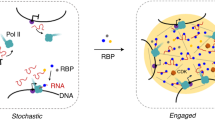
Abstract
A universal characteristic of eukaryotic transcription is that the promoter recruits RNA polymerase II (RNAPII) to produce both precursor mRNAs (pre-mRNAs) and short unstable promoter upstream transcripts (PROMPTs) toward the opposite direction. However, how the transcription machinery selects the correct direction to produce pre-mRNAs is largely unknown. Here, through multiple acute auxin-inducible degradation systems, we show that rapid depletion of an RNAPII-binding protein complex, Integrator, results in robust PROMPT accumulation throughout the genome. Interestingly, the accumulation of PROMPTs is compensated by the reduction of pre-mRNA transcripts in actively transcribed genes. Consistently, Integrator depletion alters the distribution of polymerase between the sense and antisense directions, which is marked by increased RNAPII-carboxy-terminal domain Tyr1 phosphorylation at PROMPT regions and a reduced Ser2 phosphorylation level at transcription start sites. Mechanistically, the endonuclease activity of Integrator is critical to suppress PROMPT production. Furthermore, our data indicate that the presence of U1 binding sites on nascent transcripts could counteract the cleavage activity of Integrator. In this process, the absence of robust U1 signal at most PROMPTs allows Integrator to suppress the antisense transcription and shift the transcriptional balance in favor of the sense direction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All sequencing data generated as a part of this study have been deposited to NCBI GEO under accession numbers GSE207268 and GSE207269 (NCBI tracking system no. 23081142). Source data are provided with this paper.
Code availability
All pipelines and customized codes are available on GitHub (https://github.com/Berialim/PROMPTs_analysis_visualization)77. Further information and requests for resources and reagents generated in this study are available upon request.
References
Scruggs, B. S. et al. Bidirectional transcription arises from two distinct hubs of transcription factor binding and active chromatin. Mol. Cell 58, 1101–1112 (2015).
Adelman, K. & Lis, J. T. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat. Rev. Genet. 13, 720–731 (2012).
Rhee, H. S. & Pugh, B. F. Genome-wide structure and organization of eukaryotic pre-initiation complexes. Nature 483, 295–301 (2012).
Seila, A. C. et al. Divergent transcription from active promoters. Science 322, 1849–1851 (2008).
Preker, P. et al. RNA exosome depletion reveals transcription upstream of active human promoters. Science 322, 1851–1854 (2008).
Core, L. J., Waterfall, J. J. & Lis, J. T. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322, 1845–1848 (2008).
Core, L. J. et al. Analysis of nascent RNA identifies a unified architecture of initiation regions at mammalian promoters and enhancers. Nat. Genet. 46, 1311–1320 (2014).
Kim, T. K. et al. Widespread transcription at neuronal activity-regulated enhancers. Nature 465, 182–187 (2010).
De Santa, F. et al. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 8, e1000384 (2010).
Sigova, A. A. et al. Divergent transcription of long noncoding RNA/mRNA gene pairs in embryonic stem cells. Proc. Natl Acad. Sci. USA 110, 2876–2881 (2013).
Djebali, S. et al. Landscape of transcription in human cells. Nature 489, 101–108 (2012).
Almada, A. E., Wu, X., Kriz, A. J., Burge, C. B. & Sharp, P. A. Promoter directionality is controlled by U1 snRNP and polyadenylation signals. Nature 499, 360–363 (2013).
Chiu, A. C. et al. Transcriptional pause sites delineate stable nucleosome-associated premature polyadenylation suppressed by U1 snRNP. Mol. Cell 69, 648–663.e7 (2018).
Baillat, D. et al. Integrator, a multiprotein mediator of small nuclear RNA processing, associates with the C-terminal repeat of RNA polymerase II. Cell 123, 265–276 (2005).
Welsh, S. A. & Gardini, A. Genomic regulation of transcription and RNA processing by the multitasking Integrator complex. Nat. Rev. Mol. Cell Biol. 24, 204–220 (2023).
Offley, S. R. et al. A combinatorial approach to uncover an additional Integrator subunit. Cell Rep. 42, 112244 (2023).
Elrod, N. D. et al. The Integrator complex attenuates promoter-proximal transcription at protein-coding genes. Mol. Cell 76, 738–752.e7 (2019).
Tatomer, D. C. & Wilusz, J. E. Attenuation of eukaryotic protein-coding gene expression via premature transcription termination. Cold Spring Harb. Symp. Quant. Biol. 84, 83–93 (2019).
Lykke-Andersen, S. et al. Integrator is a genome-wide attenuator of non-productive transcription. Mol. Cell 81, 514–529.e6 (2021).
Lai, F., Gardini, A., Zhang, A. & Shiekhattar, R. Integrator mediates the biogenesis of enhancer RNAs. Nature 525, 399–403 (2015).
Rubtsova, M. P. et al. Integrator is a key component of human telomerase RNA biogenesis. Sci. Rep. 9, 1701 (2019).
Barra, J. et al. Integrator restrains paraspeckles assembly by promoting isoform switching of the lncRNA NEAT1. Sci. Adv. 6, eaaz9072 (2020).
Cazalla, D., Xie, M. & Steitz, J. A. A primate herpesvirus uses the Integrator complex to generate viral microRNAs. Mol. Cell 43, 982–992 (2011).
Zheng, H. et al. Identification of Integrator-PP2A complex (INTAC), an RNA polymerase II phosphatase. Science 370, eabb5872 (2020).
Fianu, I. et al. Structural basis of Integrator-mediated transcription regulation. Science 374, 883–887 (2021).
Stein, C. B. et al. Integrator endonuclease drives promoter-proximal termination at all RNA polymerase II-transcribed loci. Mol. Cell 82, 4232–4245.e11 (2022).
Huang, K. L. et al. Integrator recruits protein phosphatase 2A to prevent pause release and facilitate transcription termination. Mol. Cell 80, 345–358.e9 (2020).
Vervoort, S. J. et al. The PP2A-Integrator–CDK9 axis fine-tunes transcription and can be targeted therapeutically in cancer. Cell 184, 3143–3162.e32 (2021).
Hu, S. et al. INTAC endonuclease and phosphatase modules differentially regulate transcription by RNA polymerase II. Mol. Cell 83, 1588–1604 (2023).
Gardini, A. et al. Integrator regulates transcriptional initiation and pause release following activation. Mol. Cell 56, 128–139 (2014).
Yue, J. et al. Integrator orchestrates RAS/ERK1/2 signaling transcriptional programs. Genes Dev. 31, 1809–1820 (2017).
Beckedorff, F. et al. The human Integrator complex facilitates transcriptional elongation by endonucleolytic cleavage of nascent transcripts. Cell Rep. 32, 107917 (2020).
Wang, H. et al. H3K4me3 regulates RNA polymerase II promoter-proximal pause-release. Nature 615, 339–348 (2023).
Stadelmayer, B. et al. Integrator complex regulates NELF-mediated RNA polymerase II pause/release and processivity at coding genes. Nat. Commun. 5, 5531 (2014).
Skaar, J. R. et al. The Integrator complex controls the termination of transcription at diverse classes of gene targets. Cell Res 25, 288–305 (2015).
Dasilva, L. F. et al. Integrator enforces the fidelity of transcriptional termination at protein-coding genes. Sci. Adv. 7, eabe3393 (2021).
Shersher, E. et al. NACK and INTEGRATOR act coordinately to activate Notch-mediated transcription in tumorigenesis. Cell Commun. Signal. 19, 96 (2021).
Nishimura, K., Fukagawa, T., Takisawa, H., Kakimoto, T. & Kanemaki, M. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat. Methods 6, 917–922 (2009).
Natsume, T., Kiyomitsu, T., Saga, Y. & Kanemaki, M. T. Rapid protein depletion in human cells by auxin-inducible degron tagging with short homology donors. Cell Rep. 15, 210–218 (2016).
Yesbolatova, A. et al. The auxin-inducible degron 2 technology provides sharp degradation control in yeast, mammalian cells, and mice. Nat. Commun. 11, 5701 (2020).
Bhatt, D. M. et al. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell 150, 279–290 (2012).
Wu, Y., Albrecht, T. R., Baillat, D., Wagner, E. J. & Tong, L. Molecular basis for the interaction between Integrator subunits IntS9 and IntS11 and its functional importance. Proc. Natl Acad. Sci. USA 114, 4394–4399 (2017).
Lourenco, C. et al. MYC protein interactors in gene transcription and cancer. Nat. Rev. Cancer 21, 579–591 (2021).
Lawson, K. A. et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 13, 424–436 (1999).
Duttke, S. H. C. et al. Human promoters are intrinsically directional. Mol. Cell 57, 674–684 (2015).
Marquardt, S. et al. A chromatin-based mechanism for limiting divergent noncoding transcription. Cell 157, 1712–1723 (2014).
Schwalb, B. et al. TT-seq maps the human transient transcriptome. Science 352, 1225–1228 (2016).
Phatnani, H. P. & Greenleaf, A. L. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 20, 2922–2936 (2006).
Hsin, J. P., Li, W., Hoque, M., Tian, B. & Manley, J. L. RNAP II CTD tyrosine 1 performs diverse functions in vertebrate cells. eLife 3, e02112 (2014).
Descostes, N. et al. Tyrosine phosphorylation of RNA polymerase II CTD is associated with antisense promoter transcription and active enhancers in mammalian cells. eLife 3, e02105 (2014).
Lai, F., Damle, S. S., Ling, K. K. & Rigo, F. Directed RNase H cleavage of nascent transcripts causes transcription termination. Mol. Cell 77, 1032–1043.e4 (2020).
Bennett, C. F. & Swayze, E. E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 50, 259–293 (2010).
Roberts, T. C., Langer, R. & Wood, M. J. A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 19, 673–694 (2020).
Colgan, D. F. & Manley, J. L. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 11, 2755–2766 (1997).
Ryan, K., Calvo, O. & Manley, J. L. Evidence that polyadenylation factor CPSF-73 is the mRNA 3′ processing endonuclease. RNA 10, 565–573 (2004).
Mimoso, C. A. & Adelman, K. U1 snRNP increases RNA Pol II elongation rate to enable synthesis of long genes. Mol. Cell 83, 1264–1279.e10 (2023).
Kaida, D. et al. U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature 468, 664–668 (2010).
Davidson, L. et al. Rapid depletion of DIS3, EXOSC10, or XRN2 reveals the immediate impact of exoribonucleolysis on nuclear RNA metabolism and transcriptional control. Cell Rep. 26, 2779–2791.e5 (2019).
Berg, M. G. et al. U1 snRNP determines mRNA length and regulates isoform expression. Cell 150, 53–64 (2012).
So, B. R. et al. A complex of U1 snRNP with cleavage and polyadenylation factors controls telescripting, regulating mRNA transcription in human cells. Mol. Cell 76, 590–599.e4 (2019).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general-purpose read summarization program. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 1–21 (2014).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, 1–9 (2008).
Gregersen, L. H., Mitter, R. & Svejstrup, J. Q. Using TTchem-seq for profiling nascent transcription and measuring transcript elongation. Nat. Protoc. 15, 604–627 (2020).
Mata, J. & Wise, J. A. 4-Thiouridine labeling to analyze mRNA turnover in Schizosaccharomyces pombe. Cold Spring Harb. Protoc. https://doi.org/10.1101/pdb.prot091645 (2017).
Wang, X. et al. RPAP2 regulates a transcription initiation checkpoint by inhibiting assembly of pre-initiation complex. Cell Rep. 39, 110732 (2022).
Lovén, J. et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 (2013).
Whyte, W. A. et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 (2013).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Grant, C. E., Bailey, T. L. & Noble, W. S. FIMO: scanning for occurrences of a given motif. Bioinformatics 27, 1017–1018 (2011).
Yeo, G. & Burge, C.B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J. Comput. Biol. 11, 377–394 (2004).
PROMPT-Finder. GitHub https://github.com/Berialim/PROMPTS_analysis_visualization (2024).
Acknowledgements
We thank the Lai and Dang lab members for constructive discussions and help with genome-wide sequencing. We also acknowledge the Chen lab at the Institute of Biomedical Research at Yunnan University for their help with high-throughput sequencing. Thanks to Z. Xue for the technical help and discussions. This work was supported by grants from the Yunnan Province Science and Technology Department (202201BF070001-015 to F.L.), the National Natural Science Foundation of China (32070626 to F.L., 32171262 and 31871254 to Y.D.), the Open Research Program of State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan (2021KF002 to F.L.) and startup funds from Yunnan University for F.L. and Y.D.
Author information
Authors and Affiliations
Contributions
F.L. and Y.D. conceived and supervised the overall project. J. Yang performed most of the experiments under the supervision of G.R. J.L. performed bioinformatics analysis. J.L. and J. Yang prepared the figures under the supervision of F.L. L.M. and X.G. generated the cell line with 3×U1 sequences. W.S. generated the CPSF73-AID2 cell lines and performed the ChromRNA-seq. L.M. performed the TT-seq. S.L. generated the HCT116:TIR1(F74A) TET-ON cell line. B.P. performed the cut-tag experiments for RNAPII and helped with the INTS11 overexpression experiments. B.P., Z.L. and W.S. generated the INTS9 cell line and performed the INTS9 ChromRNA-seq. S.C., X.G., B.W., A.D., D.H. and J. Yuan prepared all the samples for the high-throughput sequencing. F.L., Y.D., J. Yang and J.L. wrote the paper with critical feedback from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Dimitris Typas, in collaboration with the Nature Structural & Molecular Biology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Correlation of PROMPT accumulation under INTS11 or INTS9 depletion.
a. Western blot representing the protein levels of INTS11/9 in wild-type and engineered cells from three biological independent experiments with the similar results. * represents the unspecific bands. b-c. Heatmap (b) and metagene analysis (c) showing HCT116-TIR1 untagged cell ChromRNA-seq results for 10428 genes and their PROMPTs in a 10 kb window around TSS site. “CTRL” represents the untreated control, and “+IAA” represents the treatment with 500 µM IAA. d. Box plots of the expression levels of 7070 PROMPTs and pre-mRNA with/without IAA treatment in HCT116-TIR1 cell line. The two-sided Mann-Whitney test was used for statistical analyses. n.s., not significant. Boxplot shows the minimum and maximum values, the median, and the interquartile range (IQR). The IQR is represented by the box, with the lower quartile (25th percentile) at the bottom and the upper quartile (75th percentile) at the top. Whiskers extend from the box, indicating the range of data points within a certain distance from the IQR. Outliers, values outside the whiskers, were excluded for better visualization. e. Quantitative RT-PCR of RNU11, MYC and CCND1 genes in HCT116-TIR1 cell line treated with 500 µM IAA (top) or 10 µM 5-ph-IAA (bottom). Data are presented as mean ± SD. P values were calculated by unpaired t-test (n = 3 independent experiments). n.s., not significant. (*) p < 0.05. (**) p < 0.01. f-g. IGV tracks of RNU11 and RNU12 loci from INTS11 (f) or INTS9 (g) ChromRNA-seq results. h. Heatmap of 7070 genes and their PROMPTs from ChromRNA-seq with or without INTS9 depletion (left). An overlaying heatmap of +IAA/CTRL is shown on the right. Upregulation is shown in red, and down-regulation is shown in blue, the control group was treated with equal volume DMSO. i. Correlation map of the log2-fold change in PROMPTs after INTS11 or INTS9 depletion calculated with the Spearman method. j. Histogram showing the length distribution of PROMPTs (PROMPTs were identified by PROMPT-finder.) in CTRL, INTS9 and INTS11 depletion conditions.
Extended Data Fig. 2 Depletion of Integrator leads to altered transcription patterns in the sense and antisense directions.
a. Heatmap representing highly expressed genes and their PROMPTs from ChromRNA-seq results with EV or ectopic expression of INTS11 protein. “+” represents IAA treatments. The corresponding differences by log2-fold change are shown in the right panel. Upregulation is shown in red, and downregulation is shown in blue. b-c. Boxplots representing the transcript levels from PROMPT (b) and pre-mRNA (c) for 2121 active genes. P values were calculated by two-sided Mann-Whitney test. The boxplots was plotted as Extended Data Fig. 1. d. IGV tracks of the MYC and BMP4 loci from ChromRNA-seq results in INTS11 (top) or INTS9 depletion (bottom). e. Heatmap representing 2121 genes and PROMPTs from ChromRNA-seq before and after INTS9 depletion(left). Heatmap representing 2121 genes and PROMPTs from ChromRNA-seq before and after INTS11 depletion and rescue (right). f. Metagene analysis of the average density of ChromRNA-seq signals after INTS9 depletion. g. Boxplots representing the transcript levels from PROMPT (left) and pre-mRNA (right) for 2121 active genes with or without INTS9 depletion. P values were calculated by two-sided Mann-Whitney test. See Extended Data Fig. 1 for description of boxplots. h. Gene by gene transcription ratio plots representing PROMPTs (red) and gene (blue) FPKM values for high or low expressed genes. Genes categorized as Fig. 1g. Top 3 quantiles (8,9,10; 2,121 genes) for highly expressed genes. The bottom quantiles (1,2,3; 2,121 genes) for lowly expressed genes. The yellow line representing the transcription ratio as the log2 fold change of gene FPKM compared to PROMPT FPKM. i. The scatterplot illustrates the change in the transcription ratio for lowly expressed genes calculated using ChromRNA-seq. The transcription ratio represents the log2 ratio of gene FPKM versus PROMPT FPKM, indicating the directionality of transcription.
Extended Data Fig. 3 Validation of altered transcriptional changes upon INTS11 depletion by 4SU-seq and TT-seq.
a-b. IGV tracks of RNU11, RNU12, and TERC loci from TT-seq (a) and 4sU -seq (b) results before and after INTS11 depletion. c. Heatmap representing the identified 2121 active genes and PROMPTs from GSE223265 TT-seq results (left) and the overlaying heatmap of +IAA/CTRL (right). d. IGV tracks of 4sU-seq representing PROMPT accumulation at MYC or BMP4 loci. e-f. Heatmap (e) and Metagene analysis (f) showing accumulated transcripts in the PROMPT direction and reduced transcripts in the pre-mRNAs direction for 2121 active genes upon INTS11 depletion. g. Quantitative boxplots of transcription distribution at PROMPTs (left) and pre-mRNA (right) from 4sU-seq results. P values were calculated by two-sided Mann-Whitney test. Boxplots were plotted as before. h-i. The IGV track comparison of 4sU-seq (h) and TT-seq (i) at the XPO1 or EP300 loci upon INTS11 depletion.
Extended Data Fig. 4 Integrator depletion leads to the upregulation of unbiased bidirectional transcription in the enhancer RNA loci.
a. Enhancer and Super enhancer distribution map calculated by RNAPII ChIP-seq signal in units of reads per million. Enhancers are ranked by the increase in the RNAPII ChIP-seq signal. b. IGV tracks of a super-enhancer locus from ChromRNA-seq and RNAPII ChIP-seq results before and after INTS11 depletion. c. IGV tracks of ChromRNA-seq, 4sU-seq, and TT-seq of two enhancer RNA loci. d. Heatmap of 3,259 enhancer loci. from ChromRNA-seq and 4sU-seq results. e. Metagene analysis showing the average occupancy of ChromRNA-seq and 4sU-seq signals at 3259 enhancer RNA loci.
Extended Data Fig. 5 Depletion of INTS11 reduces immediate early responsive gene expression and induces PROMPT accumulation.
a. Flow diagram illustrating the EGF / IFN-β induced experiments in INTS11-AID cells. b. Selection of immediate responsive genes (FDR < 0.05). Volcano plots showing the IEG genes b. c. Venn diagram showing the overlap between EGF-induced IEG genes and genes with PROMPT after INTS11 depletion. d. Volcano plots showing the PROMPT regions with significantly upregulated RNA levels and IEG genes with downregulated pre-mRNA levels upon EGF treatment. In total 1026 IEG genes were included in the analyses. e. Quantitative boxplots of transcription distribution for 1026 EGF-induced IEG genes and their PROMPTs upon INTS11 depletion. P values were calculated by two-sided Mann-Whitney test. See Extended Data Fig. 1 for description of boxplots. f. Quantitative RT-PCR results of the EGF-induced NR4A1 gene and PROMPT locus upon INTS11 depletion. Data are presented as mean ± SD. P values were calculated by unpaired t-test (n = 3 independent experiments). (*) p < 0.05. (***) p < 0.001. g. Like in (a) but for IFN-β. h. Like in (b) but for IFN-β genes. i. IGV tracks of DDX58 and IFIT1 gene loci before and after IFN-β induction with or without INTS11 depletion. j. Quantitative RT-PCR results of the IFN-β induced IFIT1 gene and its PROMPT locus before and after the depletion of INTS11. Data are presented as mean ± SD. P values were calculated by unpaired t-test (n = 3 independent experiments). (***) p < 0.001. k-l. IEG genes and their PROMPTs before and after INTS11 depletion. The total represents the combined levels of pre-mRNA and PROMPT fold change for each locus. P values were calculated by two-sided Mann-Whitney test. See Extended Data Fig. 1 for description of boxplots.
Extended Data Fig. 6 Integrator depletion alters the total RNAP II occupancy at actively transcribed genes.
a. IGV tracks of RNU11 and RNU12 loci from total RNAPII ChIP-seq results after INTS11 depletion. b. IGV tracks of EP300 and EZH2 loci from RNAPII ChIP-seq results upon INTS11 depletion. c. Boxplots of 2121 highly expressed gene RNAPII ChIP-seq signals in PROMPTs (top) and gene bodies (bottom). P values were calculated by two-sided Mann-Whitney test. See Extended Data Fig. 1 for description of boxplots. d. Western blot of TAF1 and TAF3 protein levels after INTS1 and INTS11 depletion in whole cells and chromatin, for INTS1-AID2 cell line “-” represents equal-volume DMSO treatment as a control, and “+” represents 10 µM 5-Ph-IAA treatment, representing from three independent experiments with similar results. e. Heatmap of TAF1 ChIP-seq in a 2 kb window around TSS sites of 10428 genes and 2121 active genes. f. IGV tracks of KLF2 and NR4A1 loci from RNAP II ChIP-seq results upon EGF induction and INTS11 depletion. g. Box plots of RNAPII ChIP-seq signals at the 1026 EGF-induced gene loci (PROMPTs, left; gene bodies, right). P values were calculated by two-sided Mann-Whitney test. See Extended Data Fig. 1 for description of boxplots.
Extended Data Fig. 7 Integrator depletion alters the dynamics of RNAPII-CTD phosphorylation.
a. Western blot of total RNAP II and different CTD phosphorylation states after INTS1 depletion in whole cells, cytoplasm, nucleoplasm and chromatin, representing from three biological independent experiments with similar results (“-” equal-volume DMSO treatment, and “+” 10 µM 5-Ph-IAA treatment). b. IGV tracks of RNU11 and RNU12 loci from Tyr1P and Ser2P ChIP-seq results in INTS11-AID cells. c-d. Box plots representing the 2121 genes Tyr1P(c) and Ser2P(d) ChIP-seq results in genes and PROMPTs upon INTS11 depletion. P values were calculated by two-sided Mann-Whitney test. See Extended Data Fig. 1 for description of boxplots. e. The IGV tracks of total RNAPII, Ser2P, and Tyr1P ChIP-seq results in two enhancer loci upon INTS11 depletion. f. Heatmap representing Tyr1P ChIP-seq (left) and Ser2P ChIP-seq (right) at 3259 enhancer sites. g. Metagene analysis of Tyr1P and Ser2P ChIP-seq signals (log2) across 2121 highly expressed genes. h. Metagene analyses of the Tyr1P / RNAP II and Ser2P / RNAP II ChIP-seq ratios. i. The comparison of metagene analysis between genes and enhancers.
Extended Data Fig. 8 Endonuclease activity of Integrator is specifically required for degrading PROMPTs.
a. Heatmap representing highly expressed genes and their PROMPTs from ChromRNA-seq results with EV or ectopic expression of E203Q mutant protein. “+” represents IAA treatments. The corresponding differences by log2-fold change are shown in the right panel. Upregulation is shown in red, and downregulation is shown in blue. b-c. Boxplots representing the transcript levels from PROMPT (b) and pre-mRNA (c) for 2121 active genes. P values were calculated by two-sided Mann-Whitney test. n.s., not significant. See Extended Data Fig. 1 for description of boxplots. d. Quantitative RT-PCR representing the accumulation of 3’ end extension of RNU11 or RNU12 through the ectopic expression of INTS11 or the E203Q mutant. “+” stands for the IAA treatments. Data are presented as mean ± SD. P values were calculated by unpaired t-test (n = 3 independent experiments). (**) p < 0.01. (***) p < 0.001. e. The accumulation of PROMPTs can be rescued by gapmer ASO specific for PROMPT at the SRRT gene locus, which leads to the recovery of gene expression. (PROMPTs shown in red, gene shown in blue). Data are presented as mean ± SD. P values were calculated by unpaired t-test (n = 3 independent experiments). (**) p < 0.01. (***) p < 0.001. f. Schematic diagram showing the generation of the CPSF73-AID2 cell line (top). Western blot of CPSF73 depletion over time after 10 µM 5-Ph-IAA treatment (bottom), representing from three independent experiments with similar results. g. IGV tracks of the BMP4 and JUN locus revealing 3’ end extension after CPSF73 depletion, the control group was treated with equal volume DMSO. h. Quantitative RT-PCR results representing a 3’ extension of MYC or ACTB genes after CPSF73 depletion. Data are presented as mean ± SD. P values were calculated by unpaired t-test (n = 3 independent experiments). (*) p < 0.05. (**) p < 0.01. i. Volcano plot showing the log2 fold changes in 3847 genes showing significant 3’ extension after CPSF73 depletion (FDR < 0.05, FC > 2). j. Histogram of PROMPTs (PROMPTs were identified by PROMPT-finder.) length distribution in the CTRL, CPSF73 deception, and INTS11 depletion conditions.
Extended Data Fig. 9 The distribution and characterization of U1 sites in the human genome.
a. Density of predicted 5’ U1 splice sites within a 1-kb region flanking gene TSS. Strong and medium sites are defined in the Methods. b. Definition of U1 sites and distance with TSS. The red bar shows the predicted U1 sites. The green box shows the analyzed region. c. Quantitative boxplots of PROMPTs categorized by SOM using predicted U1 site parameters (details in Methods). Left, reads count from the CTRL sample. Right, PROMPT fold change after INTS11 depletion. P values were calculated by two-sided Mann-Whitney test. See Extended Data Fig. 1 for description of boxplots. d. IGV examples of an enhancer locus with a low U1 score together with ChromRNA-seq, TT-seq, RNAPII ChIP-seq, and predicted U1 site results. e. Schematic diagram of the endogenous 3 x U1 sequence in the CTTN gene PROMPT region. f. The sequence of the artificial 3xU1 site sequence. Predicted U1 sites are shown as green boxes below. g. The sequence of the first predicted U1 site sequence in the CTTN gene PROMPT region. The sequence was removed by homologous recombination. h. Boxplots representing the change in transcription levels of PROMPTs (left) and pre-mRNA (right) after blocking U1 snRNA using fully modified ASO. P values were calculated by two-sided Mann-Whitney test. See Extended Data Fig. 1 for description of boxplots.
Supplementary information
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 4
Unprocessed statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 6
Unprocessed statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 1
Unprocessed statistical source data.
Source Data Extended Data Fig. 5
Unprocessed statistical source sata.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 8
Unprocessed statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Li, J., Miao, L. et al. Transcription directionality is licensed by Integrator at active human promoters. Nat Struct Mol Biol (2024). https://doi.org/10.1038/s41594-024-01272-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41594-024-01272-z