments and depicted as the mean ± s.e.m. P values are from two-way ANOVA with Bonferroni’s correction for multiple testing (g) or one-way ANOVA with Tukey’s multiple-comparison test (m,n). Plots in k show data for 1 tumour representative for n = 6 tumours from 2 independent experiments.
In our concatenated scRNA-seq data, TIL clusters 1 and 2 shared high expression of stem-like T cell markers such as Tcf7 (which encodes TCF1), Slamf6 and Il7r (Fig. 2c,d and Extended Data Fig. 4d). Of note, both of these clusters displayed markedly higher expression of gene signatures of memory or tumour-reactive T cells than signatures for naive T cells (Extended Data Fig. 4e,f), which indicated that at least a substantial fraction of these cells is antigen-experienced. TCF1+ TILs in cluster 1 displayed enriched expression of Sell (which encodes CD62L), Ccr7 and Bach2 (Extended Data Fig. 4d,g,h). By contrast, TCF1+ TILs in cluster 2 lacked Sell but showed expression of markers associated with effector function (such as Gzmb, Gzmk and Fasl) and migration (S1pr1, Itga4, Gpr183, Itgb1, Cxcr3 and Ier2) (Fig. 2d and Extended Data Fig. 4d,g,h), which indicated their incipient effector differentiation. Consistently, CD62L−TCF1+ TILs but not CD62L+TCF1+ TILs stained positive for intracellular GZMB protein (Extended Data Fig. 4i), although GZMB expression in these cells was low both in terms of frequency of GZMB+ cells and total GZMB levels. The remaining scRNA-seq clusters (clusters 3–8) lacked Tcf7 expression and, in addition to Gzmb, shared expression of genes associated with T cell differentiation and effector function, including Havcr2 (which encodes TIM-3) and high expression of the chemokine receptor Cxcr6 (Fig. 2c,d and Extended Data Fig. 4g,h), which identified them as more differentiated early and/or terminally differentiated TIL populations. We confirmed co-expression of TIM-3 and CXCR6 at the protein level (Extended Data Fig. 4j) and used both molecules as overarching markers to collectively denote (TCF1−) effector TILs. Among the different clusters of TIM-3+CXCR6+ TILs, clusters 3 and 4 were marked by high expression of molecules associated with early effector-like cells; for example, Cx3cr1 (refs. 23,24) in cluster 3 and Cd7 (ref. 25) in cluster 4 (Extended Data Fig. 4d,h). By contrast, clusters 5–8 displayed increased expression of cytotoxic effector molecules and immune-inhibitory receptors (Fig. 2d and Extended Data Fig. 4h), but were distinguished by differential expression of cytokines (for example, Ifng and Tnf), molecules associated with growth arrest and DNA repair (Apex1 and Gadd45b) and type I interferon signalling (Isg15, Ifit1 and Ifit3) (Extended Data Fig. 4d,h). Notably, in contrast to tumour tissue, we did not detect any GZMB+ cells among activated CD44+CD8+ T cells in tdLNs or spleen of tumour-bearing mice (Extended Data Fig. 4k). This result supports the notion that effector differentiation of anticancer CD8+ T cells occurs within tumour tissue. Unsupervised slingshot analysis of our TIL scRNA-seq data uncovered a tree-shaped developmental trajectory that begins with TCF1+ cells and progresses over CX3CR1hiTIM-3+ effector cells into CD7hiTIM-3+ effector cells, from which it branches off into distinct terminally differentiated T cell populations (Fig. 2e). Together, these results indicate a progressive trajectory for TIL differentiation within tumours that originates from TCF1+ TILs and follows a unidirectional path of effector differentiation before ending in multiple smaller branches of terminally differentiated TIL populations.
To assess the impact of PGE2–EP2/EP4 signalling on the landscape of CD8+ TILs, we separated our scRNA-seq data on the basis of recipient mouse groups. Density analysis revealed a prominent shift towards early effector (clusters 3 and 4) and terminally differentiated TIL populations (cluster 5) in both Cd4crePtger2−/−Ptger4fl/fl mice and GzmbcrePtger2−/−Ptger4fl/fl mice compared with Ptger2−/−Ptger4fl/fl mice (Fig. 2f). We therefore quantified the frequencies TIL populations across all replicates. Ptger2−/−Ptger4fl/fl mice lacked expansion of any differentiating effector TIL populations (Fig. 2g). By contrast, in Cd4crePtger2−/−Ptger4fl/fl mice, we detected elevated frequencies of early and late effector TIL populations that further increased progressively along the common trajectory of effector differentiation (clusters 2–5; Fig. 2g). This pattern was similarly observed for GzmbcrePtger2−/−Ptger4fl/fl mice (Fig. 2g), in which TIL expansion was even more prominent, which probably reflects additional favourable activity of intratumoural GZMB+ natural killer cells15. Enhanced TIL expansion in Cd4crePtger2−/−Ptger4fl/fl mice and GzmbcrePtger2−/−Ptger4fl/fl mice correlated with the fact that TCF1+ and TIM-3+CXCR6+ TILs in both models had lost Ptger2 and Ptger4 expression (Extended Data Fig. 5a–d). Consistent with the notion that intratumoural effector differentiation causes the loss of EP4 in TCF1+ TILs in GzmbcrePtger2−/−Ptger4fl/fl mice, GzmbcrePtger2−/−Ptger4fl/f TCF1+CD8+ T cells generated in vitro displayed efficient Ptger4 ablation in an effector differentiation assay (Extended Data Fig. 5e,f). In line with enhanced TIL expansion, expression of a proliferation signature in effector TIL populations from Cd4crePtger2−/−Ptger4fl/fl mice and GzmbcrePtger2−/−Ptger4fl/fl mice was higher than from Ptger2−/−Ptger4fl/fl mice (Extended Data Fig. 5g). Consistently, EP2/EP4-deficient TCF1+ TILs and their TIM-3+ progeny displayed increased expression of the proliferation marker Ki-67 (Extended Data Fig. 5h). However, we did not detect a substantial gain in expression of a gene signature for cytotoxic effector function in these TIL populations (Extended Data Fig. 5i). Thus, PGE2 does not modify the expression of genes associated with T cell function but prevents their differentiation and expansion, which highlights a mechanistic difference to canonical factors that drive dysfunctional CD8+ T cell responses through transcriptional programming, such as TOX26,27 or MYB28. Taken together, these findings suggest that tumour-derived PGE2 locally impairs the differentiation and expansion of effector T cell populations arising from TCF1+ TILs. Moreover, EP2/EP4 deficiency rescues TILs from this inhibitory effect of PGE2.
PGE2–EP2/EP4 signalling limits clonal TIL expansion
Analyses of our scTCR-seq data revealed that many CD8+ TILs consisted of clonally expanded cells (Extended Data Fig. 6a–c), which is an indicator of tumour specificity and proliferative T cell expansion29,30. Notably, although expanded clonotypes were detectable in all TIL populations, they were more prominent among TIM-3+CXCR6+ cells (clusters 3–8) (Extended Data Fig. 6a–c), a result consistent with the notion that these differentiated TIL populations may arise locally through the proliferative expansion of a few tumour-specific TCF1+ TILs. Moreover, highly expanded TIL clones (>30) were completely absent from tumours in Ptger2−/−Ptger4fl/fl mice but were abundant in both Cd4crePtger2−/−Ptger4fl/fl mice (22.6%) and GzmbcrePtger2−/−Ptger4fl/fl mice (35.6%) (Fig. 2h,i and Extended Data Fig. 6d). Consistently, Ptger2−/−Ptger4fl/fl mice displayed an increased frequency of poorly expanded small or single clones (Fig. 2h,i and Extended Data Fig. 6d). Notably, TILs in both Cd4crePtger2−/−Ptger4fl/fl mice and GzmbcrePtger2−/−Ptger4fl/fl mice had much higher fractions of clones shared between TCF1+ cells and TIM-3+CXCR6+ effector progeny (9.1% and 14.5%, respectively) than Ptger2−/−Ptger4fl/fl mice (3.9%) (Extended Data Fig. 6e). The few shared clones detectable in Ptger2−/−Ptger4fl/fl mice, however, only showed poor expansion (Extended Data Fig. 6f). We conclude that tumour-derived PGE2 restricts clonal TIL expansion, which results in a collapse of the intratumoural CD8+ T cell response. This impairment is overcome by ablation of EP2/EP4 in CD8+ T cells, which leads to the productive differentiation and expansion of clonal effector T cell progeny within tumour tissue.
TCF1+ TIL effector expansion achieves tumour control
We next sought to provide further evidence that EP2/EP4 deficiency in CD8+ T cells permits productive effector differentiation of TILs in PGE2-producing tumours. Quantification of TIL populations across PGE2-deficient Ptgs1/Ptgs2−/− BRAFV600E tumours from WT mice and PGE2-producing control BRAFV600E from WT mice, Ptger2−/−Ptger4fl/fl mice and Cd4crePtger2−/−Ptger4fl/fl mice (Extended Data Fig. 7a–c) revealed that the numbers of TCF1+ TILs were comparable among all groups (Extended Data Fig. 7b). This result indicated that the generation of TCF1+CD8+ T cells in lymphoid tissues and their tumour infiltration is not affected by PGE2. However, whereas the numbers of differentiated TIM-3+ TILs were low in control BRAFV600E tumours in WT mice and Ptger2−/−Ptger4fl/fl mice, they were highly abundant in Cd4crePtger2−/−Ptger4fl/fl mice (Extended Data Fig. 7c) and indistinguishable from those found in Ptgs1/Ptgs2−/− BRAFV600E tumours in WT mice.
To determine whether TIM-3+ TILs were generated from TCF1+ TILs locally within tumour tissue, we made use of our finding that at early stages after implantation (day 6), tumours contain TCF1+ TILs but not (yet) differentiated TIM-3+ TILs (Fig. 2j–n). Tumour-bearing Cd4crePtger2−/−Ptger4fl/fl mice treated from day 6 onwards with the S1P1R antagonist FTY720, which prevents lymph node (LN) egress of newly primed CD8+ T cells31, showed unabated intratumoural development and prominent expansion of TIM-3+ TILs over time (Fig. 2k–m). By contrast, when initial tumour infiltration of TCF1+CD8+ T cells was blocked by FTY720 application from day 1 onwards, no intratumoural TIL expansion was detected (Fig. 2m). Notably, the proliferative response originating from TCF1+ TILs present in tumour tissue at day 6 was sufficient to achieve control of tumour growth (Fig. 2n). This result demonstrates that TCF1+ TILs locally generate potent anticancer effector responses when protected from inhibitory PGE2 signalling in tumours.
PGE2 suppresses IL-2 responsiveness of TILs
In an effort to identify the mechanisms downstream of PGE2–EP2/EP4 signalling that determine impaired TIL responses, we performed TF activity analysis of our scRNA-seq data. Deficiency of EP2/EP4 in TCF1+ TILs resulted in an increased activity of TFs associated with effector differentiation (including NFKB1, REL, JUN and TBX21), stimulatory cytokine signalling (STAT4, IRF1, NFKB1, JUN and TBX21) and survival (RUNX2 and TRP53) (Fig. 3a and Extended Data Fig. 8a,b). Most of these alterations were detectable in both TCF1+ TILs and their developing progeny (Extended Data Fig. 8c) and were highly consistent across Cd4crePtger2−/−Ptger4fl/fl mice and GzmbcrePtger2−/−Ptger4fl/fl mice (Fig. 3a and Extended Data Fig. 8c,d). Notably, we detected increased TF activity linked to IL-2 cytokine signalling (including STAT1, STAT3, STAT5B, ELK1 and NFATC2)32 in TILs from Cd4crePtger2−/−Ptger4fl/fl mice and GzmbcrePtger2−/−Ptger4fl/fl mice (Fig. 3a and Extended Data Fig. 8a,b). This result was again observed in both TCF1+ TILs and their TIM-3+CXCR6+ progeny (Extended Data Fig. 8c,d). PGE2 may therefore affect the response of TILs to IL-2, which is a notable finding given the current development of new classes of IL-2 receptor (IL-2R) agonists for cancer therapy and the emerging role of IL-2 signalling for productive anticancer responses by TCF1+ TILs33,34.
a, TF activity in TCF1+CD8+ TILs from control BRAFV600E tumours in Cd4crePtger2−/−Ptger4fl/fl mice and GzmbcrePtger2−/−Ptger4fl/fl mice (relative to Ptger2−/−Ptger4fl/fl mice). b, Effect of PGE2 on ex vivo expansion of TCF1+CD8+ TILs sorted from Ptgs1/Ptgs2−/− BRAFV600E tumours (n = 3). c,d, Effect of PGE2 on expansion (c) and proliferation (d) of repetitively activated TCF1+CD8+ T cells from in vitro T cell cultures (n = 4). e–h, Analysis of repetitively activated TCF1+CD8+ T cells by RNA-seq (n = 4). e, Experimental design. f, principal component (PC) analysis based on all DEGs. g, Volcano plot showing the effect of PGE2 exposure on gene expression in TCF1+CD8+ T cells stimulated with anti-CD3/CD28 and IL-2. h, GSEA of hallmark pathways based on g. *Pathways significantly regulated; NES, normalized enrichment score. i,j, Effect of PGE2 exposure on IL-2-dependent pSTAT5 induction in repetitively activated TCF1+CD8+ T cells. Cells were treated with 33 U ml–1 IL-2. j, n = 3. k, Expansion of repetitively activated TCF1+CD8+ T cells treated or untreated with PGE2 and stimulated as indicated (n = 3). l–n, WT or Cd4crePtger2−/−Ptger4fl/fl TCF1+CD8+ T cells from in vitro T cell cultures were incubated with or without PGE2 for 20 h before stimulation with IL-2 (l,m) or anti-CD3/CD28 and IL-2 (n). l, Flow cytometry plot showing pSTAT5 signalling after 30 min. Cells were treated with 33 U ml–1 IL-2. m, Quantification of pSTAT5 (n = 3). n, Quantification of T cell expansion (n = 3). Data in b and c are pooled from two independent experiments. Data in j, k, m and n are representative of two independent experiments. Plots in d, i and l show data for 1 T cell culture representative of n = 6 T cell cultures analysed in 2 independent experiments. For b, c, k and n, horizontal lines and error bars indicate the mean ± s.e.m. For j and m, box plots indicate the median. P values are from unpaired t-tests. In g, DEGs (P < 0.05; fold change ≥ 2) were identified by Wald test with multiple testing using the Benjamini–Hochberg method.
We therefore tested whether PGE2 controls the IL-2-mediated expansion of TCF1+ TILs sorted from PGE2-deficient Ptgs1/Ptgs2−/− tumours (identified as TIM-3−CXCR6− TILs; Extended Data Fig. 8e). PGE2 strongly compromised the capacity of TCF1+ TILs to expand and differentiate into effector cells when stimulated with high-dose IL-2 together with anti-CD3 and anti-CD28 (anti-CD3/CD28) treatment35,36 (Fig. 3b). Bypassing the scarcity of TIL numbers, we further addressed this issue using antigen-experienced, repetitively activated TCF1+CD8+ T cells generated in vitro, on which PGE2 had an identical inhibitory effect (Fig. 3c). In line with PGE2-mediated impairment of IL-2-driven proliferation and effector differentiation, PGE2-treated TCF1+CD8+ T cells from in vitro T cell cultures showed markedly reduced DNA replication early after their stimulation (Fig 3d). Transcriptional profiling by RNA-seq (Fig. 3e) revealed that PGE2 exposure resulted in distinct transcriptional changes in stimulated TCF1+CD8+ T cells and their unstimulated counterparts (Fig. 3f). Analyses of the stimulated T cell populations identified 294 differentially expressed genes (DEGs) following PGE2 exposure (Fig 3g). PGE2-treated TCF1+CD8+ T cell populations expressed increased levels of transcripts encoding for molecules related to EP2/EP4-mediated cAMP signalling (Crem and Fosl2) and T cell quiescence (for example, Phlpp1, Klf3 and Klf4) (Fig. 3g and Extended Data Fig. 8f). Gene set enrichment analysis (GSEA) showed a selective downregulation of the T cell differentiation-associated mTORC1 signalling pathway and the IL-2 signalling pathway (Fig. 3h). The latter result is consistent with the observed reduced IL-2 pathway activity in TILs identified in our scRNA-seq analysis and further supports the notion that PGE2 impairs the proliferative expansion of TILs through the inhibition of IL-2 signalling.
In line with an inhibitory effect of PGE2 on IL-2 signalling, IL-2 stimulation failed to promote STAT5 phosphorylation (pSTAT5) in PGE2-treated TCF1+CD8+ T cells (Fig. 3i). Notably, this defect was associated with reduced surface expression of the IL-2R gamma chain (IL-2Rγc) (Extended Data Fig. 8g) and could only partially be rescued by stimulation with high doses of IL-2 (Fig. 3j). This result points towards a dominant inhibitory effect of PGE2 on IL-2 signalling through the regulation of IL-2Rγc expression. Consistent with this notion, PGE2 impaired the expansion of TCF1+CD8+ T cells not only in response to IL-2 but also the γc cytokines IL-7 and IL-15 (Fig. 3k). Thus, PGE2 fundamentally affects the entire γc cytokine signalling pathway in TCF1+CD8+ T cells and their differentiating progeny. IL-2-mediated pSTAT5 (Fig. 3l,m) and IL-2-dependent T cell proliferation and expansion (Fig. 3n and Extended Data Fig. 8h,i) was rescued in TCF1+CD8+ T cells from Cd4crePtger2−/−Ptger4fl/fl mice, which demonstrates the functional relevance of EP2/EP4 signalling for the restriction of IL-2 signalling. Taken together, these data suggest that the PGE2–EP2/EP4 axis limits productive anticancer TIL responses by suppressing the IL-2 signalling pathway.
EP2/EP4-deficient TILs mediate cancer elimination
To examine antigen-specific TIL responses in more detail, we used WT (EP2/EP4-proficient) and Cd4crePtger2−/−Ptger4fl/fl (EP2/EP4-deficient) OT-I T cells. We co-transferred a small number (1 × 103 cells) of congenically marked WT and EP2/EP4-deficient OT-I T cells into recipient mice, which were subsequently challenged with MC38-OVA tumours (Fig. 4a). Consistent with the observation that PGE2 selectively inhibits CD8+ T cells within tumours, both WT and EP2/EP4-deficient OT-I T cells displayed prominent and unrestricted expansion in tdLNs (Fig. 4b,c). However, whereas the response by WT OT-I T cells collapsed after the initial phase of tumour infiltration (Fig. 4b,d), EP2/EP4-deficient OT-I T cells underwent persistent expansion in tumour tissue (Fig. 4b,d). Similarly, EP2-deficient OT-I T cells showed inefficient intratumoural expansion compared with EP2/EP4-deficient OT-I T cells (Extended Data Fig. 9a,b). Notably, EP2/EP4-deficient TCF1+ OT-I TILs over time gave rise to phenotypically distinct populations of TIM-3+CXCR6+ effector cells (Extended Data Fig. 9c,d). Re-transfer experiments (Extended Data Fig. 9e,f) confirmed that EP2/EP4-deficient TIM-3−(TCF1+) OT-I TILs but not their TIM-3+(TCF1−) descendants possessed the capacity to expand in tumours (Extended Data Fig. 9g,h) and were able to give rise to TIM-3+CXCR6+ TILs (Extended Data Fig. 9i). In separate experiments, we also observed that the development of TIM-3+ effector progeny from TCF1+ EP2/EP4-deficient OT-I T cells exclusively occurred in tumours but not in tdLNs (Fig. 4e,f and Extended Data Fig. 9j). Together, these data suggest that clonal T cell effector differentiation is restricted to tumour tissue and originates from TCF1+ TILs1,2,3. Consistent with this notion, and similar to our observations for polyclonal anticancer CD8+ T cell responses, FTY720-mediated blockade of T cell egress from LNs from day 6 onwards had no impact on the local expansion of EP2/EP4-deficient OT-I TILs (Extended Data Fig. 9k,l). We conclude that tumour-specific TCF1+ TILs expand and give rise to effector progeny within tumours, and this pivotal phase of the anticancer CD8+ T cell responses is blunted by PGE2.
a, Experimental design for b–f. b, Flow cytometric plots of CD8+ T cells from tdLNs and tumours from the indicated days. c,d, Numbers of expanded OT-I CD8+ T cells in tdLNs (c) and tumours (d) at indicated time points (n = 6). e,f, Analysis of CD44 and CXCR6 expression in Cd4crePtger2−/−Ptger4fl/fl OT-I cells. e, Flow cytometry plots. f, Subset frequencies (n = 6). g–j, Effect of CD122/CD132 blockade on OT-I T cell expansion in tumours. g–j, Effect of anti-CD122 and anti-CD132 (anti-CD122/CD132) treatment on OT-I TIL expansion in WT mice with control or Ptgs1/Ptgs2−/− BRAFV600E-OVA tumours or with MC38-OVA tumours, analysed 11 days after tumour transplantation. g,h, Flow cytometry plots (g) and OT-I TIL numbers (h) in BRAFV600E-OVA tumours (n = 6). i,j, Flow cytometry plots (i) and OT-I TIL numbers (j) in MC38-OVA tumours (n = 10). k, Experimental design for l and m with MC38-OVA tumours. l, Flow cytometry plot (left) and quantification (right) of OT-I TILs at day 10 (n = 6). m, Flow cytometry plots showing the population size of TIM-3+CXCR6+ cells among control and Cd122−/− Cd4crePtger2−/−Ptger4fl/fl OT-I TILs. n, WT mice received 1 × 103 naive OT-I T cells or 1 × 103 naive Cd4crePtger2−/−Ptger4fl/fl OT-I T cells intravenously (i.v.) and were transplanted s.c. with 2 × 106 MC38-OVA cells before analysis of tumour growth over time (n = 10). Asterisk indicates that termination criteria were reached. Data in c, d, f, h, j, l and n are pooled from two (c,d,h,l) or three (f,j,n) independent experiments and depicted as box plots extending from the 25th to 75th percentiles with the median as the centre and the whiskers corresponding to minimum and maximum values (c,d,h,j,l) or shown as the mean ± s.e.m. (f,n). Plots in b, e, g, i, l and m show data for 1 sample representative of n = 6 samples analysed in 2 (b,g,l,m) or 3 (e,i) independent experiments. P values are from paired t-tests (l), one-way ANOVA with Tukey’s multiple-comparison test (c,d) or Dunnett’s multiple-comparison test (h,j), or two-way ANOVA with Bonferroni’s correction for multiple testing (n).
In vivo blockade of IL-2R signalling using blocking antibodies against the IL-2Rβ (also known as CD122) and IL-2Rγc (also known as CD132) chains abrogated the expansion advantage of OT-I TILs in PGE2-deficient Ptgs1/Ptgs2−/− BRAFV600E-OVA tumours (Fig. 4g,h) and that of EP2/EP4-deficient OT-I TILs in PGE2-producing MC38-OVA tumours (Fig. 4i,j). Similar results were observed after T cell-specific ablation of IL-2Rβ expression (Fig. 4k), which resulted in markedly reduced expansion (Fig. 4l) and effector differentiation (Fig. 4m) of EP2/EP4-deficient OT-I TILs compared with mock-treated control EP2/EP4-deficient OT-I TILs. Therefore, the IL-2R signalling pathway drives the expansion and effector differentiation of antigen-specific CD8+ TILs in the absence of PGE2–EP2/EP4 signalling.
Finally, to specifically evaluate whether EP2/EP4-deficient antigen-specific CD8+ T cells mount protective anticancer responses, we analysed the growth of MC38-OVA tumours transplanted into WT mice with or without transfer of WT or EP2/EP4-deficient OT-I T cells. EP2/EP4-deficient OT-I T cells achieved complete rejection of MC38-OVA tumours, whereas WT OT-I T cells failed to affect progressive MC38-OVA tumour growth (Fig. 4n). Of note, EP2/EP4-deficient OT-I but not WT OT-I TILs also showed enhanced expansion that led to efficient tumour elimination in mouse melanoma D4M.3A-pOVA tumours (Extended Data Fig. 9m,n). Taken together, these results suggest that interfering with the PGE2–EP2/EP4 axis in cancer-specific CD8+ T cells can elicit their expansion and effector differentiation within tumours and result in protective T cell-mediated anticancer immunity.
Discussion
Our results demonstrate that tumour-derived PGE2 acts locally within the tumour microenvironment to limit CD8+ TIL expansion and effector differentiation originating from TCF1+ stem-like TILs. This inhibitory mechanism is crucial for cancer immune escape. We reveal that PGE2-mediated restriction of TIL responses generated from TCF1+ TILs depends on TIL-intrinsic signalling of the PGE2-receptors EP2 and EP4, which causes downregulation of functional IL-2 receptors and curtails TIL responsiveness to IL-2. As a result, interference with PGE2–EP2/EP4 signalling in CD8+ T cells enhances their IL-2 responsiveness and induces protective TIL-mediated anticancer immunity. Of note, the effect of PGE2 on TIL expansion and effector differentiation may at least in part be linked to a defect in IL-2-dependent mTORC1 signalling, as also suggested by an accompanying paper37.
Beyond highlighting that clonal expansion and effector differentiation of stem-like TCF1+CD8+ T cells occurs within tumour tissue, as recently suggested1,2,3, our results reveal that this critical phase of protective anticancer immunity is selectively targeted by tumour-derived PGE2. These findings therefore identify an intratumoural checkpoint that locally controls expansion and effector differentiation of cancer-specific CD8+ TILs. Of note, this mechanism may act in parallel to PGE2-mediated inhibition of cDC1 (ref. 38), which can support TCF1+ TIL responses within the tumour microenvironment39.
Our unbiased transcriptional profiling by scRNA-seq uncovered that protective anticancer responses by EP2/EP4-deficient TILs are coupled to a rescue of IL-2 signalling. Recent studies have highlighted the relevance of IL-2 signalling for the generation of effective CD8+ T cell responses from antigen-specific TCF1+CD8+ T cells6,34,40,41. Therefore, the discovery that the PGE2–EP2/EP4 axis antagonizes the responsiveness of TCF1+ TILs to IL-2 has important mechanistic and clinical implications. Our results provide evidence that PGE2 limits the proliferative capacity (and hence likely the self-renewal) of TCF1+ stem-like TILs and at the same time curbs effector T cell generation along the entire pathway of intratumoural TIL differentiation. Importantly, ablation of PGE2 signalling and consequently reconstitution of IL-2 signalling sufficed to achieve clonal TIL expansion and their effector differentiation within tumours that was not accompanied while preserving TCF1+ stem-like TILs. This is fundamentally different to interfering with exhaustion-inducing transcription factors such as TOX or MYB, which comes at the expense of TCF1+ stem-like CD8+ T cells and leads to a substantial change towards the development of terminally differentiated dysfunctional T cells26,27,28. Moreover, our finding that abrogating PGE2 signalling in T cells enhances clonal expansion across the entire differentiation spectrum of anticancer TILs indicates that physiological IL-2 concentrations within tumours are sufficient to drive protective anticancer immunity if IL-2 signalling in TILs is restored.
Targeting EP2 and EP4 on anticancer T cells to overcome PGE2-induced curtailing of IL-2 responsiveness might be preferential over using high concentrations of IL-2, as the latter may lead to deleterious off-target effects of IL-2 on IL-2R-expressing lung endothelial cells or CD4+ regulatory T cells42. On this note, ablation of the PGE2–EP2/EP4 signalling axis to enhance IL-2 responsiveness in adoptively transferred cancer-specific CD8+ T cells bears the promise to unleash their full potential to mount protective anticancer immunity not only in mice but also in cancer patient-derived TILs, as demonstrated in the accompanying paper37. Given the association of increased COX-mediated PGE2-production in tumours with cancer growth and poor survival rates in patients with cancer, our findings therefore identify the PGE2–EP2/EP4 signalling axis in TILs as molecular target to improve T cell immune therapy in cancer patients with PGE2-producing tumours. This strategy may further be beneficial in tumours that produce high levels of other EP2/EP4-engaging prostanoids such as PGF2α, PGD2 and PGI2 (ref. 43).
Methods
Mice
All mice used in this study were on a C57BL/6J genetic background and purchased from the Jackson Laboratory (JAX). OT-I × CD45.1 mice were generated by crossing OT-I mice (JAX, 003831) to CD45.1 (JAX, 002014) mice. Ptger2−/−Ptger4fl/fl mice were generated by crossing Ptger2−/− mice (JAX, 004376) to Ptger4fl/fl mice (JAX, 028102) and further crossed to Cd4cre mice (JAX, 022071) to generate Cd4crePtger2−/−Ptger4fl/fl mice or crossed to Gzmbcre mice (JAX, 003734) to generate GzmbcrePtger2−/−Ptger4fl/fl mice. Unless stated otherwise, mice were on a CD45.2/CD45.2 background. For some experiments, Cd4crePtger2−/−Ptger4fl/fl mice and Ptger2−/−Ptger4fl/fl mice were crossed to OT-I mice to generate Cd4crePtger2−/−Ptger4fl/fl OT-I mice and Ptger2−/−Ptger4fl/fl OT-I mice and used on a CD45.1/CD45.2 or CD45.1/CD45.1 background. WT or Rag1−/− mice (JAX, 002216) on a CD45.2/CD45.2 background were used as recipients in adoptive transfer experiments. In all experiments, mice at 6–12 weeks of age were sex-matched and randomly assigned to control or treatment groups. Mouse experiments with Ptgs1/Ptgs2−/− BRAFV600E tumours and T cell depletion were conducted without blinding; all other experiments were performed in a blinded manner. No statistical methods were used to predetermine sample sizes. Mice were killed by cervical dislocation under anaesthesia. All mice were maintained and bred at the Klinikum rechts der Isar, TUM, or at the Klinikum der Universität München, LMU, under specific-pathogen-free, controlled conditions with a 12-h light–dark cycle, ambient temperature of 24 °C and humidity maintained at 55%, and in accordance with the guidelines of the Federation of European Laboratory Animal Science Associations. All animal experiments were performed in accordance with the guidelines of the district government of upper Bavaria (Department 5–Environment, Health and Consumer Protection).
Cell lines
Control and Ptgs1/Ptgs2−/− BRAFV600E melanoma cells were generated using the CRISPR–Cas9 system as previously described14. BRAFV600E-OVA and Ptgs1/Ptgs2−/− BRAFV600E-OVA cells were generated by lentiviral transduction. In brief, OVA cDNA was subcloned into a pHIV-7 transfer vector carrying both the phosphoglycerate kinase (PGK) promoter and IRES-puromycin-resistance sequence. The production of third-generation self-inactivating lentiviral vectors, pseudotyped with VSV.G, was carried out as previously described44. Specifically, packaging cells were transfected and, after 2 days, cell supernatants were collected, filtered and used to transduce tumour cell lines in the presence of 8 µg ml–1 polybrene (Merck). After the incubation period, medium was exchanged for fresh medium, and target cells were passaged at least three times after transduction and selected using puromycin. MC38 cells were provided by A. Krüger, Institute of Experimental Oncology, TUM, and MC38-OVA and Panc02 cells were provided by V. Buchholz, Institute for Medical Microbiology, Immunology and Hygiene, TUM.
BRAFV600E, Ptgs1/Ptgs2−/− BRAFV600E, BRAFV600E-OVA and Ptgs1/Ptgs2−/− BRAFV600E-OVA cells were cultured in complete RPMI medium (RPMI 1640 medium (Thermo Fisher Scientific) supplemented with 10% FCS (Merck), 50 µM β-mercaptoethanol (Thermo Fisher Scientific), 50 U ml–1 penicillin (Thermo Fisher Scientific), 50 µg ml–1 streptomycin (Thermo Fisher Scientific) and 2 mM l-glutamine (Thermo Fisher Scientific). D4M.3A-pOVA cells were generated as previously described45 and cultured in DMEM-F12 medium (Thermo Fisher Scientific). MC38, MC38-OVA and Panc02 cells were cultured in DMEM (Thermo Fisher Scientific), with both media supplemented with 10% FCS, 50 µM β-mercaptoethanol, 50 U ml–1 penicillin, 50 mg ml–1 streptomycin, 2 mM l-glutamine, 1× MEM non-essential amino acids solution (Thermo Fisher Scientific) and 1 mM sodium pyruvate (Thermo Fisher Scientific). To generate tumour cell conditioned medium (CM), 5 × 106 tumour cells were cultured in 20 ml complete RPMI medium for 48 h and the supernatant was collected, filtered and stored at −20 °C until further use. All cell lines were routinely tested for mycoplasma contamination in-house by PCR. For Ptgs1/Ptgs2−/− BRAFV600E cells, the absence of PGE2 production was routinely confirmed by PGE2 ELISA (Cayman Chemical). No further cell line authentications were conducted in the laboratory.
Tumour cell injections
Tumour cell lines were detached by trypsinization (Thermo Fisher Scientific) and washed three times in sterile PBS (Thermo Fisher Scientific). Unless stated otherwise, 2 × 106 cells were injected s.c. in 100 µl sterile PBS into the flank of each recipient mouse. Tumour growth was measured using a digital calliper. Tumour diameters stated in the figures refer to the average values of the longest diameter and its perpendicular for each tumour. A maximal tumour diameter of 15 mm served as the humane end point and was not exceeded in any of the experiments.
CD4+ and CD8+ T cell depletion in vivo
To deplete CD4+ and CD8+ T cells, mice received intraperitoneal (i.p.) injections of 100 µl anti-mouse CD4 (100 µg per mouse, GK1.5, BioXCell) or anti-mouse CD8β (100 µg per mouse, 53-5.8, BioXCell) antibodies every 5 days, beginning on day 1 following tumour cell inoculation.
FTY720 treatment in vivo
FTY720 treatment was performed by injecting mice i.p. with 100 µl of FTY720 (20 µg per mouse, Merck) on day 1 or day 6 after tumour cell transplantation. Injection with 100 µl sterile isotonic NaCl served as control.
IL-2 receptor blockade in vivo
For blockade of IL-2Rβ and IL-2Rγc, mice received i.p. injections of 150 µl anti-mouse CD122 (300 µg per mouse, TM-Beta 1, BioXCell) and anti-mouse CD132 (300 µg per mouse, 3E12, BioXCell) antibodies on days 6 and 7 after tumour cell transplantation. Injections with 150 µl sterile isotonic NaCl served as control.
Processing of tumour tissue and lymphoid organs
Tumours, tdLNs or spleens of tumour-bearing mice were excised at the indicated time points after cell transplantation. Tumour or organ weight was determined using a microscale. For subsequent analyses by flow cytometry or cell sorting, tumour samples were mechanically dissociated and incubated with collagenase IV (200 U ml–1, Thermo Fisher Scientific) and DNase I (100 µg ml–1, Merck) for 40 min at 37 °C and filtered through a 70 µm and a 30 µm cell strainer (Miltenyi) to generate single-cell suspensions. Spleens were passed through a 70 µm cell strainer, followed by red blood cell lysis and a second filtration step using a 30 µm cell strainer. LNs were passed through a 30 µm cell strainer. For the isolation of migratory cDC1s, LNs were processed as described for tumour samples.
Antibodies and reagents for flow cytometry and cell sorting
The following antibodies and staining reagents were used for flow cytometry or cell sorting: fixable viability dye eFluor 450 (dilution: 1:500; Thermo Fisher Scientific); fixable viability dye APC-eF780 (1:1,000; Thermo Fisher Scientific); viability dye SYTOX-blue (1:2,000; Thermo Fisher Scientific); APC anti-CD3 (1:100; clone 17A2, Thermo Fisher Scientific); PE anti-CD4 (1:200; GK1.5, Biolegend); AF647 anti-CD4 (1:200; GK1.5, Biolegend); PerCP/Cy5.5 anti-CD4 (1:200; GK1.5, Biolegend); BV421 anti-CD8α (1:200; 53-6.7, Biolegend); FITC anti-CD8α (1:200; 53-6.7, Biolegend); PE-Dazzle594 anti-CD8α (1:200; 53-6.7, Biolegend); PE-Cy7 anti-CD8α (1:200; 53-6.7, Biolegend); BV605 anti-CD11b (1:200; M1/70, Biolegend); PE-Cy7 anti-CD11c (1:200; N418, Biolegend); BV570 anti-mouse/human-CD44 (1:100; IM7, Biolegend); BV711 anti-mouse/human-CD44 (1:100; IM7, Biolegend); FITC anti-mouse/human-CD44 (1:100; IM7, Biolegend); PerCP-Cy5.5 anti-mouse/human-CD44 (1:100; IM7, Biolegend); AF647 anti-CD45.1 (1:100; A20, Biolegend); PE anti-CD45.1 (1:100; A20, Biolegend); PE-Dazzle594 anti-CD45.1 (1:100; A20, Biolegend); PerCP/Cy5.5 anti-CD45.1 (1:100; A20, Biolegend); BV510 anti-CD45.2 (1:100; 104, Biolegend); FITC anti-CD45.2 (1:100; 104, Biolegend); PerCP-Cy5.5 anti-CD45.2 (1:100; 104, Biolegend); FITC anti-CD62L (1:100; MEL-14, Biolegend); PE-Dazzle594 anti-CD62L (1:100; MEL-14, Biolegend); FITC anti-CD103 (1:100; M290, BD Biosciences); APC anti-CD132/IL2Rγc (1:100; TUGm2, Biolegend); PE-Dazzle594 anti-CD186/CXCR6 (1:200; SA051D1, Biolegend); PE anti-CX3CR1 (1:100; SA011F11, Biolegend); BV605 anti-CD279/PD-1 (1:100; 29 F.1A12, Biolegend); BV421 anti-CD366/TIM-3 (1:200; RMT3-23, Biolegend); PerCP/Cy5.5 anti-TCRβ (1:100; H57-597, Biolegend); AF700 anti-I-A/I-E (1:500; MHC class II) (M5/114.15.2, Biolegend); PE anti-H-2Kb bound to SIINFEKL (1:100; 25-D1.16, Biolegend); APC anti-human GZMB (1:200; GB12, Thermo Fisher Scientific); FITC anti-Ki-67 (1:100; SolA-15, Thermo Fisher Scientific); AF700 anti-Ki-67 (1:100; SolA-15, Thermo Fisher Scientific); PE anti-TCF1/TCF7 (1:40; S33-966, BD Biosciences); AF488 anti-human pSTAT5 (0.03 µg per test, 47/Stat5(pY694); BD Biosciences); eF660 anti-TOX (1:100; TXRX10, Thermo Fisher Scientific); eFluor660 Rat-IgG2a-κ isotype-control (1:100; eBR2a, Thermo Fisher Scientific); APC mouse-IgG1κ isotype-control (1:200; P3.6.2.8.1, Thermo Fisher Scientific); AF488 mouse-IgG1κ isotype-control (0.03 µg per test; MOPC-21, Biolegend); and rabbit-anti-mouse-TCF1/TCF7 (1:100; C.725.7, Thermo Fisher Scientific). These were followed by AF647 donkey-anti-rabbit IgG (1:200; Poly4064, Biolegend) or DL488 donkey-anti-rabbit IgG (1:200; Poly4064, Biolegend). Unless stated otherwise, all antibodies were anti-mouse antibodies.
Flow cytometry and cell sorting
For staining of surface markers and viability dyes, cells were stained for 15 min at 4 °C in FACS buffer (PBS with 1% FCS and 2 mM EDTA). Staining of SIINFEKL–MHC class I complexes on cDC1s for analysis of OVA cross-presentation was performed for 40 min. For intracellular staining of GZMB, TCF1, Ki-67 and TOX, cells were fixed and permeabilized using the True-Nuclear Transcription Factor Buffer Set (Biolegend) according to the manufacturer’s protocol. Intracellular staining was performed overnight in permeabilization buffer at 4 °C. For intracellular staining of pSTAT5, cells were fixed and permeabilized using BD Cytofix (BD Biosciences) and BD Phosflow Perm Buffer III (BD Biosciences) according to the manufacturer’s instructions (protocols II and III, BD Biosciences). For the detection of EdU incorporation, EdU was added to the culture at a final concentration of 15 µM for the last 3 h of the experiment, and analysis was performed using an EdU Proliferation kit (iFluor 488, Abcam) according to the manufacturer’s protocol.
Flow cytometry analyses were performed using a LSR Fortessa Cell Analyzer (BD Biosciences, BD FACSDiva software v.8.0.1 and v.9.0.1), a SP6800 Spectral Cell Analyzer (Sony Biotechnologies, spectral analyser software v.2.0.2.14140) or a SA3800 Spectral Cell Analyzer (Sony Biotechnologies, spectral analyser software v.2.0.5.54250). For flow cytometric quantification of cell numbers, CountBright Absolute Counting Beads (Thermo Fisher Scientific) were added to samples before analyses. For some experiments, CD8+ TILs (live CD45+CD3+CD8+ cells), stem-like Cd4crePtger2−/−Ptger4fl/fl OT-I TILs (live CD45.1+CD8+CD44+TIM-3−CXCR6−) or differentiated effector Cd4crePtger2−/−Ptger4fl/fl OT-I TILs (live CD45.1+CD8+CD44+TIM-3+CXCR6+) were sorted using a FACS Aria III Cell Sorter (BD Biosciences, BD FACSDiva software v.9.0.1). Naive OT-I T cells (CD45.1+CD8+CD62L+CD44–) used in adoptive transfer experiments were sorted from blood using a SH800S Cell Sorter (Sony Biotechnologies, cell sorter software v.2.1.6). All flow cytometric data were analysed using FlowJo (BD Biosciences, v.00.8.1 and v.10.8.2).
Adoptive T cell transfer
For adoptive T cell transfer of naive T cells, 1 × 103 congenically marked naive CD8+ T cells from OT-I, Ptger2−/−Ptger4fl/fl OT-I or Cd4crePtger2−/−Ptger4fl/fl OT-I donor mice were injected i.v. in sterile PBS into sex-matched recipient WT mice 6 h before tumour cell transplantation s.c. For adoptive transfer of CRISPR-Cas9-edited T cells, 1 × 103 cells congenically marked OT-I T cells from in vitro T cell cultures were injected i.v. into recipient mice at day 2 after tumour cell transplantation s.c. For re-transfer of CD8+ TILs, 7 × 103 congenically marked stem-like (TIM-3–CXCR6–) or differentiated effector (TIM-3+CXCR6+) Cd4crePtger2−/−Ptger4fl/fl OT-I TILs were sorted from MC38-OVA tumours from WT mice and injected i.v. in sterile PBS into sex-matched recipient Rag1−/− mice inoculated with MC38-OVA tumour cells 2 days before T cell re-transfer.
Generation of repetitively activated antigen-experienced TCF1+CD8+ T cells
TCF1+CD8+ T cells were differentiated from splenic naive CD8+ T cells by repetitive activation as previously described35, with minor modifications. In brief, 1 × 106 naive CD8+ T cells were seeded in complete RPMI medium supplemented with 1× MEM non-essential amino acids solution and 1 mM sodium pyruvate. Low-dose IL-2 (85 U ml–1) and mouse anti-CD3/CD28 microbeads were added to the culture while maintaining a CD8+ T cell concentration of 1 × 106 cells per ml for multiple (re-)activation cycles over a course of 4 days, followed by purification of live cells by gradient centrifugation (Pancoll).
T cell effector differentiation
Effector differentiation of TCF1+CD8+ T cells was performed as previously described35, with minor modifications. In brief, cells were cultured with mouse anti-CD3/CD28 microbeads in the presence of high-dose IL-2 (350 U ml–1). Where indicated, PGE2 (100 ng ml–1, unless indicated otherwise in the figure legend; Thermo Fisher Scientific), tumour cell CM, IL-7 (10 ng ml–1, Miltenyi), IL-12 (10 ng ml–1, Biolegend) or IL-15/15Rα (1 ng ml–1, Thermo Fisher Scientific) was added to the culture. To assess T cell expansion, the numbers of live CD45+CD3+CD8+ T cells were quantified by flow cytometry 72 h after the incubation period.
Gene deletion by CRISPR–Cas9–gRNA complex electroporation
Cd4crePtger2−/−Ptger4fl/fl OT-I T cells were purified from spleen and cultured in complete RPMI supplemented with IL-2 (10 U ml–1) and IL-7 (5 ng ml–1) in the presence of mouse anti-CD3/CD28 microbeads. After 24 h, anti-CD3/CD28 microbeads were removed by magnetic separation and cells were electroporated (4D-Nucleofector, Lonza; pulse program CM137)46 in P3 electroporation buffer supplemented with the Cas9 electroporation enhancer (IDT), Cas9 protein (IDT) and Cd122-targeting or non-targeting gRNAs. gRNAs were generated by hybridizing trRNA (IDT) with Cd122-targeting (sequences TATGTCAAGGAGGTCCACGG and CTGGGAACGACCCGAGGATC, generated using CHOPCHOP; ref. 47) or non-targeting crRNA (IDT) (GCCTGCCCTAAACCCCGGAA; ref. 48) as mock control. Cells were rested in complete RPMI supplemented with IL-7 (5 ng ml–1, Miltenyi) at 37 °C for 48 h and validated for specific knockout by CD122 surface staining before injection into recipient mice.
Analysis of IL-2Rγc expression and IL-2 signalling
TCF1+CD8+ T cells from in vitro cultures were rested for 20 h in complete RPMI supplemented with low-dose IL-2 and purified by gradient centrifugation. Cells were stimulated with mouse anti-CD3/CD28 microbeads and low-dose IL-2 for 24 h in the absence or presence of PGE2 (100 ng ml–1). After 24 h, IL-2Rγc chain expression was analysed by flow cytometry. For analysis of IL-2-induced STAT5 signalling, anti-CD3/CD28 microbeads were removed by magnetic separation, cells were rested for 30 min at 37 °C in complete RPMI and stimulated for 30 min with different concentrations of IL-2 (10–100 U ml–1, as indicated). After the incubation period, fixation buffer was directly added to the culture to terminate the signalling process and cells were stained for flow cytometry analysis.
PGE2 measurements
Tumours and organs of tumour-bearing mice were excised 11 days after tumour cell transplantation, directly frozen in liquid nitrogen and stored at −80 °C until further processing. Samples were homogenized in homogenization buffer (0.1 M PBS, 1 mM EDTA and 10 µM indomethacin (Merck), pH 7.4) using a gentleMACS Dissociator (Miltenyi) followed by a freeze–thaw cycle. PGE2 concentrations were measured by ELISA (Cayman Chemical) according to the manufacturer’s protocol.
RNA isolation and quantitative real-time PCR
RNA was isolated using an Arcturus PicoPure RNA isolation kit (Thermo Fisher Scientific) and cDNA was generated using a SensiFAST cDNA synthesis kit (Bioline). Quantitative real-time PCR was carried out on a LightCycler 480 (Roche, LightCycler 480 software v.1.5.1) using a TAKYON No ROX SYBR MasterMix dTTP Blue kit (Eurogentec) according to the manufacturer’s protocol. Ptger4 expression was determined using the ΔCt method, with Hprt serving as reference gene. Primer sequences were from a previous study38. All primers were purchased from Eurofins.
scRNA-seq and scTCR-seq
CD8+ TILs were sorted from BRAFV600E tumours 11 days after tumour cell transplantation. A combination of cell hashing and DNA barcoding during library preparation was used for sample multiplexing, which enabled the simultaneous sequencing of four biological replicates from each group. For cell hashing, unique TotalSeq-C anti-mouse hashtag antibodies were used for hashing of cells from each experimental group as follows: WT: hashtag 1; Ptger2−/−Ptger4fl/fl: hashtag 2; Cd4crePtger2−/−Ptger4fl/fl: hashtag 3; and GzmbcrePtger2−/−Ptger4fl/fl: hashtag 4 (1:250 each, Biolegend). Hashtagged cells from one tumour-bearing mouse of each group were pooled and loaded on a Chromium Next GEM Chip (10x Genomics). RNA-seq libraries were generated using Chromium Next GEM Single Cell 5′ Reagent kits v.2 User Guide with Feature Barcode technology for Cell Surface Protein (Rev D) according to the manufacturer’s protocol (10x Genomics). Quality control was carried out using a High Sensitivity DNA kit (Agilent), a Bioanalyzer 2100 and a Qubit dsDNA HS Assay kit (Thermo Fisher Scientific). For sequencing, libraries were pooled and analysed by paired-end sequencing (2 × 150 bp) on a NovaSeq6000 platform using S4 v.1.5 (300 cycles) sequencing kits (Illumina). Libraries were sequenced to a depth of at least 2 × 104 reads per cell for gene expression libraries and 5 × 103 reads per cell for T cell receptor libraries.
Initial scRNA-seq analyses were performed for all samples from the groups Ptger2−/−Ptger4fl/fl, Cd4crePtger2−/−Ptger4fl/fl and GzmbcrePtger2−/−Ptger4fl/fl, with data from the WT group being added at a later stage for validation of Ptger2 and Ptger4 read coverage (see below). Alignment of gene expression libraries and demultiplexing were performed using cellranger multi (Cell Ranger (v.6.1.1)49; 10x Genomics) against the pre-built mouse reference v2020-A (10x Genomics, mm10/GRCm38, annotation from GENCODE Release M23) with the number of expected cells equals 21.000 as input argument. The BAM files were converted to FASTQ files using the tool bamtofastq with the argument --reads-per-fastq set to the total number of reads in the BAM file plus 10,000. After that, gene expression and TCR analysis were combined by running cellranger multi separately for each demultiplexed sample, disabling library concordance reinforcement. The algorithm was forced to find the number of cells identified in the first step of demultiplexing, and sample-specific FASTQ files were used as input for the gene expression analysis pipeline. The pre-built Ensembl GRCm38 Mouse V(D)J Reference v.5.0.0 was used for TCR analysis.
The initial downstream analysis was performed in R (v.4.0.4) with the R package Seurat (v.4.0.1)50. Only cells with more than 1,000 genes detected, less than 10% of mitochondrial genes and with UMI counts less than 3 standard deviations above the mean were kept. The data were filtered for genes detected in at least three cells in one of the samples. Filtered read counts from each sample were normalized independently using sctransform (v.0.3.2)51 with the glmGamPoi method52. Anchors between cells from different replicates were identified on the top 1,000 highly variable genes using canonical correlation analysis and 30 canonical vectors. Data integration was performed on first 20 PC analysis (PCA) dimensions. PCA was calculated for the integrated data on the top 1,000 highly variable genes and both k-nearest neighbour graph and UMAP were computed on the 30 nearest neighbours and first 20 PCA dimensions. Louvain clusters were identified using the shared nearest neighbour modularity optimization-based algorithm at resolutions 0.9, 0.65 and 0.9 for the groups Ptger2−/−Ptger4fl/fl, Cd4crePtger2−/−Ptger4fl/fl and GzmbcrePtger2−/−Ptger4fl/fl, respectively. Contaminating myeloid cells were identified based on the average cluster expression of the marker genes Cd14, Lyz2, Fcgr3, Ms4a7, Fcer1g, Cst3, H2-Aa, Ly6d, Ms4a1 and Ly6d. Cycling cells were identified based on expression of Cdk1, Mcm2, Pclaf, H2afz, Birc5 and Mki67.
The integrative analysis between groups was performed in R (v.4.2.1) with the R package Seurat (v.4.1.1)50. After general data pre-processing and regression of contaminating cells as mentioned above, filtered read counts from each sample were normalized independently using sctransform (v.0.3.2)51 with glmGamPoi method52. Anchors between cells from all groups and all their replicates were identified using a more conservative approach, which led to weaker batch correction. For that purpose, reciprocal PCA was applied on the top 1,000 highly variable genes of each sample and anchors were picked using the first 20 dimensions and 1 neighbour only. PCA was performed on the integrated data on the top 1,000 highly variable genes. A k-nearest neighbour graph and UMAP (spread of 0.4, minimum distance of 0.01) were computed on the first 20 PCs and 30 nearest neighbours. A resolution of 0.6 was used for Louvain clusters identification using the shared nearest neighbour modularity optimization-based algorithm. DEGs between two groups were identified using the Wilcoxon rank-sum test and Bonferroni correction. Gene set expression scores at single-cell level were calculated using the AddModuleScore function, including only the detected genes. Similarity scores with reference datasets were calculated using the R package SingleR (v.1.10.0)53 with the top 200 DEGs. The processed transcriptome profiles of naive CD8+ T cells, memory stem cell CD8+ T cells and central memory CD8+ T cells were from a previous study54. For tumour antigen-specific CD8+ T cells in tdLNs, tumour-infiltrating stem-like CD8+ T cells and their naive counterparts, data from a previous study3 were processed using the R package DESeq2 (v.1.36)55. Gene set expression scores at the single-cell level were calculated using the AddModuleScore function, including only the detected genes. The effector T cell gene signature was from a previous study56 (M3013: KAECH_NAIVE_VS_DAY8_EFF_CD8_TCELL_DN). The CD8+ T cell proliferation signature was obtained from MSigDB (GO:2000566). Transcriptional trajectories were inferred using the R package slingshot (v.2.4.0)57 over the UMAP calculated on the integrated data, approximating the curves by 150 points. The pseudotime was calculated as a weighted average across lineages, weighted by the assignment weight.
TCR analysis of clonotype was performed using the R package scRepertoire (v.1.6.0)58. Clonotypes were called based on a combination of VDJC genes comprising the TCR and the nucleotide sequence of the CDR3 region. Whenever the clonotype distribution is shown for individual groups, the cell number was downsampled, so that cluster 1 from all groups had the same maximum size. TF activity was inferred using the weighted mean method of decoupleR (v.2.2.2)59 and TF–target interactions available through dorothea (v.1.8.0)60, with confidence levels A to C. Normalization to Ptger2−/−Ptger4fl/fl was achieved by subtracting its scores from the scores of the other groups. The top 100 variable TFs between clusters within each group were used to draw a network graph with tidygraph (v.1.2.1)61 based on common targets with same defined mode of regulation as defined on the database. Only TFs with at least two common targets were kept for visualization. Louvain clusters were identified using igraph (v.1.3.2)62 at a resolution of 0.5.
For addition of scRNA-seq data from the WT group, samples were pre-processed as described above and mapped to a reference formed by the integrated data of the Ptger2−/−Ptger4fl/fl, Cd4crePtger2−/−Ptger4fl/fl and GzmbcrePtger2−/−Ptger4fl/fl groups using the R package Seurat (v.4.1.1)50. For that purpose, anchors between cells from the reference and the WT groups along with all replicates were identified using reciprocal PCA on top 1,000 highly variable genes. Anchors were picked using the first 20 dimensions and 1 neighbour only. Annotations were transferred using the function TransferData, and data were integrated using IntegrateEmbeddings. Cells from the added group were then projected onto the coordinates of the reference UMAP calling ProjectUMAP with 30 nearest neighbours. Read coverage was estimated using deepTools (v.3.5.4)63 with bamCoverage and a bin size of 10 bp and normalization by bins per million mapped reads. For coverage analysis on Tcf7/TCF1+ and Tcf7/TCF1− clusters, BAM files were split by cell barcodes from clusters 1–2 or clusters 3–8 using samtools (v.1.13)64 before coverage estimation. Read coverage on gene tracks was visualized using the R package trackViewer (v.1.32.1)65.
RNA-seq
In vitro generated, repetitively activated TCF1+CD8+ T cells were incubated in the presence or absence of PGE2 (100 ng ml–1) for 1 h at 37 °C followed by stimulation with IL-2 or IL-2 plus mouse anti-CD3/CD28 microbeads for an additional 4 h. Total RNA was isolated using Total RNA Miniprep (Monarch). Library preparation was carried out using a NEB Next UltraRNA Library Prep kit with i7 and i5 index reads of 8 bp each for mRNA library preparation and poly A enrichment. Sequencing was performed on a NovaSeq6000 PE150 platform in paired-end mode (read 1: 151 bp, read 2: 151 bp), using S4 (v.1.5) (300 cycles) sequencing kits (Illumina). Reads were aligned to the mouse reference genome (GRCm38/mm10, NCBI) using the Hisat2 (v.2.0.5) mapping tool. To quantify gene expression levels, featureCounts (v.1.5.0-p3) was used to count the reads mapped to each gene, followed by the calculation of fragments per kilobase of transcript sequence per million mapped reads based on gene length and read count. DEGs were identified using the DESeq2 R package (v.1.20.0). Adjusted P values were obtained using Wald test with multiple testing by the Benjamini–Hochberg method, and genes identified by DESeq2 with adjusted P values < 0.05 and fold change ≥ 2 were assigned as DEGs. Volcano plots were visualized using the ggplot2 R package ggplot2 (v.3.4.2), and PCA was conducted using the prcomp function in R and visualized using the R packages ggplot2 and ggrepel (v.0.9.3). DEGs obtained from comparing the groups ‘anti-CD3/CD28 +IL-2’ and ‘PGE2-treated + anti-CD3/CD28 +IL-2’ were ordered based on their log2 fold change values and subjected to GSEA using GSEA (v.4.3.2) probing for hallmark genes from mh.all.v2023.1.Mm (MSigDB). The PreRanked tool from GSEA (v.4.3.2) was used to determine the NES and significance by adjusted P values.
Statistical analyses
The GraphPad Prism software (v.9.5.0 and v.9.5.1) was used for statistical analyses. Affinity Designer (v.1.10.6) (Serif) was used to visualize data. Paired or unpaired two-tailed Student’s t-test, one-way ANOVA or two-way ANOVA was used to assess statistical significance, as indicated in in the figure legends. Data are shown as the mean ± s.d., mean ± s.e.m. or box and whiskers plots, as indicated in the figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data from scRNA-seq and scTCR-seq of CD8+ TILs and data from RNA-seq of TCF1+CD8+ T cells from in vitro T cell cultures have been deposited into the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/gds) under the superseries number GSE231340 (subseries numbers GSE231301 and GSE231302). The pre-built mouse reference v2020-A was provided by 10x Genomics (downloaded from https://cf.10xgenomics.com/supp/cell-exp/refdata-gex-GRCh38-2020-A.tar.gz) and is based on the mm10 GRCm38.p6 release 98 from Ensembl (http://ftp.ensembl.org/pub/release-98/fasta/mus_musculus/dna/Mus_musculus.GRCm38.dna.primary_assembly.fa.gz) with reference annotation from GENCODE Release M23 (http://ftp.ebi.ac.uk/pub/databases/gencode/Gencode_mouse/release_M23/gencode.vM23.primary_assembly.annotation.gtf.gz) provided by 10x Genomics. The pre-built GRCm38 Mouse V(D)J Reference v.5.0.0 was provided by 10x Genomics and downloaded from https://cf.10xgenomics.com/supp/cell-vdj/refdata-cellranger-vdj-GRCm38-alts-ensembl-5.0.0.tar.gz. Source data are provided with this paper.
References
Jansen, C. S. et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576, 465–470 (2019).
Siddiqui, I. et al. Intratumoral Tcf1+PD-1+ CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211.e10 (2019).
Prokhnevska, N. et al. CD8+ T cell activation in cancer comprises an initial activation phase in lymph nodes followed by effector differentiation within the tumor. Immunity 56, 107–124.e5 (2023).
Zehn, D., Thimme, R., Lugli, E., de Almeida, G. P. & Oxenius, A. ‘Stem-like’ precursors are the fount to sustain persistent CD8+ T cell responses. Nat. Immunol. 23, 836–847 (2022).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Codarri Deak, L. et al. PD-1-cis IL-2R agonism yields better effectors from stem-like CD8+ T cells. Nature 610, 161–172 (2022).
Krishna, S. et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science 370, 1328–1334 (2020).
Liu, B. et al. Temporal single-cell tracing reveals clonal revival and expansion of precursor exhausted T cells during anti-PD-1 therapy in lung cancer. Nat. Cancer 3, 108–121 (2022).
Kurtulus, S. et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1–CD8+ tumor-infiltrating T cells. Immunity 50, 181–194.e6 (2019).
Gatto, F., Schulze, A. & Nielsen, J. Systematic analysis reveals that cancer mutations converge on deregulated metabolism of arachidonate and xenobiotics. Cell Rep. 16, 878–895 (2016).
Wang, D. & DuBois, R. N. Eicosanoids and cancer. Nat. Rev. Cancer 10, 181–193 (2010).
Wang, Q., Morris, R. J., Bode, A. M. & Zhang, T. Prostaglandin pathways: opportunities for cancer prevention and therapy. Cancer Res. 82, 949–965 (2022).
Finetti, F. et al. Prostaglandin E2 and cancer: insight into tumor progression and immunity. Biology 9, 434 (2020).
Zelenay, S. et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 162, 1257–1270 (2015).
Böttcher, J. P. et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037.e14 (2018).
Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 188, 21–28 (2012).
Chen, J. H. et al. Prostaglandin E2 and programmed cell death 1 signaling coordinately impair CTL function and survival during chronic viral infection. Nat. Med. 21, 327–334 (2015).
Mosenden, R. et al. Mice with disrupted type I protein kinase A anchoring in T cells resist retrovirus-induced immunodeficiency. J. Immunol. 186, 5119–5130 (2011).
Newick, K. et al. Augmentation of CAR T-cell trafficking and antitumor efficacy by blocking protein kinase A localization. Cancer Immunol. Res. 4, 541–551 (2015).
Lone, A. M. & Taskén, K. Phosphoproteomics-based characterization of prostaglandin E2 signaling in T cells. Mol. Pharmacol. 99, 370–382 (2021).
Roberts, E. W. et al. Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30, 324–336 (2016).
Spranger, S., Bao, R. & Gajewski, T. F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015).
Böttcher, J. P. et al. Functional classification of memory CD8+ T cells by CX3CR1 expression. Nat Commun. 6, 8306 (2015).
Zander, R. et al. CD4+ T cell help is required for the formation of a cytolytic CD8+ T cell subset that protects against chronic infection and cancer. Immunity 51, 1028–1042.e4 (2019).
Aandahl, E. M. et al. CD7 is a differentiation marker that identifies multiple CD8 T cell effector subsets. J. Immunol. 170, 2349–2355 (2003).
Alfei, F. et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571, 265–269 (2019).
Scott, A. C. et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019).
Tsui, C. et al. MYB orchestrates T cell exhaustion and response to checkpoint inhibition. Nature 609, 354–360 (2022).
Yost, K. E. et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 25, 1251–1259 (2019).
Lucca, L. E. et al. Circulating clonally expanded T cells reflect functions of tumor-infiltrating T cells. J. Exp. Med. 218, e20200921 (2021).
Mandala, S. et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296, 346–349 (2002).
Ross, S. H. & Cantrell, D. A. Signaling and function of interleukin-2 in T lymphocytes. Annu. Rev. Immunol. 36, 411–433 (2018).
Hashimoto, M. et al. PD-1 combination therapy with IL-2 modifies CD8+ T cell exhaustion program. Nature 610, 173–181 (2022).
Corria-Osorio, J. et al. Orthogonal cytokine engineering enables novel synthetic effector states escaping canonical exhaustion in tumor-rejecting CD8+ T cells. Nat. Immunol. 24, 869–883 (2023).
Di Pilato, M. et al. CXCR6 positions cytotoxic T cells to receive critical survival signals in the tumor microenvironment. Cell 184, 4512–4530.e22 (2021).
Danilo, M., Chennupati, V., Silva, J. G., Siegert, S. & Held, W. Suppression of Tcf1 by inflammatory cytokines facilitates effector CD8 T cell differentiation. Cell Rep. 22, 2107–2117 (2018).
Morotti, M. et al. PGE2 inhibits TIL expansion by disrupting IL-2 signalling and mitochondrial function. Nature https://doi.org/10.1038/s41586-024-07352-w (2024).
Bayerl, F. et al. Tumor-derived prostaglandin E2 programs cDC1 dysfunction to impair intratumoral orchestration of anti-cancer T cell responses. Immunity 56, 1341–1358.e11 (2023).
Meiser, P. et al. A distinct stimulatory cDC1 subpopulation amplifies CD8+ T cell responses in tumors for protective anti-cancer immunity. Cancer Cell 41, 1498–1515.e10 (2023).
Mo, F. et al. An engineered IL-2 partial agonist promotes CD8+ T cell stemness. Nature 597, 544–548 (2021).
Tichet, M. et al. Bispecific PD1-IL2v and anti-PD-L1 break tumor immunity resistance by enhancing stem-like tumor-reactive CD8+ T cells and reprogramming macrophages. Immunity 56, 162–179.e6 (2023).
Leonard, W. J., Lin, J.-X. & O’Shea, J. J. The γc family of cytokines: basic biology to therapeutic ramifications. Immunity 50, 832–850 (2019).
Biringer, R. G. A review of prostanoid receptors: expression, characterization, regulation, and mechanism of action. J. Cell Commun. Signal. 15, 155–184 (2021).
Wübbenhorst, D. et al. Tetracycline-regulated bone morphogenetic protein 2 gene expression in lentivirally transduced primary rabbit chondrocytes for treatment of cartilage defects. Arthritis Rheum. 62, 2037–2046 (2010).
Di Pilato, M. et al. Targeting the CBM complex causes Treg cells to prime tumours for immune checkpoint therapy. Nature 570, 112–116 (2019).
Oh, S. A., Seki, A. & Rutz, S. Ribonucleoprotein transfection for CRISPR/Cas9-mediated gene knockout in primary T cells. Curr. Protoc. Immunol. 124, e69 (2019).
Labun, K. et al. CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res. 47, W171–W174 (2019).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Ahlmann-Eltze, C. & Huber, W. glmGamPoi: fitting Gamma–Poisson generalized linear models on single cell count data. Bioinformatics 36, 5701–5702 (2021).
Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20, 163–172 (2019).
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297 (2011).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Kaech, S. M., Hemby, S., Kersh, E. & Ahmed, R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111, 837–851 (2002).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Borcherding, N., Bormann, N. L. & Kraus, G. scRepertoire: an R-based toolkit for single-cell immune receptor analysis. F1000Research 9, 47 (2020).
Badia-I-Mompel, P. et al. decoupleR: ensemble of computational methods to infer biological activities from omics data. Bioinform. Adv. 2, vbac016 (2022).
Holland, C. H., Szalai, B. & Saez-Rodriguez, J. Transfer of regulatory knowledge from human to mouse for functional genomics analysis. Biochim. Biophys. Acta Gene Regul. Mech. 1863, 194431 (2020).
Pedersen, T. tidygraph: A tidy API for graph manipulation. GitHub https://github.com/thomasp85/tidygraph (2023).
Csárdi, G. and Nepusz, T. The igraph software package for complex network research. Gigascience https://doi.org/10.1093/gigascience/giab008 (2006).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Danecek, P. et al. Twelve years of SAMtools and BCFtools. GigaScience 10, giab008 (2021).
Ou, J. & Zhu, L. J. trackViewer: a Bioconductor package for interactive and integrative visualization of multi-omics data. Nat. Methods 16, 453–454 (2019).
Acknowledgements
We are grateful to C. Reis e Sousa and R. Marais for sharing the BRAFV600E cell lines and T. Mempel for providing D4M.3A-pOVA cells; and members of the Institute of Molecular Immunology and G. Coukos for discussions and suggestions. This work was supported by the Elite Network of Bavaria (N-LW-2016-370 awarded to J.P.B., i-Target to S.K.); the German research foundation (DFG) project numbers 424926990, 442405234, 449174900 and 461704785–SPP awarded to J.P.B., KO5055-2-1 and 510821390 to S.K., ZE 832/8-1, ZE 832/6-1 and SFB 1371 project P07 to D.Z. and SFB-TRR 338/1 2021–452881907 projects A01 (D.H.B.), B01 (S.K.), B02 (V.R.B), start-up funding (J.P.B.); the European Research Council (ERC, 756017, 101100460 and 101124203 to S.K.); and the German Cancer Aid (DKH, 70113918 to V.R.B., AvantCAR.de to S.K.); the Wilhelm-Sander Foundation to S.K.; the Else Kröner-Fresenius-Stiftung (IOLIN to S.S. and S.K.) and the Bavarian Research Foundation (BAYCELLATOR to D.H.B. and S.K.). M.M., A.J.G and D.D.J received support from the Lausanne Branch of the Ludwig Institute for Cancer Research.
Funding
Open access funding provided by Technische Universität München.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.B.L., J.D., M.M., A.J.G., V.R.B., D.Z., P.A.K., D.D.L., S.K. and J.P.B. Investigation: S.B.L., J.D., P.M., F.B., A.-M.P., A.H., J.H., L.R., T.J.R., N.S., A.O., L.G., S.L., S.M., D.B., L.F., L.M. and S.S. Formal analysis: S.B.L., J.D., G.P.d.A., P.M., F.B. and J.H. Methodology: S.B.L., J.D., S.J., G.P.d.A., D.H.B., V.R.B., D.Z., S.K. and J.P.B. Resources: G.P.d.A., D.H.B., V.R.B., P.K. and D.Z. Supervision: S.K. and J.P.B. Writing: S.B.L., J.D., S.K. and J.P.B. All authors contributed to feedback and proofreading.
Corresponding author
Ethics declarations
Competing interests
J.P.B., S.B.L., A.-M.P., P.K. and S.K. are preparing a patent application that includes work in this manuscript.
Peer review
Peer review information
Nature thanks Tyler Curiel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Cd4crePtger2−/−Ptger4fl/fl, GzmbcrePtger2−/−Ptger4fl/fl and Ptger2−/−Ptger4fl/fl mice exhibit normal T cell profiles.
Flow cytometric analysis of CD8+ and CD4+ T cells in spleens and inguinal lymph nodes (LN) of WT, Ptger2−/−Ptger4fl/fl, Cd4crePtger2−/−Ptger4fl/fl and GzmbcPtger2−/−Ptger4fl/fl mice (n = 8 per group). a-c, Representative plots showing a, CD4+ and CD8+ T cell frequency among live CD45+ cells or T cell subset composition among b, CD4+ T cells or c, CD8+ T cells as measured by CD44 and CD62L marker expression. d-g, Quantification of total cell numbers and subset composition for d, CD8+ T cells in LN (n = 8 per group), e, CD4+ T cells in LN (n = 8 per group), f, splenic CD8+ T cells (n = 5 per group) and g, splenic CD4+ T cells (n = 5 per group), based on a-c. Plots in a-c show data for one sample representative for n = 5–8 samples from two independent experiments. Data in d-g are pooled from two independent experiments and depicted as box plots extending from the 25th to 75th percentiles with the median as centre and whiskers corresponding to minimum and maximum values. P ≥ 0.05, not significant (NS) as determined by one-way ANOVA with Dunnett’s multiple-comparison test.
Extended Data Fig. 2 Cd4crePtger2−/−Ptger4fl/fl mice reject PGE2-producing tumours.
Growth profiles following s.c. inoculation of cancer cell lines into WT, Ptger2−/−Ptger4fl/fl or Cd4crePtger2−/−Ptger4fl/fl mice. a, Individual profiles of 2 × 105 control or Ptgs1/Ptgs2−/− BRAFV600E tumour cells (n = 10). b, Growth of 2 × 105 Ptgs1/Ptgs2−/− BRAFV600E melanoma cells transplanted into Rag1−/− mice (n = 4) or WT mice with or without CD8+ T cell depletion (n = 5 per group). c, Individual profiles of 2 × 106 Panc02 tumour cells (n = 8). d, Individual profiles of 2 × 105 MC38 tumour cells (n = 6). e, Representation of all profiles shown in d. Data in a-e are pooled from two (b,c,d,e) or three (a) independent experiments and depicted as mean ± s.e.m. P values are from two-way ANOVA with Bonferroni’s multiple-comparison test. P ≥ 0.05, not significant (NS).
Extended Data Fig. 3 Tumour-derived PGE2 does not impact on priming of anticancer CD8+ T cells in lymph nodes.
a, Experimental design for b-e. WT mice received 1 × 103 naive OVA-specific CD45.1+ OT-I T cells followed by inoculation with 2 × 106 control or Ptgs1/Ptgs2−/− BRAFV600E-OVA cells. 6 days later, tdLNs were analysed by flow cytometry. Mice injected with Ptgs1/Ptgs2−/− BRAFV600E cells (lacking OVA expression) served as control. b, Representative plot showing S8:H-2Kb surface staining on migratory cDC1 (identified as live CD45+CD11c+MHCIIhiCD103+CD8α−CD11b− cells). c, Quantification based on b, with n = 5 for control and Ptgs1/Ptgs2−/− BRAFV600E-OVA, n = 4 for Ptgs1/Ptgs2−/−BRAFV600E. d, Flow cytometry plots showing the sorting strategy for naive OT-I T cells. e, Flow cytometry plots showing expression of CD44 and TCF1 in polyclonal CD8+ T cells and OVA-specific OT-I T cells. f, Quantification of antigen-experienced CD44+ TCF1+ OT-I T cells based on e. n = 5. g, PGE2 concentration in lysates from tumours and indicated organs analysed 11 days after s.c. inoculation of WT mice with control or Ptgs1/Ptgs2−/− BRAFV600E melanoma cells (n = 5 per group). h, Effect of equilateral co-transplantation of 2 × 105 control and Ptgs1/Ptgs2−/− BRAFV600E tumours on tumour growth (n = 4 per group). Data in c and f-h are pooled from two (c,f,h) or three (g) independent experiments and depicted as mean ± s.e.m. Plots in b,e show data for one sample representative for n = 5 samples from two independent experiments. P values in c,f are from one-way ANOVA with Tukey’s multiple-comparison test, P values in g are from unpaired t-tests, P values in h are from two-way ANOVA with Bonferroni’s multiple-comparison test. P ≥ 0.05, not significant (NS).
Extended Data Fig. 4 Isolation of CD8+ TILs for scRNA-seq and phenotypic characterisation of CD8+ TIL populations.
Ptger2−/−Ptger4fl/fl, Cd4crePtger2−/−Ptger4fl/fl or GzmbcrePtger2−/−Ptger4fl/fl mice were transplanted with 2 × 106 control BRAFV600E melanoma cells. After 11 days, CD8+ TIL populations were sorted from n = 4 tumours per group and analysed by scRNA-seq (b,d-h) or flow cytometry (c,i-k). a, Flow cytometry plots showing the sorting strategy. b, UMAP plots showing transcript expression of Cd44, Tox and Pdcd1 (encoding PD-1) as determined by scRNA-seq. c, Flow cytometric analysis of CD44, TOX and PD-1 protein expression in CD8+ TILs. d, Heatmap showing the expression of cluster signature transcripts (top 50). e,f, Correlation of TCF1+CD8+ TIL cluster gene expression with gene signatures of e naive CD8+ T cells, memory stem cell CD8+ T cells (TSCM) and central memory CD8+ T cells (TCM) or f naive CD8+ T cells, tumour antigen-specific CD8+ T cells in tdLNs and tumour-infiltrating stem-like CD8+ T cells. g, UMAP visualisation of transcript expression of indicated immune genes as determined by scRNA-seq. h, Expression levels of selected immune genes across CD8+ TIL clusters. i, Flow cytometric analysis of GZMB and CD62L expression among TCF1+ and TIM-3+ CD8+ TIL populations from a WT mouse. j, Flow cytometric analysis of TIM-3 and CXCR6 protein expression in CD8+ TILs from a WT mouse. k, Analysis of GZMB expression in activated (CD44+) CD8+ T cells isolated from tumours, tdLNs and spleen. Numbers indicate percentage of GZMB+ cells compared to isotype control. Plots in a,c,i-k show data for one tumour representative for n = 6 tumours from one (a,b), two (c,i,k), or three (j) independent experiments or pooled data from n = 4 biological replicates from one experiment (d-h). P values in e,f are from pairwise comparisons using Wilcoxon rank sum test and Bonferroni correction for multiple testing. P ≥ 0.05, not significant (NS).
Extended Data Fig. 5 GzmbcrePtger2−/−Ptger4fl/fl mice exhibit Ptger4 knockout in TCF1+CD8+ TILs; increased proliferation of TCF1+CD8+ TILs and their progeny in T cell-specific EP2/EP4 double deficient mice.
a, Gene tracks showing the average scRNA-seq read coverage on Ptger2 and Ptger4 loci in CD8+ TILs from BRAFV600E melanoma tumours in WT, Ptger2−/−Ptger4fl/fl, Cd4crePtger2−/−Ptger4fl/fl and GzmbcrePtger2−/−Ptger4fl/fl mice across all replicates. Dashed boxes highlight knockout(KO)-specific target regions. b, Quantification of Ptger2 and Ptger4 read coverage for KO-specific target regions based on a. n = 4. c,d, Gene tracks showing the scRNA-seq read coverage on Ptger2 and Ptger4 loci in c, TCF1+CD8+ TILs and d, TIM-3+ TIL progeny from WT, Ptger2−/−Ptger4fl/fl, Cd4crePtger2−/−Ptger4fl/fl and GzmbcrePtger2−/−Ptger4fl/fl mice. Data for one representative replicate with comparable cell numbers is shown. e,f, WT or GzmbcrePtger2−/−Ptger4fl/fl TCF1+ stem-like and TCF1− effector CD8+ T cells from in vitro T cell cultures were stimulated or not with anti-CD3/CD28 for 24 h. e, Representative flow cytometry plots showing TCF1, CD62L and GZMB protein expression. f, Ptger4 mRNA expression as measured by knockout-sensitive RT-PCR (n = 2). g, Expression of a CD8+ T cell proliferation signature across the distinct populations of CD8+ TILs from Ptger2−/−Ptger4fl/fl, Cd4crePtger2−/−Ptger4fl/fl and GzmbcrePtger2−/−Ptger4fl/fl mice based on scRNA-seq. h, Analysis of Ki-67 protein expression in TCF1+ and TIM-3+ CD8+ TILs 11 days after inoculation of Ptger2−/−Ptger4fl/fl, Cd4crePtger2−/−Ptger4fl/fl and GzmbcrePtger2−/−Ptger4fl/fl mice with 2 × 106 control BRAFV600E melanoma cells. n = 7. i, Expression of a gene signature for T cell effector function, analysed as in g. Data in a-e,g,i are from one experiment. Data in b,h are pooled from two (b) or three (h) independent experiments and depicted as mean ± s.e.m. Data in f are depicted as mean ± s.d. from one experiment. P values are from one-way ANOVA with Tukey’s multiple-comparison test. P ≥ 0.05, not significant (NS).
Extended Data Fig. 6 EP2/EP4 double deficiency permits clonal differentiation and expansion of CD8+ TILs in PGE2-producing tumours.
a, UMAP visualisations of T cell clonotype distribution across CD8+ TILs based on scTCR-seq. b, Clonotype frequency across CD8+ TIL clusters based on a. c, UMAP visualisation of expanded tumour-infiltrating CD8+ T cell clonotypes coloured according to cluster classification. d, Comparison of clonotype frequencies between Ptger2−/−Ptger4fl/fl, Cd4crePtger2−/−Ptger4fl/fl and GzmbcrePtger2−/−Ptger4fl/fl mice. e, Frequencies of T cell clones shared between TCF1+CD8+ TILs and TIM-3+ TIL progeny. f, UMAP plots visualising the top 3 most frequent shared clonotypes for Ptger2−/−Ptger4fl/fl, Cd4crePtger2−/−Ptger4fl/fl and GzmbcrePtger2−/−Ptger4fl/fl mice. Data are from one experiment.
Extended Data Fig. 7 EP2/EP4 double deficiency rescues development of early and terminally differentiated effector CD8+ T cells within tumour tissue.
a-c, WT, Ptger2−/−Ptger4fl/fl or Cd4crePtger2−/−Ptger4fl/fl mice were injected s.c. with 2×106 control or Ptgs1/Ptgs2−/− BRAFV600E cells and CD44+ CD8+ TILs were analysed 11 days later by flow cytometry. a, Representative plots showing the frequency of TCF1+ stem-like and TIM-3+ effector CD8+ TIL populations. b, Quantification of TCF1+CD8+ TILs (Ptgs1/Ptgs2−/− into WT, n = 9; Control into WT, n = 11; Control into Ptger2−/−Ptger4fl/fl, n = 7; Control into Cd4crePtger2−/−Ptger4fl/fl, n = 10). c, Quantification of TIM-3+CD8+ TILs (Ptgs1/Ptgs2−/− into WT, n = 8; Control into WT, n = 10; Control into Ptger2−/−Ptger4fl/fl, n = 7; Control into Cd4crePtger2−/−Ptger4fl/fl, n = 10). Data in b,c are pooled from three independent experiments and depicted as mean ± s.e.m. Plots in a show data for one tumour sample representative for at least n = 7 samples from three independent experiments. P values are from one-way ANOVA with Tukey’s multiple-comparison test. P ≥ 0.05, not significant (NS).
Extended Data Fig. 8 PGE2-mediated changes in TCF1+CD8+ T cells, effect on γc expression and proliferation.
a-d, scRNA-seq based analysis of TF activity alterations in TCF1+CD8+ TILs and their TIM-3+CXCR6+ effector progeny between control BRAFV600E melanoma tumours in Cd4crePtger2−/−Ptger4fl/fl and GzmbcrePtger2−/−Ptger4fl/fl mice compared to Ptger2−/−Ptger4fl/fl mice. a,b, Alterations of TF network activity in TCF1+CD8+ TILs from BRAFV600E melanoma tumours in a, Cd4crePtger2−/−Ptger4fl/fl mice compared to Ptger2−/−Ptger4fl/fl mice or b, GzmbcrePtger2−/−Ptger4fl/fl mice compared to Ptger2−/−Ptger4fl/fl mice. c, Heatmap visualisation, d, Correlation of TF activity alterations for TIL populations from Cd4crePtger2−/−Ptger4fl/fl and GzmbBcrePtger2−/−Ptger4fl/fl mice. e, Flow cytometry plots showing the sorting strategy for TCF1+ TILs (identified as TIM-3−CXCR6− cells) from Ptgs1/Ptgs2−/− BRAFV600E tumours. f, Volcano plot showing the effect of PGE2 exposure on gene expression in TCF1+CD8+ T cells from in vitro T cell cultures, based on RNA-seq analysis. g, Effect of PGE2 treatment on IL-2Rγc protein expression (n = 3). h, WT or Cd4crePtger2−/−Ptger4fl/fl TCF1+CD8+ T cells from in vitro T cell cultures were stimulated with anti-CD3/CD28 and high-dose IL-2 in presence or absence of PGE2. After 24 h cells were analysed for T cell proliferation by EdU incorporation. i, WT, Ptger2−/−Ptger4fl/fl or Cd4crePtger2−/−Ptger4fl/fl TCF1+CD8+ T cells were treated or not with tumour cell conditioned medium (CM) or PGE2 and stimulated as indicated. After 72 h, T cell expansion was analysed by flow cytometry. n = 3. Data in a-d, f are from one experiment. Data in g,i show data for one representative of two independent experiments with n = 3 biological replicates. In f), DEGs (P < 0.05; fold change ≥ 2) were identified by Wald test with multiple testing by the Benjamini-Hochberg method. Horizontal lines and error bars in (g,i) indicate mean ± s.d. P-values are from d, Fitted linear regression models or g, and i, unpaired t-test. P ≥ 0.05, not significant (NS). Plots in (h) show data for one sample representative for n = 3 samples.
Extended Data Fig. 9 PGE2 impairs proliferative expansion and effector differentiation of antigen-specific TCF1+CD8+ TILs.
a, Experimental design for b-d b, Quantification of OT-I cell expansion in tumours at different time points (day 6 and day 8: n = 8 per group; day 10: n = 7 per group). c, Histograms showing expression of indicated molecules in Cd4crePtger2−/−Ptger4fl/fl OT-I TILs at different time points. Naive Cd4crePtger2−/−Ptger4fl/fl OT-I T cells served as control. d, Flow cytometry plots showing subpopulation composition among Cd4crePtger2−/−Ptger4fl/fl OT-I TILs. e-h, WT mice received 1 × 103 naive congenically marked CD45.1+ Cd4crePtger2−/−Ptger4fl/fl OT-I T cells followed by inoculation with 2 × 106 MC38-OVA cells. 8 days later, the indicated subpopulations of OT-I TILs were sorted and 7 × 103 cells were re-transferred into MC38-OVA bearing Rag1−/− recipient mice. TILs in Rag1−/− recipients were analysed at day 8 post re-transfer. e, Experimental design. f, Flow cytometry plots showing the sorting strategy. g, Flow cytometry plots showing the frequency of OT-I TILs recovered at day 8 post re-transfer. h, Quantification of OT-I TIL progenies based on (g), with TIM-3−CXCR6− stem-like, n = 7; TIM-3+CXCR6+ differentiated, n = 8. i, Flow cytometry plots showing the expression of TIM-3 and CXCR6 among recovered OT-I TILs. j, Analysis of TCF1 expression in Cd4crePtger2−/−Ptger4fl/fl OT-I T cells in tdLNs and tumours over time. k, Experimental design for l. l, Effect of FTY720 treatment from day 6 on OT-I T cell expansion in tumours (n = 6). m,n, WT mice received 1 × 103 naive OT-I T cells or 1 × 103 naive Cd4crePtger2−/−Ptger4fl/fl OT-I T cells i.v. and were transplanted s.c. with 2 × 106 D4M.3A-pOVA melanoma cells before m, quantification of OT-I cell expansion in tumours at different time points (n = 6) or n, analysis of tumour growth over time (n = 4). Data in b,h,l-n are pooled from two (h,l-n) or three (b) independent experiments and depicted as box plots extending from the 25th to 75th percentiles with the median as centre and whiskers corresponding to minimum and maximum values (b,h,l,m) or shown as mean ± s.e.m. (n). Plots in c,d,g,i,j show data for one sample representative for (c,d,g,i) n = 7 or (j) n = 6 samples from two independent experiments. P values in b,l are from one-way ANOVA with Tukey’s multiple-comparison test, P values in h,m are from unpaired t-test, P-values in n are from two-way ANOVA with Bonferroni’s correction for multiple testing. P ≥ 0.05, not significant (NS).
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lacher, S.B., Dörr, J., de Almeida, G.P. et al. PGE2 limits effector expansion of tumour-infiltrating stem-like CD8+ T cells. Nature (2024). https://doi.org/10.1038/s41586-024-07254-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-024-07254-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.