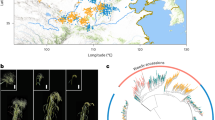
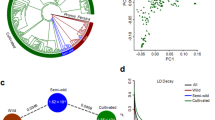
Abstract
Cowpeas (tropical legumes) are important in ensuring food and nutritional security in developing countries, especially in sub-Saharan Africa. Herein, we report two high-quality genome assemblies of grain and vegetable cowpeas and we re-sequenced 344 accessions to characterize the genomic variations landscape. We identified 39 loci for ten important agronomic traits and more than 541 potential loci that underwent selection during cowpea domestication and improvement. In particular, the synchronous selections of the pod-shattering loci and their neighboring stress-relevant loci probably led to the enhancement of pod-shattering resistance and the compromise of stress resistance during the domestication from grain to vegetable cowpeas. Moreover, differential selections on multiple loci associated with pod length, grain number per pod, seed weight, pod and seed soluble sugars, and seed crude proteins shaped the yield and quality diversity in cowpeas. Our findings provide genomic insights into cowpea domestication and improvement footprints, enabling further genome-informed cultivar improvement of cowpeas.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The genome assemblies of G98 (accession number JBALLC000000000) and G323 (accession number JAZDUG000000000) and the re-sequencing data of 344 accessions have been deposited in the NCBI Sequence Read Archive under the BioProject accession number PRJNA889224; the RNA-seq data for gene annotation have been deposited in the NCBI Sequence Read Archive under BioProject accession number PRJNA954189; the transcriptome data of three cowpea accessions have been deposited in the NCBI Sequence Read Archive under BioProject accession number PRJNA970477. The genotype and phenotype data can be accessed in figshare (https://doi.org/10.6084/m9.figshare.21646556)141.
Code availability
All codes and tools used in this study are described in Methods and Reporting Summary.
References
Singh, B. B. Cowpea: The Food Legume of the 21st Century (Crop Science Society of America, 2014).
Timko, M. P & Singh, B. in Plant Genetics and Genomics: Crops and Models (eds Moore, P. H. & Ming, R.) 227–258 (Springer, 2008).
Pasquet, R. S. & Padulosi, S. Genus Vigna and cowpea (Vigna unguiculata (L.) Walp.) taxonomy: current status and prospects. In Proc. 5th World Cowpea Conference (eds Boukara, O. et al.) 66–87 (International Institute of Tropical Agriculture, 2012).
Herniter, I. A., Muñoz-Amatriaín, M. & Close, T. J. Genetic, textual, and archeological evidence of the historical global spread of cowpea (Vigna unguiculata (L.) Walp.). Legume Sci. 2, e57 (2020).
Timko, M. P., Ehlers, J. D. & Roberts, P. A. in Genome Mapping and Molecular Breeding in Plants, Vol. 3 (ed. Kole, C.) 49–67 (Springer, 2007).
National Research Council. Lost Crops of Africa, Vol. 2 (The National Academies Press, 2006).
Som, M. G. & Hazra, P. in Genetic Improvement of Vegetable Crops (eds Kalloo G. & Bergh, B. O.) 339–354 (Elsevier, 1993).
Kongjaimun, A. et al. The genetics of domestication of yardlong bean, Vigna unguiculata (L.) Walp. ssp. unguiculata cv.-gr. sesquipedalis. Ann. Bot. 109, 1185–1200 (2012).
Lo, S. et al. Identification of QTL controlling domestication-related traits in cowpea (Vigna unguiculata L. Walp). Sci. Rep. 8, 6261 (2018).
Lo, S. et al. A genome-wide association and meta-analysis reveal regions associated with seed size in cowpea [Vigna unguiculata (L.) Walp]. Theor. Appl. Genet. 132, 3079–3087 (2019).
Suanum, W. et al. Co-localization of QTLs for pod fiber content and pod shattering in F2 and backcross populations between yardlong bean and wild cowpea. Mol. Breed. 36, 80 (2016).
Andargie, M., Pasquet, R. S., Gowda, B. S., Muluvi, G. M. & Timko, M. P. Molecular mapping of QTLs for domestication-related traits in cowpea (V. unguiculata (L.) Walp.). Euphytica 200, 401–412 (2014).
Herniter, I. A., Muñoz-Amatriaín, M., Lo, S., Guo, Y.-N. & Close, T. J. Identification of candidate genes controlling black seed coat and pod tip color in cowpea (Vigna unguiculata [L.] Walp). G3 8, 3347–3355 (2018).
Kongjaimun, A. et al. QTL mapping of pod tenderness and total soluble solid in yardlong bean [Vigna unguiculata (L.) Walp. subsp. unguiculata cv.-gr. sesquipedalis]. Euphytica 189, 217–223 (2013).
Xu, P. et al. Genomic regions, cellular components and gene regulatory basis underlying pod length variations in cowpea (V. unguiculata L. Walp). Plant Biotechnol. J. 15, 547–557 (2017).
Wu, X. B., Cortés, A. J. & Blair, M. W. Genetic differentiation of grain, fodder and pod vegetable type cowpeas (Vigna unguiculata L.) identified through single nucleotide polymorphisms from genotyping-by-sequencing. Mol. Hortic. 2, 8 (2022).
Lonardi, S. et al. The genome of cowpea (Vigna unguiculata [L.] Walp.). Plant J. 98, 767–782 (2019).
Yang, T. et al. Improved pea reference genome and pan-genome highlight genomic features and evolutionary characteristics. Nat. Genet. 54, 1553–1563 (2022).
Pan, L. et al. Comprehensive genomic analyses of Vigna unguiculata provide insights into population differentiation and the genetic basis of key agricultural traits. Plant Biotechnol. J. 21, 1426–1439 (2023).
Guan, J. T. et al. Genomic analyses of rice bean landraces reveal adaptation and yield related loci to accelerate breeding. Nat. Commun. 13, 5707 (2022).
Rennie, E. A. et al. Identification of a sphingolipid α-glucuronosyltransferase that is essential for pollen function in Arabidopsis. Plant Cell 26, 3314–3325 (2014).
Chen, L. Y. et al. The Arabidopsis alkaline ceramidase TOD1 is a key turgor pressure regulator in plant cells. Nat. Commun. 6, 6030 (2015).
Haslam, T. M. & Feussner, I. Diversity in sphingolipid metabolism across land plants. J. Exp. Bot. 73, 2785–2798 (2022).
Liu, J. et al. Natural variation in ARF18 gene simultaneously affects seed weight and silique length in polyploid rapeseed. Proc. Natl Acad. Sci. USA 112, E5123–E5132 (2015).
Shi, L. L. et al. A CACTA-like transposable element in the upstream region of BnaA9.CYP78A9 acts as an enhancer to increase silique length and seed weight in rapeseed. Plant J. 98, 524–539 (2019).
Abbo, S. et al. Plant domestication versus crop evolution: a conceptual framework for cereals and grain legumes. Trends Plant Sci. 19, 351–360 (2014).
Yang, J. H. et al. Genomic signatures of vegetable and oilseed allopolyploid Brassica juncea and genetic loci controlling the accumulation of glucosinolates. Plant Biotechnol. J. 19, 2619–2628 (2021).
Kang, L. et al. Genomic insights into the origin, domestication and diversification of Brassica juncea. Nat. Genet. 53, 1392–1402 (2021).
Lo, S. et al. Genetic, anatomical, and environmental patterns related to pod shattering resistance in domesticated cowpea [Vigna unguiculata (L.) Walp]. J. Exp. Bot. 72, 6219–6229 (2021).
Taylor-Teeples, M. et al. An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature 517, 571–575 (2015).
Zhang, D. M. et al. An uncanonical CCCH-tandem zinc-finger protein represses secondary wall synthesis and controls mechanical strength in rice. Mol. Plant. 11, 163–174 (2018).
Huang, L. C., Tan, H. Y., Zhang, C. Q., Li, Q. F. & Liu, Q. Q. Starch biosynthesis in cereal endosperms: an updated review over the last decade. Plant Commun. 2, 100237 (2021).
Tian, H. et al. Arabidopsis NPCC6/NaKR1 is a phloem mobile metal binding protein necessary for phloem function and root meristem maintenance. Plant Cell 22, 3963–3979 (2010).
Su, S. et al. Gibberellins orchestrate panicle architecture mediated by DELLA-KNOX signalling in rice. Plant Biotechnol. J. 19, 2304–2318 (2021).
Léran, S. et al. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci. 19, 5–9 (2014).
Corratgé-Faillie, C. & Lacombe, B. Substrate (un)specificity of Arabidopsis NRT1/PTR FAMILY (NPF) proteins. J. Exp. Bot. 68, 3107–3113 (2017).
Kanneganti, V. & Gupta, A. K. Wall associated kinases from plants—an overview. Physiol. Mol. Biol. Plants 14, 109–118 (2008).
Kohorn, B. D. et al. An Arabidopsis cell wall-associated kinase required for invertase activity and cell growth. Plant J. 46, 307–316 (2006).
Barry, C. S., Llop-Tous, M. I. & Grierson, D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol. 123, 979–986 (2000).
Kaga, A., Isemura, T., Tomooka, N. & Vaughan, D. A. The genetics of domestication of the azuki bean (Vigna angularis). Genetics 178, 1013–1036 (2008).
Robles, P. & Quesada, V. Research progress in the molecular functions of plant mTERF proteins. Cells 10, 205 (2021).
Schwarz, D. S. & Blower, M. D. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell. Mol. Life Sci. 73, 79–94 (2016).
Zang, J. Z., Kriechbaumer, V. & Wang, P. W. Plant cytoskeletons and the endoplasmic reticulum network organization. J. Plant Physiol. 264, 153473 (2021).
Bouchenak, M. & Lamri-Senhadji, M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: a review. J. Med. Food 16, 185–198 (2013).
Zhang, M. et al. Progress in soybean functional genomics over the past decade. Plant Biotechnol. J. 20, 256–282 (2022).
Chen, L. Q. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468, 527–532 (2010).
Yang, J. L. et al. SWEET11 and 15 as key players in seed filling in rice. N. Phytol. 218, 604–615 (2018).
Sosso, D. et al. Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 47, 1489–1493 (2015).
Wang, S. D. et al. Simultaneous changes in seed size, oil content and protein content driven by selection of SWEET homologues during soybean domestication. Natl Sci. Rev. 7, 1776–1786 (2020).
Duan, Z. B. et al. Natural allelic variation of GmST05 controlling seed size and quality in soybean. Plant Biotechnol. J. 20, 1807–1818 (2022).
Paniagua, C. et al. Antisense down-regulation of the strawberry β-galactosidase gene FaβGal4 increases cell wall galactose levels and reduces fruit softening. J. Exp. Bot. 67, 619–631 (2016).
Ma, L., Xue, N., Fu, X. Y., Zhang, H. S. & Li, G. Arabidopsis thaliana FAR-RED ELONGATED HYPOCOTYLS3 (FHY3) and FAR-RED-IMPAIRED RESPONSE1 (FAR1) modulate starch synthesis in response to light and sugar. N. Phytol. 213, 1682–1696 (2017).
Zhong, R. Q. & Ye, Z. H. The SAC domain-containing protein gene family in Arabidopsis. Plant Physiol. 132, 544–555 (2003).
Ilsley, J. L., Sudol, M. & Winder, S. J. The WW domain: linking cell signalling to the membrane cytoskeleton. Cell. Signal. 14, 183–189 (2002).
Yao, H. Y. & Xue, H. W. Phosphatidic acid plays key roles regulating plant development and stress responses. J. Integr. Plant Biol. 60, 851–863 (2018).
Zhang, H., Lu, Y., Zhao, Y. & Zhou, D. X. OsSRT1 is involved in rice seed development through regulation of starch metabolism gene expression. Plant Sci. 248, 28–36 (2016).
Parker, T. A., Lo, S. & Gepts, P. Pod shattering in grain legumes: emerging genetic and environment-related patterns. Plant Cell 33, 179–199 (2021).
Xu, P. et al. Natural variation and gene regulatory basis for the responses of asparagus beans to soil drought. Front. Plant Sci. 6, 891 (2015).
Santos, J. R. P., Ndeve, A. D., Huynh, B. L., Matthews, W. C. & Roberts, P. A. QTL mapping and transcriptome analysis of cowpea reveals candidate genes for root-knot nematode resistance. PLoS ONE 13, e0189185 (2018).
Huynh, B. L. et al. Genetic mapping and legume synteny of aphid resistance in African cowpea (Vigna unguiculata L. Walp.) grown in California. Mol. Breed. 35, 36 (2015).
Huynh, B. L. et al. A major QTL corresponding to the Rk locus for resistance to root‑knot nematodes in cowpea (Vigna unguiculata L. Walp.). Theor. Appl. Genet. 129, 87–95 (2016).
Muchero, W., Ehlers, J. D., Close, T. J. & Roberts, P. A. Mapping QTL for drought stress-induced premature senescence and maturity in cowpea [Vigna unguiculata (L.) Walp.]. Theor. Appl. Genet. 118, 849–863 (2009).
Muchero, W., Ehlers, J. D. & Roberts, P. A. Restriction site polymorphism-based candidate gene mapping for seedling drought tolerance in cowpea [Vigna unguiculata (L.) Walp.]. Theor. Appl. Genet. 120, 509–518 (2010).
Muchero, W., Ehlers, J. D., Close, T. J. & Roberts, P. A. Genic SNP markers and legume synteny reveal candidate genes underlying QTL for Macrophomina phaseolina resistance and maturity in cowpea [Vigna unguiculata (L) Walp.]. BMC Genom. 12, 8 (2011).
Pottorff, M. et al. Genetic and physical mapping of candidate genes for resistance to Fusarium oxysporum f. sp. tracheiphilum race 3 in cowpea [Vigna unguiculata (L.) Walp.]. PLoS ONE 7, e41600 (2012).
Pottorff, M. et al. Identification of candidate genes and molecular markers for heat-induced brown discoloration of seed coats in cowpea [Vigna unguiculata (L.) Walp.]. BMC Genom. 15, 328 (2014).
Ravelombola, W., Shi, A. & Huynh, B. L. Loci discovery, network-guided approach, and genomic prediction for drought tolerance index in a multi-parent advanced generation intercross (MAGIC) cowpea population. Hortic. Res. 8, 24 (2021).
Wu, X. Y. et al. Association mapping for fusarium wilt resistance in Chinese asparagus bean germplasm. Plant Genome 8, 1–6 (2015).
Wu, X. Y. et al. SNP marker-based genetic mapping of rust resistance gene in the vegetable cowpea landrace ZN016. Legum. Res. 41, 222–225 (2018).
Heng, T., Kaga, A., Chen, X. & Somta, P. Two tightly linked genes coding for NAD-dependent malic enzyme and dynamin-related protein are associated with resistance to Cercospora leaf spot disease in cowpea (Vigna unguiculata (L.) Walp.). Theor. Appl. Genet. 133, 395–407 (2020).
Geater, C. W. & Fehr, W. R. Association of total sugar content with other seed traits of diverse soybean cultivars. Crop Sci. 40, 1552–1555 (2000).
Yoshikawa, Y., Chen, P. Y., Zhang, B., Scaboo, A. & Orazaly, M. Evaluation of seed chemical quality traits and sensory properties of natto soybean. Food Chem. 153, 186–192 (2014).
Bu, Y. P. et al. Conditional and unconditional QTL analyses of seed hardness in vegetable soybean (Glycine max L. Merr.). Euphytica 214, 237 (2018).
Murray, M. G. & Thompson, W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8, 4321–4325 (1980).
Koren, S. et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 27, 722–736 (2017).
Cheng, H. Y., Concepcion, G. T., Feng, X. W., Zhang, H. W. & Li, H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat. Methods 18, 170–175 (2021).
Burton, J. N. et al. Chromosome-scale scaffolding of de novo genome assemblies based on chromatin interactions. Nat. Biotechnol. 31, 1119–1125 (2013).
Rhie, A., Walenz, B. P., Koren, S. & Phillippy, A. M. Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 21, 245 (2020).
Flynn, J. M. et al. RepeatModeler2 for automated genomic discovery of transposable element families. Proc. Natl Acad. Sci. USA 117, 9451–9457 (2020).
Jurka, J. et al. Repbase Update, a database of eukaryotic repetitive elements. Cytogenet. Genome Res. 110, 462–467 (2005).
Neumann, P., Novák, P., Hoštáková, N. & Macas, J. Systematic survey of plant LTR-retrotransposons elucidates phylogenetic relationships of their polyprotein domains and provides a reference for element classification. Mob. DNA 10, 1 (2019).
Wheeler, T. J. et al. Dfam: a database of repetitive DNA based on profile hidden Markov models. Nucleic Acids Res. 41, D70–D82 (2013).
Ellinghaus, D., Kurtz, S. & Willhoeft, U. LTRharvest, an efficient and flexible software for de novo detection of LTR retrotransposons. BMC Bioinform. 9, 18 (2008).
Xu, Z. & Wang, H. LTR_FINDER: an efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res. 35, W265–W268 (2007).
Tarailo‐Graovac, M. & Chen, N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinformatics 4, 4.10.1–4.10.14 (2009).
Benson, G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27, 573–580 (1999).
Beier, S., Thiel, T., Münch, T., Scholz, U. & Mascher, M. MISA-web: a web server for microsatellite prediction. Bioinformatics 33, 2583–2585 (2017).
Stanke, M., Diekhans, M., Baertsch, R. & Haussler, D. Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics 24, 637–644 (2008).
Korf, I. Gene finding in novel genomes. BMC Bioinform. 5, 59 (2004).
Keilwagen, J. et al. Using intron position conservation for homology-based gene prediction. Nucleic Acids Res. 44, E89 (2016).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295 (2015).
Tang, S., Lomsadze, A. & Borodovsky, M. Identification of protein coding regions in RNA transcripts. Nucleic Acids Res. 43, E78 (2015).
Haas, B. J. et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the program to assemble spliced alignments. Genome Biol. 9, R7 (2008).
Haas, B. J. et al. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Res. 31, 5654–5666 (2003).
Lowe, T. M. & Eddy, S. R. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25, 955–964 (1997).
Loman, T. A Novel Method for Predicting Ribosomal RNA Genes in Prokaryotic Genomes. MSc thesis, Lund Univ. (2017).
Griffiths-Jones, S. et al. Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res. 33, D121–D124 (2005).
Griffiths-Jones, S., Grocock, R. J., van Dongen, S., Bateman, A. & Enright, A. J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 34, D140–D144 (2006).
She, R., Chu, J. S., Wang, K., Pei, J. & Chen, N. GenBlastA: enabling BLAST to identify homologous gene sequences. Genome Res. 19, 143–149 (2009).
Birney, E., Clamp, M. & Durbin, R. GeneWise and genomewise. Genome Res. 14, 988–995 (2004).
Song, J. M. et al. Two gap-free reference genomes and a global view of the centromere architecture in rice. Mol. Plant 14, 1757–1767 (2021).
Swarbreck, D. et al. The Arabidopsis Information Resource (TAIR): gene structure and function annotation. Nucleic Acids Res. 36, D1009–D1014 (2008).
Kang, Y. J. et al. Genome sequence of mungbean and insights into evolution within Vigna species. Nat. Commun. 5, 5443 (2014).
Yang, K. et al. Genome sequencing of adzuki bean (Vigna angularis) provides insight into high starch and low fat accumulation and domestication. Proc. Natl Acad. Sci. USA 112, 13213–13218 (2015).
Schmutz, J. et al. A reference genome for common bean and genome-wide analysis of dual domestications. Nat. Genet. 46, 707–713 (2014).
Garcia, T. et al. Comprehensive genomic resources related to domestication and crop improvement traits in Lima bean. Nat. Commun. 12, 702 (2021).
Valliyodan, B. et al. Construction and comparison of three reference-quality genome assemblies for soybean. Plant J. 100, 1066–1082 (2019).
Varshney, R. K. et al. Draft genome sequence of pigeonpea (Cajanus cajan), an orphan legume crop of resource-poor farmers. Nat. Biotechnol. 30, 83–89 (2012).
Qin, S. et al. A draft genome for Spatholobus suberectus. Sci. Data 6, 113 (2019).
Kamal, N. et al. Insights into the evolution of symbiosis gene copy number and distribution from a chromosome-scale Lotus japonicus Gifu genome sequence. DNA Res. 27, dass015 (2020).
Kreplak, J. et al. A reference genome for pea provides insight into legume genome evolution. Nat. Genet. 51, 1411–1422 (2019).
De Vega, J. J. et al. Red clover (Trifolium pratense L.) draft genome provides a platform for trait improvement. Sci. Rep. 5, 17394 (2015).
Young, N. D. et al. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 480, 520–524 (2011).
Varshney, R. K. et al. Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat. Biotechnol. 31, 240–246 (2013).
Hane, J. K. et al. A comprehensive draft genome sequence for lupin (Lupinus angustifolius), an emerging health food: insights into plant–microbe interactions and legume evolution. Plant Biotechnol. J. 15, 318–330 (2017).
Bertioli, D. J. et al. The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat. Genet. 48, 438–446 (2016).
Griesmann, M. et al. Phylogenomics reveals multiple losses of nitrogen-fixing root nodule symbiosis. Science 361, eaat1743 (2018).
Emms, D. M. & Kelly, S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20, 238 (2019).
Nguyen, L. T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Yang, Z. H. PAML: a program package for phylogenetic analysis by maximum likelihood. Bioinformatics 13, 555–556 (1997).
Yu, G. C., Wang, L. G., Han, Y. Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Marçais, G. et al. MUMmer4: a fast and versatile genome alignment system. PLoS Comput. Biol. 14, e1005944 (2018).
Wang, K., Li, M. Y. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Danecek, P. et al. Twelve years of SAMtools and BCFtools. GigaScience 10, giab008 (2021).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Cingolani, P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6, 80–92 (2012).
Kumar, S. et al. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664 (2009).
Zhang, C., Dong, S. S., Xu, J. Y., He, W. M. & Yang, T. L. PopLDdecay: a fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics 35, 1786–1788 (2019).
Varelis, P. Food Chemistry and Analysis (Elsevier, 2016).
Kang, H. M. et al. Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 42, 348–354 (2010).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (1995).
Chen, H., Patterson, N. & Reich, D. Population differentiation as a test for selective sweeps. Genome Res. 20, 393–402 (2010).
Shiroguchi, K., Jia, T. Z., Sims, P. A. & Xie, X. S. Digital RNA sequencing minimizes sequence-dependent bias and amplification noise with optimized single-molecule barcodes. Proc. Natl Acad. Sci. USA 109, 1347–1352 (2012).
Kivioja, T. et al. Counting absolute numbers of molecules using unique molecular identifiers. Nat. Methods 9, 72–74 (2012).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. EdgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Wu, X. Y. et al. Development of a core set of single nucleotide polymorphism markers for genetic diversity analysis and cultivar fingerprinting in cowpea. Legume Sci. 3, e93 (2021).
Wu, Xi. & Li, G. Differential selection of yield and quality traits has shaped genomic signatures of cowpea domestication and improvement. figshare https://doi.org/10.6084/m9.figshare.21646556 (2024).
Acknowledgements
We thank J. Yang from Zhejiang University for valuable comments on the paper. This work was supported by the Key R&D Program of Zhejiang Province (2022C02016 to Xinyi Wu), the Major Science and Technology Project of Plant Breeding in Zhejiang Province (2021C02065 to B.W.), Key R&D Program of Guangdong Province (2020B020220002 to Xinyi Wu), the Crop Germplasm Identification Project of General Seed Management Station of Agriculture and Rural Affairs Department in Zhejiang Province (2022R23T60D01 to Xinyi Wu), the Fundamental Research Funds for the Central Universities (+226-2022-00100 to M.Z.) and Biological Breeding Project of ZAAS Program for Transdisciplinary Research (to Xinyi Wu).
Author information
Authors and Affiliations
Contributions
Xinyi Wu, M.Z. and G.L. conceived and designed the project. Xinyi Wu, Z.H. and N.L. performed genome assembly and assessment, comparative genome analysis and other bioinformatic analyses. Xinyi Wu, B.W., Xiaohua Wu, Y.W., J.W., Z.L., Y.S. and W.D. performed field cultivation and phenotype investigation in Hangzhou, and Y.Z. and Y.Y. performed field cultivation and phenotype investigation in Guangzhou. M.L., J.D. and J.W. worked on quality testing and GWAS data analysis. J.D. performed gene expression analysis and gene function validation on the bi-parental population. Xinyi Wu and Z.H. wrote the paper, M.Z. revised the paper and all authors read and approved the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Caroline Belser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10.
Supplementary Tables
The contents of each supplementary table are provided in the first sheet.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, X., Hu, Z., Zhang, Y. et al. Differential selection of yield and quality traits has shaped genomic signatures of cowpea domestication and improvement. Nat Genet (2024). https://doi.org/10.1038/s41588-024-01722-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41588-024-01722-w