Abstract
Bacille Calmette–Guérin (BCG) vaccination can confer nonspecific protection against heterologous pathogens. However, the underlying mechanisms remain mysterious. We show that mice vaccinated intravenously with BCG exhibited reduced weight loss and/or improved viral clearance when challenged with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 B.1.351) or PR8 influenza. Protection was first evident between 14 and 21 d post-vaccination and lasted ∼3 months. Notably, BCG induced a biphasic innate response and robust antigen-specific type 1 helper T cell (TH1 cell) responses in the lungs. MyD88 signaling was essential for innate and TH1 cell responses, and protection against SARS-CoV-2. Depletion of CD4+ T cells or interferon (IFN)-γ activity before infection obliterated innate activation and protection. Single-cell and spatial transcriptomics revealed CD4-dependent expression of IFN-stimulated genes in lung myeloid and epithelial cells. Notably, BCG also induced protection against weight loss after mouse-adapted SARS-CoV-2 BA.5, SARS-CoV and SHC014 coronavirus infections. Thus, BCG elicits integrated organ immunity, where CD4+ T cells feed back on tissue myeloid and epithelial cells to imprint prolonged and broad innate antiviral resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The scRNA-seq data generated in the present study are publicly accessible in the Gene Expression Omnibus under accession no. GSE244126. GeoMx data generated in this study are publicly accessible under GEO accession code GSE247145. Source data are provided with this paper.
Code availability
Computer code is available upon reasonable request.
References
Pulendran, B. & Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 12, 509–517 (2011).
Aaby, P. et al. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ 311, 481 (1995).
Aaby, P. et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 204, 245–252 (2011).
Lund, N. et al. The effect of oral polio vaccine at birth on infant mortality: a randomized trial. Clin. Infect. Dis. 61, 1504–1511 (2015).
Moorlag, S. J. C. F. M., Arts, R. J. W., van Crevel, R. & Netea, M. G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 25, 1473–1478 (2019).
Faustman, D. L. et al. Multiple BCG vaccinations for the prevention of COVID-19 and other infectious diseases in type 1 diabetes. Cell Rep. Med. 3, 100728 (2022).
Hilligan, K. L. et al. Intravenous administration of BCG protects mice against lethal SARS-CoV-2 challenge. J. Exp. Med 219, e20211862 (2021).
Escobar, L. E., Molina-Cruz, A. & Barillas-Mury, C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc. Natl Acad. Sci. USA 117, 17720–17726 (2020).
O’Neill, L. A. J. & Netea, M. G. BCG-induced trained immunity: can it offer protection against COVID-19? Nat. Rev. Immunol. 20, 335–337 (2020).
Hensel, J. et al. Protection against SARS-CoV-2 by BCG vaccination is not supported by epidemiological analyses. Sci. Rep. 10, 18377 (2020).
Kaufmann, E. et al. BCG vaccination provides protection against IAV but not SARS-CoV-2. Cell Rep. 38, 110502 (2022).
Pittet, L. F. et al. Randomized trial of BCG vaccine to protect against Covid-19 in health care workers. N. Engl. J. Med. 388, 1582–1596 (2023).
Darrah, P. A. et al. Article airway T cells are a correlate of i.v. Bacille Calmette–Guerin-mediated protection against tuberculosis in rhesus macaques. Cell Host Microbe 31, 1–16 (2023).
Dijkman, K. et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med. 25, 255–262 (2019).
Kaufmann, S. H. E. Tuberculosis vaccines: time to think about the next generation. Semin. Immunol. 25, 172–181 (2013).
Cowley, S. C. & Elkins, K. L. CD4+ T cells mediate IFN-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo tuberculosis. J. Immunol. 171, 4689–4699 (2003).
Kagina, B. M. N. et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette–Guérin vaccination of newborns. Am. J. Respir. Crit. Care Med. 182, 1073–1079 (2010).
Mittrücker, H. W. et al. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc. Natl Acad. Sci. USA 104, 12434–12439 (2007).
Mata, E. et al. Pulmonary BCG induces lung-resident macrophage activation and confers long-term protection against tuberculosis. Sci. Immunol. 6, eabc2934 (2021).
Steigler, P., Verrall, A. J. & Kirman, J. R. Beyond memory T cells: mechanisms of protective immunity to tuberculosis infection. Immunol. Cell Biol. 97, 647–655 (2019).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Arts, R. J. W., Joosten, L. A. B. & Netea, M. G. Immunometabolic circuits in trained immunity. Semin. Immunol. 28, 425–430 (2016).
Cirovic, B. et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe 28, 322–334.e5 (2020).
Kleinnijenhuis, J. et al. Long-lasting effects of bcg vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 6, 152–158 (2014).
Kaufmann, E. et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190.e19 (2018).
Arts, R. J. W. et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe https://doi.org/10.1016/j.chom.2017.12.010 (2018).
Kleinnijenhuis, J. et al. Bacille Calmette–Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1202870109 (2012).
Darrah, P. A. et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 577, 95–102 (2020).
Leist, S. R. et al. A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell 183, 1070–1085.e12 (2020).
Roberts, A. et al. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 3, 0023–0037 (2007).
Menachery, V. D. et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 21, 1508–1513 (2015).
Mitroulis, I. et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell https://doi.org/10.1016/j.cell.2017.11.034 (2018).
Saraiva, M., Vieira, P. & O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 217, e20190418 (2020).
Covián, C. et al. BCG-induced cross-protection and development of trained immunity: implication for vaccine design. Front. Immunol. 10, 2806 (2019).
Israelow, B. et al. Adaptive immune determinants of viral clearance and protection in mouse models of SARS-CoV-2. Sci. Immunol. 6, eabl4509 (2021).
Li, S. et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat. Immunol. https://doi.org/10.1038/ni.2789 (2014).
Aleksander, S. A. et al. The Gene Ontology knowledge base in 2023. Genetics 224, iyad018 (2023).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29 (2000).
Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Merritt, C. R. et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat. Biotechnol. 38, 586–599 (2020).
Lee, A. et al. A molecular atlas of innate immunity to adjuvanted and live attenuated vaccines, in mice. Nat. Commun. 13, 549 (2022).
Wimmers, F. et al. The single-cell epigenomic and transcriptional landscape of immunity to influenza vaccination. Cell Press 184, 3915–3935.e21 (2021).
Lee, A., Wimmers, F. & Pulendran, B. Epigenetic adjuvants: durable reprogramming of the innate immune systemsy with adjuvants. Curr. Opin. Immunol. 77, 102189 (2022).
Singhania, A. et al. CD4+CCR6+ T cells dominate the BCG-induced transcriptional signature. eBioMedicine 74, 103746 (2021).
Dalton, D. K. et al. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259, 1739–1742 (1993).
Green, A. M., DiFazio, R. & Flynn, J. L. IFN-γ from CD4+ T cells Is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J. Immunol. 190, 270–277 (2013).
Lee, A. J. & Ashkar, A. A. The dual nature of type I and type II interferons. Front. Immunol. 9, 1–10 (2018).
Yao, Y. et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell 175, 1634–1650.e17 (2018).
Arunachalam, P. S. et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 596, 410–416 (2021).
Li, C. et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 23, 543–555 (2022).
Gu, H. et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science 369, 1603–1607 (2020).
Stegemann-Koniszewski, S. et al. Alveolar type II epithelial cells contribute to the anti-influenza A virus response in the lung by integrating pathogen- and microenvironment-derived signals. mBio 7, e00276–16 (2016).
Balachandran, S. et al. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13, 129–41 (2000).
Sims, S. H. et al. A novel interferon-inducible domain: structural and functional analysis of the human interferon regulatory factor 1 gene promoter. Mol. Cell. Biol. 13, 690–702 (1993).
de Queiroz, N. M. G. P., Marinho, F. V., de Araujo, A. C. V. S. C., Fahel, J. S. & Oliveira, S. C. MyD88-dependent BCG immunotherapy reduces tumor and regulates tumor microenvironment in bladder cancer murine model. Sci. Rep. 11, 15648 (2021).
Kaveh, D. A., Carmen Garcia-Pelayo, M. & Hogarth, P. J. Persistent BCG bacilli perpetuate CD4+ T effector memory and optimal protection against tuberculosis. Vaccine 32, 6911–6918 (2014).
Zhang, L. et al. Variable virulence and efficacy of BCG vaccine strains in mice and correlation with genome polymorphisms. Mol. Ther. 24, 398–405 (2016).
Castillo-Rodal, A. I. et al. Mycobacterium bovis BCG substrains confer different levels of protection against Mycobacterium tuberculosis infection in a BALB/c model of progressive pulmonary tuberculosis. Infect. Immun. 74, 1718–1724 (2006).
Kremenovic, M., Schenk, M. & Lee, D. J. Clinical and molecular insights into BCG immunotherapy for melanoma. J. Intern. Med. 288, 625–640 (2020).
Vierboom, M. P. M. et al. Stronger induction of trained immunity by mucosal BCG or MTBVAC vaccination compared to standard intradermal vaccination. Cell Rep. Med. 2, 100185 (2021).
Lu, X. et al. Engineered PLGA microparticles for long-term, pulsatile release of STING agonist for cancer immunotherapy. Sci. Transl. Med. 12, eaaz6606 (2020).
Zens, K. D. & Farber, D. L. Memory CD4+ T cells in influenza. Curr. Top. Microbiol. Immunol. 386, 399–421 (2015).
Asanuma, H., Sharp, M., Maecker, H. T., Maino, V. C. & Arvin, A. M. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J. Infect. Dis. 181, 859–866 (1999).
Bruxvoort, K. J. et al. Recombinant adjuvanted zoster vaccine and reduced risk of COVID-19 diagnosis and hospitalization in older adults. J. Infect. Dis. 225, 1915–1922 (2021).
Edara, V. V. et al. Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe 29, 516–521.e3 (2021).
Vanderheiden, A. et al. Type I and type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures. J. Virol. 94, e00985-20 (2020).
Vanderheiden, A. et al. CCR2 signaling restricts SARS-CoV-2 infection. mBio https://doi.org/10.1128/mBio.02749-21 (2021).
Feng, Y. et al. Broadly neutralizing antibodies against sarbecoviruses generated by immunization of macaques with an AS03-adjuvanted COVID-19 vaccine. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.adg74 (2023).
Szretter, K. J., Balish, A. L. & Katz, J. M. Influenza: propagation, quantification, and storage. Curr. Protoc. Microbiol. https://doi.org/10.1002/0471729256.mc15g01s3 (2006).
Kovacs, S. B., Oh, C., Aachoui, Y. & Miao, E. A. Evaluating cytokine production by flow cytometry using brefeldin A in mice. STAR Protoc. 2, 100244 (2021).
Hartmann, F. J. et al. Scalable conjugation and characterization of immunoglobulins with stable mass isotope reporters for single-cell mass cytometry analysis. Methods Mol. Biol. 1989, 55–81 (2019).
Fienberg, H. G., Simonds, E. F., Fantl, W. J., Nolan, G. P. & Bodenmiller, B. A platinum-based covalent viability reagent for single-cell mass cytometry. Cytometry 81A, 467–475 (2012).
Zunder, E. R. et al. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nat. Protoc. 10, 316–333 (2015).
Van Gassen, S. et al. FlowSOM: using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A87, 636–645 (2015).
Nakano, H., Nakano, K. & Cook, D. N. Isolation and purification of epithelial and endothelial cells from mouse lung. Methods Mol. Biol. 1799, 59–69 (2018).
Acknowledgements
Work in the laboratory of B.P. is supported in part by grants and contracts from the NIH (R01 AI048638, U19 AI057266, U19 AI167903 and ACC 75N93022C00052), the Bill and Melinda Gates Foundation, Open Philanthropy, the Violetta L. Horton and Soffer Endowments, and other anonymous donors to B.P. We thank P. Jagannathan for his help in obtaining the BCG TICE vaccine. We thank the Stanford Shared FACS Facility for technical support. Data were collected on instruments in the Shared FACS Facility purchased by the Parker Institute for Cancer Immunotherapy or obtained using National Institutes of Health (NIH) S10 Shared Instrument Grant (Symphony, grant no. 1S10OD026831-01). We thank the Stanford Functional Genomics Facility for technical assistance, D. Wagh and E. Kim for library preparation and J. Coller for data analysis. The sequencing data were generated with instrumentation purchased with NIH funds (grant nos. S10OD025212 and 1S10OD021763) at the Stanford Genomics Facility. We thank the Stanford Human Immune Monitoring Core for technical assistance in performing Luminex assays. We thank B. Franco from Stanford Veterinary Service Center for technical support. We acknowledge the pathological services provided by HistoWitz, Inc. and pathological analysis provided by J. Feldstein at HistoWitz, Inc. Diagrams in Figs. 1a, 4a, 5a,c, 8 and Extended Data Fig. 2a were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
A.L. and B.P. designed the study, interpreted the data and wrote the manuscript. A.L. led and planned the research and analyzed the data. K.F. and M.S.S. performed SARS-CoV-2 mouse experiments including challenge, viral load quantification and sample analysis. S.W. performed mouse injections and helped with tissue harvesting and processing. Z.F. contributed to scRNA-seq analysis. T.K.T. helped with CyTOF staining and data acquisition. D.S., A.D.R., Y.L. and A.P. contributed to and performed GeoMx. H.H. and V.L. helped with tissue harvesting and processing. H.M.F. and K.L.G. vaccinated mice for mouse-adapted coronavirus challenge. H.M.F., J.M.P., S.R.L. and M.L.H. performed viral infections, tissue harvesting and sample analysis for mouse-adapted coronavirus challenge. J.M.P. developed the mouse-adapted SARS-CoV-2 BA.5 virus. C.L. propagated the PR8 virus. R.S.B. provided advice and guidance to SARS-CoV-2 challenge experiments. P.S.A. provided guidance and advice. G.P.N. provided CyTOF instrument.
Corresponding author
Ethics declarations
Competing interests
B.P. has served or is serving on the External Immunology Network of GSK and on the scientific advisory boards of Sanofi, Medicago, CircBio and Boehringer-Ingelheim. R.S.B. serves on the Scientific Advisory Board of Takeda, Vaxart, Inc. and Invivyd, and has collaborations with Janssen Pharmaceuticals, Gilead, Chimerix and Pardes Biosciences. S.R.L. and R.S.B. are listed on a patent for the SARS-CoV-2 MA10 virus (US 11225508 B1, ‘Mouse-adapted SARS-CoV-2 viruses and methods of use thereof’). G.P.N is a co-founder and stockholder of Ionpath Inc., a co-founder and stockholder of Akoya Biosciences, Inc. and an inventor on patent US9909167., a Scientific Advisory Board member for Akoya Biosciences, Inc., received research grants from Pfizer, Inc., Vaxart, Inc., Celgene, Inc. and Juno Therapeutics, Inc. and is a co-founder of Scale Biosciences Inc. M.S.S. has served in an advisory role for Ocugen, Inc. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Rino Rappuoli, Maria Rescigno and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jamie Wilson, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
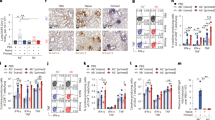
Extended Data Fig. 1 BCG delivered via multiple routes of vaccination conferred protection against SARS-CoV-2 and influenza A PR8.
a, Representative immunohistochemisry images depicting staining of SARS-CoV-2 nucleocapsid protein (NP). Blue circles represent expression of SARS-CoV-2 NP in focal alveoli, alveolar macrophages, alveolar pneumocytes and rare bronchiolar epithelial cells. BCG IV: Intravenous BCG vaccinated. b, Left, weight loss of intranasal BCG vaccinated mice followed 3 days post-SARS-CoV-2 challenge. Right, SARS-CoV-2 RNA-dependent RNA polymerse (RdRp) viral load as fold-change over mock-infected, in the lungs and nasal turbinates. Statistical analysis was performed by two-tailed Mann-Whitney test. c, Survival plot and weight loss of mice following PR8 infection. Mice were vaccinated via various routes including intravenous (IV), intranasal (IN), intramuscular (IM), subcutaneous (SC). BCG IV and IN, data combined from 2 independent experiments, n = 11 (BCG IV), n = 12 (BCG IN); BCG IM, data combined from 3 independent experiments (n = 16 for naïve and n = 17 for BCG IM). BCG SC, data from one independent experiment (n = 5). Survival analysis was performed using log-rank (Mantel-Cox) test. Statistical analysis for weight loss was performed by Two-way ANOVA with Sidak’s multiple comparisons test. **, P < 0.01.
Extended Data Fig. 2 BCG vaccination protects mice from weight loss against various strains of mouse-adapted Sarbecoviruses.
a, Experimental outline and weight loss of mice following challenge. b, Gross lung discoloration score at day 2 or 4 post-challenge. c, Lung viral titer at day 2 or 4 post-challenge, as assessed by plaque assay. Data representative of one independent experiment; In a, b, c, for SHC014 MA15, n = 8 (BCG); For SARS-CoV MA15 n = 9 (BCG); n = 7 for all other groups. Mean±SEM values are plotted. Statistical analysis was performed by Two-way ANOVA with Sidak’s multiple comparisons test. *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.0001.
Extended Data Fig. 3 BCG induced prolonged serum cytokine and lung innate activation.
a, Extended data of kinetics of serum cytokine responses measured by Luminex. Data representative of two independent experiments; n = 10 (day 0), n = 4 (day 21), n = 5 (all other time points). b, Flow cytometry gating strategy of lung populations (Top) and cell frequencies (CD45+ live cells) of innate cells in lungs (Bottom). Data is representative of two independent experiments for day 21 and one representative experiment for other timepoints (n = 5-8). Data representative of two independent experiments at day 21 (n = 4) and one independent experiment for all other timepoints; n = 9 (day 0), n = 5 for all other timepoints. Statistical analysis was performed by One-way ANOVA with Tukey’s multiple comparisons test (a), One-way ANOVA with Dunnett’s multiple comparison test for each timepoint compared to day 0 (b). *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.0001.
Extended Data Fig. 4 BCG induced systemic innate activation and bone marrow stem and progenitor expansion.
a, Flow cytometry gating strategy, cell frequencies (CD45+ live cells), and CD86 MFI of innate cells in spleen. Data is representative of two independent experiments for day 21 and one representative experiment for other timepoints; n = 4 for day 21, n = 8 (day 0), n = 5 for all other timepoints. b, Flow cytometry gating strategy and frequencies of bone marrow cells (% of live cells). Data from one representative experiment. Abbreviations, LSK: Lin−Sca-1+c-Kit+ cells, LT-HSC: Long-term HSC. ST-HSC: Short-term HSC. MPP: Multipotent progenitor. One-way ANOVA with Dunnett’s multiple comparison test for each timepoint compared to day 0 (a and b). *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.0001.
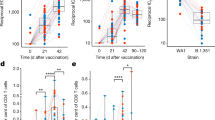
Extended Data Fig. 5 Extended data on CyTOF analysis in the spleen.
UMAP embedding of the cell clusters identified by FlowSOM clustering (left), heatmap depicting the normalized values of key surface markers across identified clusters (right). (bottom) Frequencies of CD4+ T cell and Ly6Chi monocyte in the spleen and normalized and scaled values of intracellular markers detected by CyTOF. The box plots show median, first and third quartiles and the whiskers show 1.5x interquartile range (IQR) on either side. Statistical analysis was performed by two-tailed Wilcoxon rank-sum test. *, P < 0.05; ***, P < 0.005.
Extended Data Fig. 6 Differential immune responses to BCG vaccination in knock-out (KO) mice.
a and b, Serum cytokine responses in wild-type (WT) and Myd88−/− mice vaccinated with BCG intravenously at baseline, 6 h and 18 days post-vaccination (a), and day 3 post-SARS-CoV-2 challenge (b). c and d, Serum cytokine responses in wild-type (WT) and ASC−/− (c) and STING−/− (d) mice vaccinated with BCG intravenously. Data representative of two independent experiments for Myd88−/− and ASC−/− (n = 6). Two-way ANOVA with Tukey’s multiple comparison test (panel b, e, f). Multiple Mann-Whitney with Holm-Sidak multiple comparisons test (panel c, d). *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
Extended Data Fig. 7 SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) level in CD4 and/or CD8 antibody depleted mice.
Viral load was measured at day 3 post-challenge by quantitative PCR and is represented as fold-change over mock-infected mice (mean±SEM). Data representative of one independent experiment, n = 6. Statistical analysis by two-tailed Mann-Whitney test; ns, not significant.
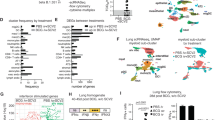
Extended Data Fig. 8 Extended data from scRNA-seq analysis of lungs in mice at day 0 and 21 post-vaccination.
a, Top variable genes defining cell clusters identified from scRNA-seq data. b, Distribution of cells identified day 0 and 21 post-vaccination on the UMAP embedding. c, Overrepresentation analysis and enrichment of Gene Ontology (GO) biological processes across identified cell clusters, using differentially expressed genes (DEGs; log2FC cutoff > 0.25; FDR cutoff < 0.05). Enrichment was performed using hypergeometric distribution with BH correction.
Extended Data Fig. 9 Protein level expression of interferon-stimulated gene (Mx1) and IFN-γ in lung cells, and CD4+ T cells, respectively.
a, Mx1 median fluorescent intensity (MFI) of lung myeloid and alveolar type II epithelial cells detected using Mx1-GFP reporter mice at various timepoints post-vaccination with BCG intravenously (n = 3-5). Data representative of two independent experiments in lung myeloid cells and one representative experiment in alveolar type II cells; n = 5 for day 21, n = 4 for all other timepoints. b, Frequency of IFN-g+ cells (% of cell population of interest) in vivo, as measured by flow cytometry staining 6 h following Brefeldin A injection in mice. Data from one representative experiment; n = 7 for BCG, n = 4 for Unimmunized. Statistical analysis was performed by Two-way ANOVA with Dunnet’s test (a; myeloid cells) Sidak’s multiple comparisons test (b), and with two-tailed Mann-Whitney test (a; alveolar type II cells). *, P < 0.01; ****, P < 0.0001.
Extended Data Fig. 10 Extended data showing impaired activation of lung myeloid cells following CD4+ T cell or IFN-γ depletion.
CD86 median fluorescence intensity (MFI) of DCs in the lungs at day 21 post-BCG IV vaccination following CD4 depletion (top) and IFN-g depletion (bottom). Data representative of one independent experiment; n = 5 (BCG+Isotype), n = 6 (Unvaccinated and BCG+anti-CD4), n = 9 (BCG) in CD4 depletion; n = 4 (Unvaccinated) and n = 5 (rest of the groups) in IFN-g depletion. Statistical analysis was performed by One-way ANOVA with Tukey’s multiple comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.0001.
Supplementary information
Supplementary Table 1
Metal-conjugated antibody panel used for CyTOF.
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Fig. 5
Source data for Fig. 5.
Source Data Fig. 6
Source data for Fig. 6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, A., Floyd, K., Wu, S. et al. BCG vaccination stimulates integrated organ immunity by feedback of the adaptive immune response to imprint prolonged innate antiviral resistance. Nat Immunol 25, 41–53 (2024). https://doi.org/10.1038/s41590-023-01700-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01700-0
This article is cited by
-
BCG-mediated viral protection goes biphasic
Nature Immunology (2024)
-
Integrated organ immunity: a path to a universal vaccine
Nature Reviews Immunology (2024)