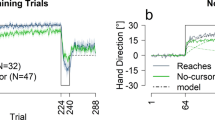
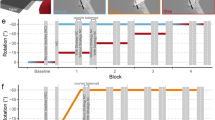
Abstract
Sports are replete with strategies, yet coaching lore often emphasizes ‘quieting the mind’, ‘trusting the body’ and ‘avoiding overthinking’ in referring to the importance of relying less on high-level explicit strategies in favor of low-level implicit motor learning. We investigated the interactions between explicit strategy and implicit motor adaptation by designing a sensorimotor learning paradigm that drives adaptive changes in some dimensions but not others. We find that strategy and implicit adaptation synergize in driven dimensions, but effectively cancel each other in undriven dimensions. Independent analyses—based on time lags, the correlational structure in the data and computational modeling—demonstrate that this cancellation occurs because implicit adaptation effectively compensates for noise in explicit strategy rather than the converse, acting to clean up the motor noise resulting from low-fidelity explicit strategy during motor learning. These results provide new insight into why implicit learning increasingly takes over from explicit strategy as skill learning proceeds.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data generated and analyzed in the current study are available from the corresponding author upon reasonable request.
Code availability
All analysis code is available from the corresponding author upon reasonable request.
References
Ellis, N. Rules and instances in foreign language learning: interactions of explicit and implicit knowledge. Eur. J. Cogn. Psychol. 5, 289–318 (1993).
Gugerty, L. J. Situation awareness during driving: explicit and implicit knowledge in dynamic spatial memory. J. Exp. Psychol. Appl. 3, 42–66 (1997).
Schendan, H. E., Searl, M. M., Melrose, R. J. & Stern, C. E. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron 37, 1013–1025 (2003).
Taylor, J. A., Krakauer, J. W. & Ivry, R. B. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J. Neurosci. 34, 3023–3032 (2014).
Kahneman, D. & Egan, P. Thinking, Fast and Slow Vol. 1 (Farrar, Straus and Giroux New York, 2011).
Mazzoni, P. & Krakauer, J. W. An implicit plan overrides an explicit strategy during visuomotor adaptation. J. Neurosci. 26, 3642–3645 (2006).
Taylor, J. A., Klemfuss, N. M. & Ivry, R. B. An explicit strategy prevails when the cerebellum fails to compute movement errors. Cerebellum 9, 580–586 (2010).
Benson, B. L., Anguera, J. A. & Seidler, R. D. A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. J. Neurophysiol. 105, 2843–2851 (2011).
Bond, K. M. & Taylor, J. A. Flexible explicit but rigid implicit learning in a visuomotor adaptation task. J. Neurophysiol. 113, 3836–3849 (2015).
Krakauer, J. W., Pine, Z. M., Ghilardi, M.-F. & Ghez, C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J. Neurosci. 20, 8916–8924 (2000).
Krakauer, J. W. in Progress in Motor Control Vol. 629 (ed. Sternad, D.) 405–421 (Springer US, 2009).
Brayanov, J. B., Press, D. Z. & Smith, M. A. Motor memory is encoded as a gain-field combination of intrinsic and extrinsic action representations. J. Neurosci. 32, 14951–14965 (2012).
Wu, H. G. & Smith, M. A. The generalization of visuomotor learning to untrained movements and movement sequences based on movement vector and goal location remapping. J. Neurosci. 33, 10772–10789 (2013).
Von Helmholtz, H. Handbuch der Physiologischen Optik Vol. 9 (Voss, 1867).
Held, R. & Freedman, S. J. Plasticity in human sensorimotor control. Science 142, 455–462 (1963).
Shadmehr, R. & Mussa-Ivaldi, F. A. Adaptive representation of dynamics during learning of a motor task. J. Neurosci. 14, 3208–3224 (1994).
Smith, M. A. & Shadmehr, R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J. Neurophysiol. 93, 2809–2821 (2005).
Smith, M. A., Ghazizadeh, A. & Shadmehr, R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 4, e179 (2006).
Joiner, W. M. & Smith, M. A. Long-term retention explained by a model of short-term learning in the adaptive control of reaching. J. Neurophysiol. 100, 2948–2955 (2008).
Wagner, M. J. & Smith, M. A. Shared internal models for feedforward and feedback control. J. Neurosci. 28, 10663–10673 (2008).
Sing, G. C., Joiner, W. M., Nanayakkara, T., Brayanov, J. B. & Smith, M. A. Primitives for motor adaptation reflect correlated neural tuning to position and velocity. Neuron 64, 575–589 (2009).
Kagerer, F. A., Contreras-Vidal, J. L. & Stelmach, G. E. Adaptation to gradual as compared with sudden visuo-motor distortions. Exp. Brain Res. 115, 557–561 (1997).
Taylor, J. A., Wojaczynski, G. J. & Ivry, R. B. Trial-by-trial analysis of intermanual transfer during visuomotor adaptation. J. Neurophysiol. 106, 3157–3172 (2011).
Alhussein, L., Hosseini, E. A., Nguyen, K. P., Smith, M. A. & Joiner, W. M. Dissociating effects of error size, training duration, and amount of adaptation on the ability to retain motor memories. J. Neurophysiol. 122, 2027–2042 (2019).
Taylor, J. A. & Ivry, R. B. Flexible cognitive strategies during motor learning. PLoS Comput. Biol. 7, e1001096 (2011).
Jakobson, L. S. & Goodale, M. A. Trajectories of reaches to prismatically-displaced targets: evidence for ‘automatic’ visuomotor recalibration. Exp. Brain Res. 78, 575–587 (1989).
Hudson, T. E. & Landy, M. S. Measuring adaptation with a sinusoidal perturbation function. J. Neurosci. Methods 208, 48–58 (2012).
Kettner, R. E. et al. Prediction of complex two-dimensional trajectories by a cerebellar model of smooth pursuit eye movement. J. Neurophysiol. 77, 2115–2130 (1997).
Stein, C. M., Morris, N. J. & Nock, N. L. Structural equation modeling. Methods Mol. Biol. 850, 495–512 (2017).
de Marco, G. et al. Principle of structural equation modeling for exploring functional interactivity within a putative network of interconnected brain areas. Magn. Reson. Imaging 27, 1–12 (2009).
Harris, C. M. & Wolpert, D. M. Signal-dependent noise determines motor planning. Nature 394, 780–784 (1998).
Jones, K. E., Hamilton, A. F. C. & Wolpert, D. M. Sources of signal-dependent noise during isometric force production. J. Neurophysiol. 88, 1533–1544 (2002).
Shadmehr, R., Smith, M. A. & Krakauer, J. W. Error correction, sensory prediction, and adaptation in motor control. Annu. Rev. Neurosci. 33, 89–108 (2010).
Krakauer, J. W. & Mazzoni, P. Human sensorimotor learning: adaptation, skill, and beyond. Curr. Opin. Neurobiol. 21, 636–644 (2011).
Hadjiosif, A. et al. Cerebellar damage reduces the stability of motor memories. in Proc. Transl. Comput. Mot. Control https://sites.google.com/site/acmcconference/2014/30.pdf (2014).
Haith, A. M., Huberdeau, D. M. & Krakauer, J. W. The influence of movement preparation time on the expression of visuomotor learning and savings. J. Neurosci. 35, 5109–5117 (2015).
Fitts, P. M. & Posner, M. I. Human Performance (Brooks/Cole, 1967).
Anderson, J. R. Acquisition of cognitive skill. Psychol. Rev. 89, 369–406 (1982).
Beilock, S. L., Wierenga, S. A. & Carr, T. H. Expertise, attention, and memory in sensorimotor skill execution: impact of novel task constraints on dual-task performance and episodic memory. Q. J. Exp. Psychol. Sect. A 55, 1211–1240 (2002).
Gray, R. Attending to the execution of a complex sensorimotor skill: expertise differences, choking, and slumps. J. Exp. Psychol. Appl. 10, 42–54 (2004).
Jackson, R. C., Ashford, K. J. & Norsworthy, G. Attentional focus, dispositional reinvestment, and skilled motor performance under pressure. J. Sport Exerc. Psychol. 28, 49–68 (2006).
Flegal, K. E. & Anderson, M. C. Overthinking skilled motor performance: or why those who teach can’t do. Psychon. Bull. Rev. 15, 927–932 (2008).
Poldrack, R. A. et al. Interactive memory systems in the human brain. Nature 414, 546–550 (2001).
Poldrack, R. A. & Packard, M. G. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia 41, 245–251 (2003).
Packard, M. G., Hirsh, R. & White, N. M. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J. Neurosci. 9, 1465–1472 (1989).
Boyd, L. A. & Winstein, C. J. Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learn. Mem. 11, 388–396 (2004).
Karni, A. et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc. Natl Acad. Sci. USA 95, 861–868 (1998).
Martin, T. A., Keating, J. G., Goodkin, H. P., Bastian, A. J. & Thach, W. T. Throwing while looking through prisms: I. Focal olivocerebellar lesions impair adaptation. Brain 119, 1183–1198 (1996).
Diedrichsen, J., Verstynen, T., Lehman, S. L. & Ivry, R. B. Cerebellar involvement in anticipating the consequences of self-produced actions during bimanual movements. J. Neurophysiol. 93, 801–812 (2005).
Breska, A. & Ivry, R. B. Double dissociation of single-interval and rhythmic temporal prediction in cerebellar degeneration and Parkinson’s disease. Proc. Natl Acad. Sci. USA 115, 12283–12288 (2018).
Bollen, K. Structural Equations with Latent Variables (John Wiley, 1989).
Kline, R. B. Principles and Practice of Structural Equation Modeling (Guilford Publications, 2015).
Rosseel, Y. lavaan: an R package for structural equation modeling. J. Stat. Softw. 48, 1–36 (2012).
Acknowledgements
The authors thanks A. Brennan and L. Alhussein for helpful discussions. This work was supported by the National Institutes of Health (NIH) grants R01 AG041878 and R01 NS105839 to M.A.S.
Author information
Authors and Affiliations
Contributions
Y.R.M., S.W. and M.A.S. designed the experiments. Y.R.M. and M.A.S. analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Joern Diedrichsen, Ned Jenkinson, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The presence of time lag between adaptive responses is necessary to suppress the combined learning response at the perturbation-free frequencies in a two-process linear system.
Left panel: Error amplitude resulting from the combination of both processes as a function of the relative noise level between processes. Right panel: Correlation between time series of processes when the processes are shifted from each other by −1, 0, or 1 trial. Black line indicates Lag-0 correlation. Blue line indicates Lag-1 correlation, that is corr(x2(n-1), x1(n)). Red line indicates Lead-1 correlation, that is corr(x2(n + 1), vs x1(n)). Thus the blue line being below the black & red indicates that x2 lags x1.
Extended Data Fig. 2 Simulation of two processes using same noise levels between processes and independently distributed learning rate parameters across participants.
This simulation removes both the difference in noise levels and the across-participant learning rate anti-correlation from the simulation shown in Fig. 6 in the main paper. A_1=0.9, B_1=0.34+x_i, eps_1=1.75, A_2=0.9, B_2=0.34+y_i, eps_2=1.75, where x_i, y_i ~ Unif(−0.16, 0.16). Panels analogous to those in Fig. 6b, c in the manuscript. Upper left: simulated perturbation-driven learning curves for one example, simulated individual. Upper right: Histogram of simulated (n=69) within-participant correlations between perturbation-driven strategic and implicit learning curves. Lower left: analogous to upper left, but for perturbation-free learning curves. Lower right: analogous to upper right, but for perturbation-free learning curves. Please note that Supplementary Fig 1b on the following page presents panels analogous to those in Fig. 6d-f in the manuscript. Fig E1b. Simulation of two processes using same noise levels between processes and independently distributed learning rate parameters across participants (Like Supplementary Fig. 1a). This removes both the difference in noise levels and the across-participant learning rate anti-correlation from the simulation shown in Fig. 6 in the main paper. A_1=0.9, B_1=0.34+x_i, eps_1=1.75, A_2=0.9, B_2=0.34+y_i, eps_2=1.75, where x_i, y_i ~ Unif(−0.16, 0.16). Panels analogous to those in Fig. 6d–f in the manuscript. We performed 100 runs of the simulation, each with n=69. Top 2-by-2 panels: inter-individual relationships among perturbation-driven & perturbation-free strategic and implicit learning from one run of the simulation. Bottom left: log-likelihoods from the SEM analysis across 100 simulations (error bars indicate 95% CI, calculated across 100 simulations). Bottom right: histogram of lags from one run of the simulation. Text on histogram indicates the fraction of the 100 simulation runs for which the mean lag was greater than 0.
Extended Data Fig. 4 Simulation of two processes using different noise levels between processes and independently distributed learning rate parameters across participants.
This simulation removes the across-participant learning rate anti-correlation from the simulation shown in the main paper. A_1 = 0.9, B_1 = 0.34 + x_i, eps_1 = 1.5, A_2 = 0.9, B_2 = 0.34 + y_i, eps_2 = 2, where x_i, y_i ~ Unif(−0.16, 0.16). Panels analogous to those of Supplementary Fig. 2, 1b, and Fig. 6d–f in the main paper.
Supplementary information
Supplementary Information
Supplementary Math Note.
Rights and permissions
About this article
Cite this article
Miyamoto, Y.R., Wang, S. & Smith, M.A. Implicit adaptation compensates for erratic explicit strategy in human motor learning. Nat Neurosci 23, 443–455 (2020). https://doi.org/10.1038/s41593-020-0600-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0600-3
This article is cited by
-
NSF DARE—transforming modeling in neurorehabilitation: a patient-in-the-loop framework
Journal of NeuroEngineering and Rehabilitation (2024)
-
Prediction error in implicit adaptation during visually- and memory-guided reaching tasks
Scientific Reports (2024)
-
Large-scale citizen science reveals predictors of sensorimotor adaptation
Nature Human Behaviour (2024)
-
Adapting to visuomotor rotations in stepped increments increases implicit motor learning
Scientific Reports (2023)
-
An implicit memory of errors limits human sensorimotor adaptation
Nature Human Behaviour (2021)