Abstract
How chromatin enzymes work in condensed chromatin and how they maintain diffusional mobility inside remains unexplored. Here we investigated these challenges using the Drosophila ISWI remodeling ATPase, which slides nucleosomes along DNA. Folding of chromatin fibers did not affect sliding in vitro. Catalytic rates were also comparable in- and outside of chromatin condensates. ISWI cross-links and thereby stiffens condensates, except when ATP hydrolysis is possible. Active hydrolysis is also required for ISWI’s mobility in condensates. Energy from ATP hydrolysis therefore fuels ISWI’s diffusion through chromatin and prevents ISWI from cross-linking chromatin. Molecular dynamics simulations of a ‘monkey-bar’ model in which ISWI grabs onto neighboring nucleosomes, then withdraws from one before rebinding another in an ATP hydrolysis-dependent manner, qualitatively agree with our data. We speculate that monkey-bar mechanisms could be shared with other chromatin factors and that changes in chromatin dynamics caused by mutations in remodelers could contribute to pathologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Large data files that exceed the size limitations or data that underlie more peripheral parts of the study are available upon request from the corresponding authors. Source data are provided with this paper.
Code availability
Code is available from GitHub at https://github.com/StiglerLab/Vizjak_2023 (ref. 86).
References
Burak, Y., Ariel, G. & Andelman, D. Onset of DNA aggregation in presence of monovalent and multivalent counterions. Biophys. J. 85, 2100–2110 (2003).
Post, C. B. & Zimm, B. H. Theory of DNA condensation: collapse versus aggregation. Biopolymers 21, 2123–2137 (1982).
Woodcock, C. L. F. Ultrastructure of inactive chromatin. J. Cell Biol. 59, A368 (1973).
Olins, A. L. & Olins, D. E. Spheroid chromatin units (v bodies). Science 183, 330–332 (1974).
Finch, J. T. & Klug, A. Solenoidal model for superstructure in chromatin. Proc. Natl Acad. Sci. USA 73, 1897 (1976).
Ou, H. D. et al. ChromEMT: visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science 357, eaag0025 (2017).
Maeshima, K. et al. Nucleosomal arrays self-assemble into supramolecular globular structures lacking 30-nm fibers. EMBO J. 35, 1115–1132 (2016).
Adhireksan, Z., Sharma, D., Lee, P. L. & Davey, C. A. Near-atomic resolution structures of interdigitated nucleosome fibres. Nat. Commun. 11, 4747 (2020).
Hsieh, T. H. S. et al. Mapping nucleosome resolution chromosome folding in yeast by Micro-C. Cell 162, 108 (2015).
Ricci, M. A., Manzo, C., García-Parajo, M. F., Lakadamyali, M. & Cosma, M. P. Chromatin fibers are formed by heterogeneous groups of nucleosomes in vivo. Cell 160, 1145–1158 (2015).
Gibson, B. A. et al. Organization of chromatin by intrinsic and regulated phase separation. Cell 179, 470–484.e21 (2019).
Strickfaden, H. et al. Condensed chromatin behaves like a solid on the mesoscale in vitro and in living cells. Cell 183, 1772–1784.e13 (2020).
Zhang, Y., Narlikar, G. J. & Kutateladze, T. G. Enzymatic reactions inside biological condensates. J. Mol. Biol. 433, 166624 (2021).
Hihara, S. et al. Local nucleosome dynamics facilitate chromatin accessibility in living mammalian cells. Cell Rep. 2, 1645–1656 (2012).
Kornberg, R. D. & Lorch, Y. Primary role of the nucleosome. Mol. Cell 79, 371–375 (2020).
Kim, J. M. et al. Single-molecule imaging of chromatin remodelers reveals role of ATPase in promoting fast kinetics of target search and dissociation from chromatin. eLife 10, e69387 (2021).
Corona, D. F. V. et al. ISWI is an ATP-dependent nucleosome remodeling factor. Mol. Cell 3, 239–245 (1999).
Hamiche, A., Sandaltzopoulos, R., Gdula, D. A. & Wu, C. ATP-dependent histone octamer sliding mediated by the chromatin remodeling complex NURF. Cell 97, 833–842 (1999).
Ludwigsen, J., Hepp, N., Klinker, H., Pfennig, S. & Mueller-Planitz, F. Remodeling and repositioning of nucleosomes in nucleosomal arrays. Methods Mol. Biol. 1805, 349–370 (2018).
Mueller-Planitz, F., Klinker, H., Ludwigsen, J. & Becker, P. B. The ATPase domain of ISWI is an autonomous nucleosome remodeling machine. Nat. Struct. Mol. Biol. 20, 82–89 (2013).
Schram, R. D., Klinker, H., Becker, P. B. & Schiessel, H. Computational study of remodeling in a nucleosomal array. Eur. Phys. J. E 38, 85 (2015).
Klinker, H. et al. ISWI remodelling of physiological chromatin fibres acetylated at lysine 16 of histone H4. PLoS ONE 9, e88411 (2014).
Boyer, L. A. et al. Functional delineation of three groups of the ATP-dependent family of chromatin remodeling enzymes. J. Biol. Chem. 275, 18864–18870 (2000).
Logie, C., Tse, C., Hansen, J. C. & Peterson, C. L. The core histone N-terminal domains are required for multiple rounds of catalytic chromatin remodeling by the SWI/SNF and RSC complexes. Biochemistry 38, 2514–2522 (1999).
Peeples, W. & Rosen, M. K. Mechanistic dissection of increased enzymatic rate in a phase-separated compartment. Nat. Chem. Biol. 17, 693–702 (2021).
Poirier, M. G., Bussiek, M., Langowski, J. & Widom, J. Spontaneous access to DNA target sites in folded chromatin fibers. J. Mol. Biol. 379, 772–786 (2008).
Poirier, M. G., Oh, E., Tims, H. S. & Widom, J. Dynamics and function of compact nucleosome arrays. Nat. Struct. Mol. Biol. 16, 938–944 (2009).
Hagerman, T. A. et al. Chromatin stability at low concentration depends on histone octamer saturation levels. Biophys. J. 96, 1944–1951 (2009).
Gibson, B. A. et al. In diverse conditions, intrinsic chromatin condensates have liquid-like material properties. Proc. Natl Acad. Sci. USA 120, e2218085120 (2023).
Goins, A. B., Sanabria, H. & Waxham, M. N. Macromolecular crowding and size effects on probe microviscosity. Biophys. J. 95, 5362–5373 (2008).
Yang, J. G. & Narlikar, G. J. FRET-based methods to study ATP-dependent changes in chromatin structure. Methods 41, 291–295 (2007).
Zhang, M. et al. Molecular organization of the early stages of nucleosome phase separation visualized by cryo-electron tomography. Mol. Cell 82, 3000 (2022).
Weidemann, T. et al. Counting nucleosomes in living cells with a combination of fluorescence correlation spectroscopy and confocal imaging. J. Mol. Biol. 334, 229–240 (2003).
Leonard, J. D. & Narlikar, G. J. A nucleotide-driven switch regulates flanking DNA length sensing by a dimeric chromatin remodeler. Mol. Cell 57, 850–859 (2015).
Larson, A. G. & Narlikar, G. J. The role of phase separation in heterochromatin formation, function, and regulation. Biochemistry 57, 2540–2548 (2018).
Grüne, T. et al. Crystal structure and functional analysis of a nucleosome recognition module of the remodeling factor ISWI. Mol. Cell 12, 449–460 (2003).
Bhardwaj, S. K. et al. Dinucleosome specificity and allosteric switch of the ISW1a ATP-dependent chromatin remodeler in transcription regulation. Nat. Commun. 11, 5913 (2020).
Yamada, K. et al. Structure and mechanism of the chromatin remodelling factor ISW1a. Nature 472, 448–453 (2011).
Li, L. et al. Structure of the ISW1a complex bound to the dinucleosome. Nat. Struct. Mol. Biol. 31, 266–274 (2024). https://doi.org/10.1038/s41594-023-01174-6
Wang, J. et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174, 688–699.e16 (2018).
Muzzopappa, F., Hertzog, M. & Erdel, F. DNA length tunes the fluidity of DNA-based condensates. Biophys. J. 120, 1288–1300 (2021).
Ludwigsen, J., Klinker, H. & Mueller-Planitz, F. No need for a power stroke in ISWI-mediated nucleosome sliding. EMBO Rep. 14, 1092–1097 (2013).
Harrer, N. et al. Structural architecture of the nucleosome remodeler ISWI determined from cross-linking, mass spectrometry, SAXS, and modeling. Structure 26, 282–294.e6 (2018).
Rudolph, J., Mahadevan, J., Dyer, P. & Luger, K. Poly(ADP-ribose) polymerase 1 searches DNA via a ‘monkey bar’ mechanism. Elife 7, e37818 (2018).
Deindl, S. et al. ISWI remodelers slide nucleosomes with coordinated multi-base-pair entry steps and single-base-pair exit steps. Cell 152, 442–452 (2013).
Gamarra, N., Johnson, S. L., Trnka, M. J., Burlingame, A. L. & Narlikar, G. J. The nucleosomal acidic patch relieves auto-inhibition by the ISWI remodeler SNF2h. eLife 7, e35322 (2018).
Dann, G. P. et al. ISWI chromatin remodellers sense nucleosome modifications to determine substrate preference. Nature 548, 607–611 (2017).
Clapier, C. R., Längst, G., Corona, D. F., Becker, P. B. & Nightingale, K. P. Critical role for the histone H4 N terminus in nucleosome remodeling by ISWI. Mol. Cell. Biol. 21, 875–883 (2001).
Schalch, T., Duda, S., Sargent, D. F. & Richmond, T. J. X-ray structure of a tetranucleosome and its implications for the chromatin fibre. Nature 436, 138–141 (2005).
Verschure, P. J. et al. Condensed chromatin domains in the mammalian nucleus are accessible to large macromolecules. EMBO Rep. 4, 861–866 (2003).
Beaudouin, J., Mora-Bermúdez, F., Klee, T., Daigle, N. & Ellenberg, J. Dissecting the contribution of diffusion and interactions to the mobility of nuclear proteins. Biophys. J. 90, 1878–1894 (2006).
Erdel, F., Baum, M. & Rippe, K. The viscoelastic properties of chromatin and the nucleoplasm revealed by scale-dependent protein mobility. J. Phys. Condens. Matter 27, 064115 (2015).
Maeshima, K. et al. A transient rise in free Mg2+ ions released from ATP-Mg hydrolysis contributes to mitotic chromosome condensation. Curr. Biol. 28, 444–451.e6 (2018).
Shimamoto, Y., Tamura, S., Masumoto, H. & Maeshima, K. Nucleosome–nucleosome interactions via histone tails and linker DNA regulate nuclear rigidity. Mol. Biol. Cell 28, 1580–1589 (2017).
Kroschwald, S. et al. Different material states of Pub1 condensates define distinct modes of stress adaptation and recovery. Cell Rep. 23, 3327–3339 (2018).
Munder, M. C. et al. A pH-driven transition of the cytoplasm from a fluid- to a solid-like state promotes entry into dormancy. eLife 5, e09347 (2016).
Erdel, F. & Rippe, K. Formation of chromatin subcompartments by phase separation. Biophys. J. 114, 2262–2270 (2018).
Schneider, M. W. G. et al. A mitotic chromatin phase transition prevents perforation by microtubules. Nature 609, 183 (2022).
Keizer, V. I. P. et al. Live-cell micromanipulation of a genomic locus reveals interphase chromatin mechanics. Science 377, 489–495 (2022).
Erdel, F. et al. Mouse heterochromatin adopts digital compaction states without showing hallmarks of HP1-driven liquid–liquid phase separation. Mol. Cell 78, 236–249.e7 (2020).
Irgen-Gioro, S., Yoshida, S., Walling, V. & Chong, S. Fixation can change the appearance of phase separation in living cells. Elife 11, e79903 (2022).
Hansen, J. C., Maeshima, K. & Hendzel, M. J. The solid and liquid states of chromatin. Epigenetics Chromatin 14, 50 (2021).
Korber, P. & Becker, P. B. Nucleosome dynamics and epigenetic stability. Essays Biochem 48, 63–74 (2010).
Muzzopappa, F. et al. Detecting and quantifying liquid–liquid phase separation in living cells by model-free calibrated half-bleaching. Nat. Commun. 13, 1–15 (2022).
Whitehouse, I., Rando, O. J., Delrow, J. & Tsukiyama, T. Chromatin remodelling at promoters suppresses antisense transcription. Nature 450, 1031–1035 (2007).
Gelbart, M. E., Bachman, N., Delrow, J., Boeke, J. D. & Tsukiyama, T. Genome-wide identification of Isw2 chromatin-remodeling targets by localization of a catalytically inactive mutant. Genes Dev. 19, 942 (2005).
Blosser, T. R., Yang, J. G., Stone, M. D., Narlikar, G. J. & Zhuang, X. Dynamics of nucleosome remodelling by individual ACF complexes. Nature 462, 1022–1027 (2009).
Tilly, B. C. et al. In vivo analysis reveals that ATP-hydrolysis couples remodeling to SWI/SNF release from chromatin. eLife 10, e69424 (2021).
Erdel, F., Schubert, T., Marth, C., Längst, G. & Rippe, K. Human ISWI chromatin-remodeling complexes sample nucleosomes via transient binding reactions and become immobilized at active sites. Proc. Natl Acad. Sci. USA 107, 19873–19878 (2010).
Oppikofer, M. et al. Expansion of the ISWI chromatin remodeler family with new active complexes. EMBO Rep. 18, 1697–1706 (2017).
Clapier, C. R., Verma, N., Parnell, T. J. & Cairns, B. R. Cancer-associated gain-of-function mutations activate a SWI/SNF-family regulatory hub. Mol. Cell 80, 712–725.e5 (2020).
Hodges, H. C. et al. Dominant-negative SMARCA4 mutants alter the accessibility landscape of tissue-unrestricted enhancers. Nat. Struct. Mol. Biol. 25, 61–72 (2018).
Elfring, L. K. et al. Genetic analysis of brahma: the Drosophila homolog of the yeast chromatin remodeling factor SWI2/SNF2. Genetics 148, 251–265 (1998).
Li, W. et al. Biophysical properties of AKAP95 protein condensates regulate splicing and tumorigenesis. Nat. Cell Biol. 22, 960–972 (2020).
Shi, B. et al. UTX condensation underlies its tumour-suppressive activity. Nature 597, 726–731 (2021).
Pédelacq, J.-D., Cabantous, S., Tran, T., Terwilliger, T. C. & Waldo, G. S. Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 24, 79–88 (2006).
Klinker, H., Haas, C., Harrer, N., Becker, P. B. & Mueller-Planitz, F. Rapid purification of recombinant histones. PLoS ONE 9, e104029 (2014).
Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys. J. 78, 1606–1619 (2000).
Demeler, B. & Gorbet, G. E. in Analytical Ultracentrifugation (eds Uchiyama, S. et al.) 119–143 (Springer, 2016).
Goins, A. B., Sanabria, H. & Waxham, M. N. Macromolecular crowding and size effects on probe microviscosity. Biophys J. 95, 5362–5373 (2008).
Digman, M. A., Caiolfa, V. R., Zamai, M. & Gratton, E. The phasor approach to fluorescence lifetime imaging analysis. Biophys. J. 94, 14–16 (2008).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
MultiStackReg (BioImage Informatics Index, 2022); https://biii.eu/multistackreg
Koulouras, G. et al. EasyFRAP-web: a web-based tool for the analysis of fluorescence recovery after photobleaching data. Nucleic Acids Res. 46, 467–472 (2018).
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing https://www.R-project.org/ (2022).
Vizjak_2023 (GitHub, 2023); https://github.com/StiglerLab/Vizjak_2023
Acknowledgements
We thank the following members of the Mueller-Planitz lab: S. Albig, M. Khare and S. Härtel for histone purification, M. A. Shegane for donating nucleosome arrays, and A. Lentz for purifying GFP and cloning ISWI–GFP. We thank M. Muernseer (Nanolive) for collecting holotomography data and the Ökten group (TU Munich) for providing a sfGFP plasmid. P.V. acknowledges support from the IRTG SFB 1064. F.M.-P. acknowledges financial support from the Deutsche Forschungsgemeinschaft (SFB1064 A07, MU3613/3-1 and MU3613/8-1); J.S. from the LMU Center for Nanoscience CeNS, a DFG Emmy Noether grant (STI673/2-1) and an ERC Starting Grant (758124); P.B.B. from SFB1064 A01 and BE1140/6-1; and M.H. by St. Jude Children’s Research Hospital, the American Lebanese Syrian Associated Charities and NIH awards R01GM141694 and R01GM135599. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: F.M.-P., J.S. and P.V. Methodology and formal analysis: P.V., J.S., M.G.P., N.H., A.S., D.K. and M.S. Investigation: P.V., M.G.P., A.S., J.B., J.S., D.K., N.H. and M.S. Writing of original draft: F.M.-P., P.V., M.G.P., D.K., M.S., A.S., J.B. and J.S. Visualization: P.V., J.S., D.K., M.S. and M.G.P. Funding acquisition: F.M.-P., J.S., P.B.B. and M.H. Writing—review and editing: all authors. N.H. and A.S. contributed equally.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks John van Noort and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Sara Osman, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Catalytic activity of ISWI for varying Mg-concentrations.
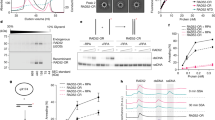
a, Quality controls for 25mer nucleosome arrays. Left: agarose gel after magnesium precipitation of assembled arrays and the resolubilized pellet. IN, input; SN, supernatant; P, pellet. Competitor DNA derived from the plasmid backbone (<1 kb) was excluded from P. Middle: Not1 digestion (a Not1 site is present in each linker) liberated mostly mononucleosomes, running around 400 bp, but little 197 bp fragments, confirming saturation of most 601 repeats with octamers. Right: BsiWI digestion (all nucleosomes occlude a BsiWI restriction site) for arrays assembled with different octamer amounts. As 601 sites become saturated, digestion is hindered. Arrays were reconstituted 16 times with similar results. b, Increasing Mg2+-concentrations reduce mononucleosome-stimulated ATP hydrolysis rates. Saturating concentrations of ATP (1 mM) and mononucleosomes were used (1.33 µM). Control experiments with three times lower mononucleosome concentrations gave the analogous results. c, Nucleosome array as in Fig. 1a but with different orientation of restriction sites such that the BamHI site is now more peripheral. The array was cut out from the plasmid with HincII (light green) and EcoRI (magenta). d, BamHI accessibility assay as in Fig. 1f but for the array shown in c. e, Left: quantification of gel in d and exponential fits of time courses. Right: rate coefficients from single exponential fits. Bars in b and e are mean values of two independent experiments (dots).
Extended Data Fig. 2 ISWI-GFP partitions into condensates.
a, GST-labeled GFP does not partition into chromatin condensates. The colocalization experiment was performed with 40 nM of unlabeled 25mer, 10 nM of 25mer-Cy3 and 1.125 µM of GFP-GST. N = 2, with similar results. b, ISWI-GFP concentrations were determined inside condensates and, after centrifugation, in the surrounding solution (bottom) from fluorescence intensities using calibration curves with ISWI-GFP dilutions (top). Two different microscope settings were used to image lower and higher dilutions. Means and SD of two independent replicates are shown. c, Nucleosome sliding time courses as in Fig. 2d. Conditions were identical except that reactions were started by addition of Mg-ATP, not by enzyme. N = 1.
Extended Data Fig. 3 FLIM-FRET controls.
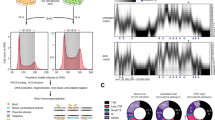
a, Enrichment of labeled 0N60 mononucleosomes in chromatin condensates. N = 3 biological replicates; bars are averages. The mononucleosome concentration in solution was determined from z-plane above the condensates. b, Whole condensate FRAP of FRET-0N60 nucleosomes to assess their exchange between condensate and solution. Line is an average and shadow SD of eight bleached condensates. One of two independent replicates with similar results is shown. c, Phasor representation of data in Fig. 3b. Upon introduction of the acceptor, the donor’s lifetime distribution moves away from the universal circle line (single exponential lifetimes), consistent with at least two donor populations in different FRET states. The phasor representation makes no assumptions on the number of decay rates nor on specific decay model 75. d, Acceptor bleaching enhances donor fluorescence and lifetime, indicative of FRET. Imaging of FRET-0N60 nucleosomes in chromatin condensates. The acceptor fluorophore was bleached in the left half of the field of view, leading to an increase in donor fluorescence and donor lifetime (right most panel). N = 1. e, FLIM-FRET measurements as in Fig. 3d, but with 5 mM Mg-ATP. Bars are averages of three independent experiments (dots).
Extended Data Fig. 4 ISWI and nucleosome array mobility inside condensates.
a, Intra-condensate mobility of ISWI-GFP measured by partial-condensate FRAP. Half of a condensate was bleached in presence and absence of Mg-ATP (1 mM). Line is an average and shadow SD of 15 bleached condensates for each condition. N = 4 biological replicates. b, Partial-condensate FRAP of ISWI-GFP (500 nM) in presence of indicated nucleotides (all 0.77 mM), 37 nM 25mer and 1.75 nM Cy3-25mer. c, chromatin and ISWI concentrations as in Fig. 4b, but without Cy3-25mer (80 nM unlabeled 25mer). No nucleotide (8 independent condensates) and ADP-data (7 condensates) were collected in the absence of ATP regenerating system, ATP data in its presence (5 independent condensates). d, ISWI-GFP is more mobile than chromatin in condensates. Dual FRAP of ISWI-GFP and Cy3-labeled condensates formed by 25mer arrays in presence of Mg-ATP (1 mM) and ISWI (100 nM). Four independent replicates show similar results.
Extended Data Fig. 5 ISWI and Mg2+ affect biophysical properties of condensates.
a, Optical tweezer force readout during condensate fusion. The fusion velocity was determined as the slope of the normalized force data at the inflection point. Incurred forces during fusion were on the order of 1 pN (see Methods). b, Fraction of successful fusion events in 1 mM MgCl2 (orange) and 5 mM MgCl2 (red). Nucleosome concentration: 1170 nM. c, Fusion velocities of condensates measured by optical tweezers containing indicated ISWI concentrations. Data was obtained from the indicated number of independent condensates measured in one experiment. d, ISWI increased viscosity of condensates as reported by enhanced fluorescence of the molecular rotor thioflavin T (ThT). In a high viscosity medium, rotation around the C-C bond (green arrow) is constrained, and the excitation energy is released as fluorescence. Bars: average ThT fluorescence intensities relative to outside medium; error: SD. Six condensates were analyzed for 0 nM and 293 nM ISWI, five for 1170 nM ISWI red circles). One of two independent replicates with similar results is shown. e, Nucleotides (1 mM) and 2 mM instead of 1 mM free Mg2+ show only modest effects on fusion velocities compared to ISWI-AMPPNP. Data were obtained from the indicated number of independent condensates derived from two experiments conducted on different days. Statistical significance was determined by two-sided t-test (p-values: UTP: 3.5e-4, ADP: 0.053, AMPPNP: 4.6e-4, 2 mM MgCl2: 1.4e-12, AMPPNP + ISWI: 2.3e-14). Box plots in c and e show medians and the 25th/75th percentiles, whiskers the 9th and 91st percentiles; asterisks indicate significance levels (n.s.: p > 0.05, *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001, ****: p ≤ 1e-4).
Extended Data Fig. 6 Mechanistic details of the simulated monkey bar mechanism.
a, A model for the independent switching of the strengths of the two nucleosome interaction sites during ISWI´s ATPase cycle. Escape rates (black) and transition rates (red) in timestep-1 are indicated. b, Schematic representation of the implementation of the model in Fig. 6a for molecular dynamics simulations. c, Modified Lennard-Jones potential used in simulations. Below distances of 2R0 a regular Lennard-Jones potential is used. Between 2R0 and 3R0 the potential is described using a linear approximation, while interactions with range above 3R0 are set to 0. d, Conversion of the strength of the modified Lennard-Jones potential to escape rates based on the mean first passage time of potential escape76.
Supplementary information
Supplementary Information
Supplementary Table 1.
Supplementary Video 1
FLIM–FRET time lapse with ATP.
Supplementary Video 2
FLIM–FRET time lapse with AMPPNP.
Supplementary Video 3
Controlled fusion with optical tweezers of chromatin condensates containing ISWI and ADP–BeFx.
Supplementary Video 4
Controlled fusion with optical tweezers of chromatin condensates containing ISWI and ATP.
Supplementary Video 5
Simulation of ISWI FRAP with graph.
Supplementary Video 6
Simulation of condensate fusion with graph.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Raw data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Figs. 1 and 2 and Source Data Extended Figs. 1 and 2
Unprocessed gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vizjak, P., Kamp, D., Hepp, N. et al. ISWI catalyzes nucleosome sliding in condensed nucleosome arrays. Nat Struct Mol Biol (2024). https://doi.org/10.1038/s41594-024-01290-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41594-024-01290-x