Abstract
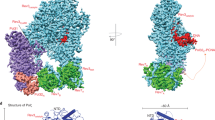
Rev1–Polζ-dependent translesion synthesis (TLS) of DNA is crucial for maintaining genome integrity. To elucidate the mechanism by which the two polymerases cooperate in TLS, we determined the cryogenic electron microscopic structure of the Saccharomyces cerevisiae Rev1–Polζ holocomplex in the act of DNA synthesis (3.53 Å). We discovered that a composite N-helix-BRCT module in Rev1 is the keystone of Rev1–Polζ cooperativity, interacting directly with the DNA template–primer and with the Rev3 catalytic subunit of Polζ. The module is positioned akin to the polymerase-associated domain in Y-family TLS polymerases and is set ideally to interact with PCNA. We delineate the full extent of interactions that the carboxy-terminal domain of Rev1 makes with Polζ and identify potential new druggable sites to suppress chemoresistance from first-line chemotherapeutics. Collectively, our results provide fundamental new insights into the mechanism of cooperativity between Rev1 and Polζ in TLS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support this study are available from the corresponding author upon reasonable request. The cryo-EM density maps of Rev1-Full–Polζ-DNA-dCTP and Rev1-ΔN–Polζ-DNA-dCTP generated in this study have been deposited in the Electron Microscopy Data Bank (EMDB) under accession numbers EMD-41371 and EMD-41372, respectively. The resulting atomic coordinates for Rev1-Full–Polζ-DNA-dCTP and Rev1-ΔN–Polζ-DNA-dCTP have been deposited in the Protein Data Bank (PDB) with accession numbers PDB 8TLQ and PDB 8TLT, respectively. Atomic coordinates from previously published work used in the study are available in the PDB with accession numbers PDB 6V93 and PDB 4ID3. Source data are provided with this paper.
References
Johnson, R. E., Washington, M. T., Haracska, L., Prakash, S. & Prakash, L. Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature 406, 1015–1019 (2000).
Jain, R., Aggarwal, A. K. & Rechkoblit, O. Eukaryotic DNA polymerases. Curr. Opin. Struct. Biol. 53, 77–87 (2018).
Prakash, S., Johnson, R. E. & Prakash, L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74, 317–353 (2005).
Johnson, R. E., Prakash, L. & Prakash, S. Pol31 and Pol32 subunits of yeast DNA polymerase ẟ are also essential subunits of DNA polymerase ζ. Proc. Natl Acad. Sci. USA 109, 12455–12460 (2012).
Makarova, A. V., Stodola, J. L. & Burgers, P. M. A four-subunit DNA polymerase ζ complex containing Pol ẟ accessory subunits is essential for PCNA-mediated mutagenesis. Nucleic Acids Res. 40, 11618–11626 (2012).
Makarova, A. V. & Burgers, P. M. Eukaryotic DNA polymerase ζ. DNA Repair (Amst.) 29, 47–55 (2015).
Lange, S. S., Takata, K. & Wood, R. D. DNA polymerases and cancer. Nat. Rev. Cancer 11, 96–110 (2011).
Nelson, J. R., Lawrence, C. W. & Hinkle, D. C. Thymine–thymine dimer bypass by yeast DNA polymerase ζ. Science 272, 1646–1649 (1996).
Malik, R. et al. Cryo-EM structure of translesion DNA synthesis polymerase ζ with a base pair mismatch. Nat. Commun. 13, 1050 (2022).
Malik, R. et al. Structure and mechanism of B-family DNA polymerase ζ specialized for translesion DNA synthesis. Nat. Struct. Mol. Biol. 27, 913–924 (2020).
Hashimoto, K. et al. The vital role of polymerase ζ and REV1 in mutagenic, but not correct, DNA synthesis across benzo[a]pyrene-dG and recruitment of polymerase ζ by REV1 to replication-stalled site. J. Biol. Chem. 287, 9613–9622 (2012).
Doles, J. et al. Suppression of Rev3, the catalytic subunit of Polζ, sensitizes drug-resistant lung tumors to chemotherapy. Proc. Natl Acad. Sci. USA 107, 20786–20791 (2010).
Xie, K., Doles, J., Hemann, M. T. & Walker, G. C. Error-prone translesion synthesis mediates acquired chemoresistance. Proc. Natl Acad. Sci. USA 107, 20792–20797 (2010).
Xu, X. et al. Enhancing tumor cell response to chemotherapy through nanoparticle-mediated codelivery of siRNA and cisplatin prodrug. Proc. Natl Acad. Sci. USA 110, 18638–18643 (2013).
Nelson, J. R., Lawrence, C. W. & Hinkle, D. C. Deoxycytidyl transferase activity of yeast REV1 protein. Nature 382, 729–731 (1996).
Nair, D. T., Johnson, R. E., Prakash, L., Prakash, S. & Aggarwal, A. K. Rev1 employs a novel mechanism of DNA synthesis using a protein template. Science 309, 2219–2222 (2005).
Guo, C. et al. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. EMBO J. 22, 6621–6630 (2003).
Ohashi, E. et al. Interaction of hREV1 with three human Y-family DNA polymerases. Genes Cells 9, 523–531 (2004).
Tissier, A. et al. Co-localization in replication foci and interaction of human Y-family members, DNA polymerase polη and REV1 protein. DNA Repair 3, 1503–1514 (2004).
Haracska, L. et al. Roles of yeast DNA polymerases ẟ and ζ and of Rev1 in the bypass of abasic sites. Genes Dev. 15, 945–954 (2001).
Pozhidaeva, A. et al. NMR structure and dynamics of the C-terminal domain from human Rev1 and its complex with Rev1 interacting region of DNA polymerase η. Biochemistry 51, 5506–5520 (2012).
Xie, W., Yang, X., Xu, M. & Jiang, T. Structural insights into the assembly of human translesion polymerase complexes. Protein Cell 3, 864–874 (2012).
Wojtaszek, J. et al. Multifaceted recognition of vertebrate Rev1 by translesion polymerases ζ and ϰ. J. Biol. Chem. 287, 26400–26408 (2012).
Futreal, P. A. et al. BRCA1 mutations in primary breast and ovarian carcinomas. Science 266, 120–122 (1994).
Miki, Y. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266, 66–71 (1994).
Callebaut, I. & Mornon, J. P. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 400, 25–30 (1997).
Bork, P. et al. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 11, 68–76 (1997).
Manke, I. A., Lowery, D. M., Nguyen, A. & Yaffe, M. B. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science 302, 636–639 (2003).
Yu, X., Chini, C. C., He, M., Mer, G. & Chen, J. The BRCT domain is a phospho-protein binding domain. Science 302, 639–642 (2003).
Lawrence, C. W. Cellular functions of DNA polymerase ζ and Rev1 protein. Adv. Protein Chem. 69, 167–203 (2004).
Guo, C. et al. REV1 protein interacts with PCNA: significance of the REV1 BRCT domain in vitro and in vivo. Mol. Cell 23, 265–271 (2006).
Kobayashi, M., Figaroa, F., Meeuwenoord, N., Jansen, L. E. & Siegal, G. Characterization of the DNA binding and structural properties of the BRCT region of human replication factor C p140 subunit. J. Biol. Chem. 281, 4308–4317 (2006).
de Groote, F. H. et al. The Rev1 translesion synthesis polymerase has multiple distinct DNA binding modes. DNA Repair (Amst.) 10, 915–925 (2011).
Kochenova, O. V. et al. Yeast DNA polymerase ζ maintains consistent activity and mutagenicity across a wide range of physiological dNTP concentrations. Nucleic Acids Res. 45, 1200–1218 (2017).
Pages, V. et al. Requirement of Rad5 for DNA polymerase ζ-dependent translesion synthesis in Saccharomyces cerevisiae. Genetics 180, 73–82 (2008).
Kuang, L. et al. A non-catalytic function of Rev1 in translesion DNA synthesis and mutagenesis is mediated by its stable interaction with Rad5. DNA Repair (Amst.) 12, 27–37 (2013).
Pryor, J. M., Gakhar, L. & Washington, M. T. Structure and functional analysis of the BRCT domain of translesion synthesis DNA polymerase Rev1. Biochemistry 52, 254–263 (2013).
Yamane, K. & Tsuruo, T. Conserved BRCT regions of TopBP1 and of the tumor suppressor BRCA1 bind strand breaks and termini of DNA. Oncogene 18, 5194–5203 (1999).
Kobayashi, M., Ab, E., Bonvin, A. & Siegal, G. Structure of the DNA-bound BRCA1 C-terminal region from human replication factor C p140 and model of the protein–DNA complex. J. Biol. Chem. 285, 10087–10097 (2010).
Feng, H., Parker, J. M., Lu, J. & Cao, W. Effects of deletion and site-directed mutations on ligation steps of NAD+-dependent DNA ligase: a biochemical analysis of BRCA1 C-terminal domain. Biochemistry 43, 12648–12659 (2004).
Ma, Y. et al. A biochemically defined system for mammalian nonhomologous DNA end joining. Mol. Cell 16, 701–713 (2004).
Leung, C. C. & Glover, J. N. BRCT domains: easy as one, two, three. Cell Cycle 10, 2461–2470 (2011).
Mok, M. C. Y. et al. Identification of an XRCC1 DNA binding activity essential for retention at sites of DNA damage. Sci. Rep. 9, 3095 (2019).
Rudolph, J. et al. The BRCT domain of PARP1 binds intact DNA and mediates intrastrand transfer. Mol. Cell 81, 4994–5006.e5 (2021).
Liu, X., Gaubitz, C., Pajak, J. & Kelch, B. A. A second DNA binding site on RFC facilitates clamp loading at gapped or nicked DNA. eLife 11, e77483 (2022).
Wilkinson, A. et al. Analysis of ligation and DNA binding by Escherichia coli DNA ligase (LigA). Biochim. Biophys. Acta 1749, 113–122 (2005).
Kikuchi, S., Hara, K., Shimizu, T., Sato, M. & Hashimoto, H. Structural basis of recruitment of DNA polymerase ζ by interaction between REV1 and REV7 proteins. J. Biol. Chem. 287, 33847–33852 (2012).
Wojtaszek, J. et al. Structural basis of Rev1-mediated assembly of quaternary vertebrate translesion polymerase complex consisting of Rev1, heterodimeric polymerase (Pol) ζ and Pol κ. J. Biol. Chem. 287, 33836–33846 (2012).
Acharya, N., Johnson, R. E., Prakash, S. & Prakash, L. Complex formation with Rev1 enhances the proficiency of Saccharomyces cerevisiae DNA polymerase ζ for mismatch extension and for extension opposite from DNA lesions. Mol. Cell. Biol. 26, 9555–9563 (2006).
Pustovalova, Y., Maciejewski, M. W. & Korzhnev, D. M. NMR mapping of PCNA interaction with translesion synthesis DNA polymerase Rev1 mediated by Rev1-BRCT domain. J. Mol. Biol. 425, 3091–3105 (2013).
Krishna, T. S., Kong, X. P., Gary, S., Burgers, P. M. & Kuriyan, J. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell 79, 1233–1243 (1994).
Zheng, F., Georgescu, R. E., Li, H. & O’Donnell, M. E. Structure of eukaryotic DNA polymerase ẟ bound to the PCNA clamp while encircling DNA. Proc. Natl Acad. Sci. USA 117, 30344–30353 (2020).
Lancey, C. et al. Structure of the processive human Pol ẟ holoenzyme. Nat. Commun. 11, 1109 (2020).
Pustovalova, Y. et al. Interaction between the Rev1 C-terminal domain and the PolD3 subunit of Polζ suggests a mechanism of polymerase exchange upon Rev1/Polζ-dependent translesion synthesis. Biochemistry 55, 2043–2053 (2016).
Wojtaszek, J. L. et al. A small molecule targeting mutagenic translesion synthesis improves chemotherapy. Cell 178, 152–159.e11 (2019).
Gangavarapu, V. et al. Mms2-Ubc13-dependent and -independent roles of Rad5 ubiquitin ligase in postreplication repair and translesion DNA synthesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 26, 7783–7790 (2006).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Afonine, P. V. et al. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D Struct. Biol. 74, 814–840 (2018).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Kozakov, D. et al. The FTMap family of web servers for determining and characterizing ligand-binding hot spots of proteins. Nat. Protoc. 10, 733–755 (2015).
Acknowledgements
This work was funded by grant R35-GM131780 (to A.K.A) from the National Institutes of Health (NIH). I.U.-B. acknowledges support from the Spanish Ministry of Science, Innovation and Universities. Cryo-EM data collection was performed at the Simons Electron Microscopy Center and National Resource for Automated Molecular Microscopy, located at the New York Structural Biology Center, supported by grants from the Simons Foundation (SF349247), NYSTAR and the NIH National Institute of General Medical Sciences (GM103310), with additional support from Agouron Institute (F00316), NIH (OD019994) and NIH (RR029300). We thank J. Wang, J. Mendez and E. T. Eng for assistance with cryo-EM data collection. Computing resources needed for this work were provided in part by the High Performance Computing facility of the Icahn School of Medicine at Mount Sinai. Molecular graphics and analyses were performed with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311.
Author information
Authors and Affiliations
Contributions
R.M., A.K.A., R.E.J. and S.P. designed the experiments; R.E.J. characterized, expressed and assembled the Rev1–Polζ complexes in yeast; R.M. purified the Rev1–Polζ complexes and performed all the cryo-EM studies. R.M. built, characterized and refined the atomic models. A.K.A. guided the overall project; S.P. and L.P. guided the biochemical and protein expression studies. A.K.A., R.M., R.E.J., S.P. and L.P. prepared the manuscript with input from I.U.-B.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Dimitris Typas, in collaboration with the Nature Structural & Molecular Biology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Purification of a Rev1-Polζ holoenzyme complex.
a, Superdex 200 PC3.2/30 Gel filtration purification of the Rev1-Polζ holocomplex. A summary of the purification steps up to the gel filtration step are shown. 1 μl each from fractions 7-20 were separated on a 4-20% gradient SDS-PAGE gel. Molecular weight marker sizes are shown on the left and protein identities are shown on the right. b, Purified Rev1-Polζ. Fractions 8-10 from the gel filtration step (panel A) containing stoichiometric amounts of each subunit of Rev1-Polζ were pooled and incubated with 200 μl Glutathione Sepharose beads to remove any residual prescission protease and the resulting protein preparation was concentrated using a microcon MWCO-100 concentrator. Lane 1, 1.0 μl Rev1-Polζ holocomplex. Molecular weight marker sizes are shown on the left and protein identities are shown on the right. c, SDS gel profile of Rev1-ΔN-Polζ. Coomassie stained 4-20% SDS PAGE of purified Rev1-ΔN-Polζ holocomplex employed for vitrification. d, SDS gel profile of Rev1-Full-Polζ. Coomassie blue stained 4-20% SDS PAGE of purified Rev1-Full-Polζ holocomplex employed for vitrification. All the gels were run twice. Subunit identities and molecular weight markers are shown for each gel.
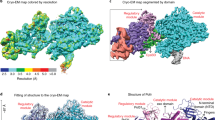
Extended Data Fig. 2 Cryo-EM data processing of the Rev1-ΔN-Polζ holocomplex.
a, Representative electron micrograph is shown. Scale bar is 200 nm. 8353 micrographs were collected and processed for the cryo-EM reconstruction b, An overview of the workflow for the cryo-EM data processing is shown. Schematic shows particle picking using blob picker and Topaz. Various stages of data processing includes 2-D classification and Ab-initio clean-up using cryoSPARC. The representative 2-D classes are shown and the major 3-D class is used for refinement. The map calculated after non-uniform refinement has a FSC0.143 of 2.85 Å. Number of Particles used at key stages of data processing are shown in blue. c, Cryo-EM density map of the Rev1-ΔN-Polζ holocomplex colored by local resolution is shown. Scale bar is in Å.
Extended Data Fig. 3 Cryo-EM data processing of the Rev1-Full-Polζ holocomplex.
a, Representative electron micrograph is shown. Scale bar is 200 nm. 13,404 micrographs were collected and processed for the cryo-EM reconstruction. b, An overview of the workflow for the cryo-EM data processing shows particle picking using Topaz and processing including 2-D classification and Ab-initio reconstruction using cryoSPARC. The representative 2-D classes are shown and the major 3-D class is used for refinement. The map calculated after non-uniform refinement has a FSC0.143 of 3.53 Å. Number of particles at key stages of data processing are highlighted in blue. c, Cryo-EM density map of the Rev1-Full-Polζ holocomplex colored by local resolution. Scale bar is in Å.
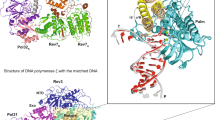
Extended Data Fig. 4 Catalytic Rev3 structure in the Rev1-Polζ holocomplex.
a, Structure of Rev3 colored by domain is shown. Dark blue, brown, magenta, cyan, yellow, orange and gray denote, respectively, the N-terminal, RIR, exo, palm, fingers, thumb and C-terminal domains of Rev3. The DNA is highlighted in red. b, Cryo-EM density of the T0-Po, T1-P1 and T2-P2 bases of the DNA for Rev1-Full-Polζ (left) as well as Rev1-ΔN-Polζ (right) holocomplexes.
Extended Data Fig. 5 The assembly of Rev1 into the Polζ holocomplex in yeast cells depends upon Rev1 BRCT.
Polζ complexes were purified by α-Flag affinity purification of Flag-tagged Rev3 from protein extracts of yeast cells overexpressing individual subunits of the Rev1-Full-Polζ holocomplex and analyzed by SDS-PAGE and Coomassie staining. The complexes were eluted with prescission protease, and free GST and prescission protease were subsequently removed by incubation with Glutathione Sepharose. Lane 1, Rev1-Polζ holocomplex purified from cells expressing wild type Rev3/Rev7/Pol31/Pol32/Rev1 subunits. Rev1 copurifies with the Polζ holoenzyme. Lane 2, Polζ complex from cells as in lane 1, but lacking overexpression of the Pol32 subunit. Rev1 copurifies with the Polζ complex, despite the lack of Pol32. Lane 3, Rev1-Polζ holocomplex from cells as in lane 1, but overexpressing the rev1-1 mutant protein which harbors the G193R mutation within the BRCT domain. The rev1-1 mutant does not copurify with the Polζ complex. Expression of the rev1-1 protein was confirmed by Western blotting of total protein extract using affinity purified α-Rev1 antibodies. The gel was run once. The Protein identities and molecular weight sizes (kDa) are shown on the right and left, respectively.
Extended Data Fig. 6 Overlay of the palm domains of the Rev1-Polζ holocomplex and Pol3 highlighting the outward conformation of the NTD-palm linker in Rev3.
Superimposition of the palm domains of Rev3 and Pol3 shows the NTD-palm linker of Pol3 trekking a path closer to the template DNA (shown in red; T0 and T1 bases are highlighted) in comparison to Rev3.
Extended Data Fig. 7 Comparison of surface representation of BRCT domains of Rev1 with PARP1, RFC, and BRCA1.
a, Structural alignment of the BRCT domains of Rev1, PARP1, RFC and BRCA1. b, Electrostatic surface potential of the Rev1 N-helix-BRCT module shows an extensive positively charged surface (blue) for binding DNA (red). c, d, Electrostatic surface potentials of the PARP1 (c) and RFC BRCT (d) domains are also positively charged (blue) for interacting with DNA (red) e, Electrostatic surface potential of the BRCA1 BRCT1 differs from that of Rev1/PARP1/RFC BRCT domains.
Extended Data Fig. 8 Sequence alignment of BRCT domains from various proteins.
Amino acid sequence alignment of conventional as well as DNA binding BRCT domains is shown. Equivalent residues among all the BRCTs are highlighted in yellow. Secondary structure elements of the Rev1 N-helix-BRCT module are shown above and are derived from the coordinates of Rev1-Polζ holocomplex structure (PDB ID: 8TLQ) using ESpript. Helices are designated as black coils and beta sheets are indicated by blue arrows. Key DNA binding residues in Rev1 BRCT are highlighted by a purple star and those conserved between the yeast and human Rev1 BRCT are highlighted in red.
Extended Data Fig. 9 Druggable ligand-binding sites predicted for the Rev1 CTD/Polζ interface.
Rev1 CTD, Rev7B and a portion of the Rev3-RIR are shown in yellow, green and brown, respectively. Potential ligand binding Site 1 and Site 2, as predicted by FTsite, are shown in salmon and grey colored mesh, respectively. The cluster of probes at each site are shown in sticks. Site 1 represents the Rev1 CTD/Rev7B interface whereas Site 2 highlights the ligand-binding site at Rev1 CTD/Rev7B/Rev3RIR interface.
Extended Data Fig. 10 Model-map FSC curves.
The curves for Rev1-Full-Polζ (a) as well as Rev1-ΔN-Polζ (b) holocomplexes are shown where the FSC0.5 for each of the complexes are 3.8 Å and 3.1 Å, respectively.
Supplementary information
Source data
Source Data Extended Data Fig. 1
Source Data file for Extended Data Fig. 1
Source Data Extended Data Fig. 5
Source Data file for Extended Data Fig. 5
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malik, R., Johnson, R.E., Ubarretxena-Belandia, I. et al. Cryo-EM structure of the Rev1–Polζ holocomplex reveals the mechanism of their cooperativity in translesion DNA synthesis. Nat Struct Mol Biol (2024). https://doi.org/10.1038/s41594-024-01302-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41594-024-01302-w