Abstract
The effects of the amide-linked (lidocaine (LDC), mepivacaine (MPV), prilocaine (PLC)) and ester-bound local anesthetics (benzocaine (BZC), procaine (PRC), and tetracaine (TTC)) on the pore-forming activity of the antifungal lipopeptide syringomycin E (SRE) in lipid bilayers were studied. Independently on electrolyte concentration in the membrane bathing solution the observed changes in conductance of SRE channels agreed with the altered membrane dipole potential under the action of ester-bound local anesthetics. Effects of aminoamides in diluted and concentrated solutions were completely different. At 0.1 M KCl (pH 7.4) the effects of amide-linked anesthetics were in accordance with changes in the membrane surface potential, while at 2 M KCl aminoamides blocked ion passage through the SRE channels, leading to sharp reductions in pore conductance at negative voltages and 100-fold decreases in the channel lifetimes. The effects were not practically influenced by the membrane lipid composition. The interaction cooperativity implied the existence of specific binding sites for amide-bound anesthetics in SRE channels.
Similar content being viewed by others
Introduction
Local anesthetics are compounds causing the suspension of impulse transmission along nerve fibers, and their molecular structure comprises hydrophobic and lipophilic parts connected by amide or ether bonds. Correspondingly, they are divided into amide-linked (e.g., LDC, MPV, and PLC) and ester-bound (e.g., BZC, PRC, and TTC) groups. The chemical structures of local anesthetics are presented on the Fig. 1a. As a rule, at physiological pH, these drugs exist in both neutral and cationic forms. Among the listed anesthetics, only BZC is excluded, which is almost completely uncharged under physiological conditions.
(a) Chemical structures of local anesthetics (LDC, PLC, MPV, PRC, BZC, and TTC). (b) Structures of tested lipopeptides (SRE, syringostatin A, syringotoxin B, and syringopeptin 22 A). The first four lipopeptides differ in the amino acid sequence between positions 2 and 6. The 3-hydroxy fatty acyl group is a derivative of decane, dodecanoic, tetra- or hexadecanoic acid. Abbreviations for nonproteinogenic amino acids: Asp(3-OH) – 3-hydroxyaspartic acid; Dab – 2,4-diaminobutyric acid; Dhb – 2,3-dehydroaminobutyric acid; Hse – homoserine; Orn – ornithine; Thr(4-Chl) – 4-chlorothreonine; aThr – allothreonine.
Local anesthetics interfere with impulse conductions in nerves and muscles by binding to voltage-gated sodium channels and blocking the transient Na+ inward current1. While the major drug mechanism of action is not resolved, experimental evidence favors steric blocking, closed state stabilization, or some combination of the two. Extensive site-directed mutagenesis experiments have provided proof that LDC-like drugs bind in the inner pore2,3,4. However, substantial evidence supports that anesthetics generally perturb bulk membrane structure5,6,7 and, consequently, affect membrane transport, especially ion channels8,9,10. Specifically, a good correlation between the partition coefficients in lipid/water systems (and, consequently, their ability to rearrange membrane lipids) and the clinical potency of the drugs support the hypothesis that membrane lipids are the primary sites of anesthetic action11,12. Lee et al.8 proposed that local anesthetics trigger the transition of surrounding lipids to the more fluid, liquid crystalline phase, allowing the sodium channel to close, resulting in local anesthesia.
Since anesthetics are present in both cationic and non-ionic forms under physiological conditions, the question of whether uncharged or charged species affect the physical properties of the membrane has been raised. Fraceto et al.13 showed that the uncharged versions of LDC and MPV increase the mobility of choline nuclei and decrease the mobility of glycerol-region hydrogens, thus modulating lipid packing. Hata et al.14,15 studied the effects of local anesthetics on the phase transition temperatures of dipalmitoylphosphatidylcholine bilayer membranes by optical and calorimetrical methods. TTC, LDC and PRC depressed the main and pre-transition temperatures of the DPPC bilayers. Moreover, TTC induced complex phase behaviors of dipalmitoylphosphatidylcholine, including the formation of mixed lipid/anesthetic micelles and transition from the interdigated gel phase (rather than the ripple phase) to the liquid crystalline phase at relatively high TTC concentrations. Using high-pressure Fourier transform infrared spectroscopy, Auger et al.16 demonstrated smaller interchain interactions for dimyristoylphosphatidylcholine due to increases in both orientational and conformational disorder caused by uncharged TTC intercalation between lipid acyl chains. The authors concluded that uncharged TTC disorders myristoyl chains, while the charged form induces the formation of an interdigitated gel phase. The authors also showed that cholesterol (CHOL) prevents the formation of the interdigitated phase.
In addition to modifying the elastic properties of membranes, anesthetics electrostatically interact with the lipid bilayer, i.e., the drugs alter the electrical potential at the water/lipid boundary17, termed the membrane boundary potential. The boundary potential consists of two components18,19,20,21,22,23: a surface component (φs), related to the surface charge of the membrane, and the dipole component (φd), due to specifically orientated lipid and water dipoles at the interface, which imparts a highly positive electrostatic potential to the membrane interior membrane in respect to the adjacent aqueous phase and, consequently, regulation of reconstituted ion channels24,25,26,27,28,29,30,31,32,33,34,35. Furthermore, TTC was found to neutralize the negative surface charges of cardiolipin-containing liposomes36. The authors also concluded that TTC increases surface potential more effectively than LDC37. Furthermore, both the charged and uncharged forms of TTC and LDC induce substantial changes in the membrane dipole potential38,39.
This study aimed to establish the mechanism underlying the influence of local anesthetics on SYRingomycin E channels. The antifungal lipopeptide SRE was previously shown to form voltage-gated, predominantly anion-selective asymmetric cone-shaped channels with a narrow peptide and a wide lipid mouth40. The topology of SRE channel water pores is like that of sodium channels in open state, with a relatively wide vestibule and a narrower region, including the selectivity filter41. Moreover, SRE channels are characterized by potential and mechanical sensitivity. Altogether, these facts make SRE channels extremely convenient for studying the binding and lipid-mediated effects of local anesthetics. To test the possibility of binding, we used the drugs of two types, amide-linked (LDC, PLC, and MPV) and ester-bound anesthetics (BZC, PRC, and TTC). Binding cooperativities were evaluated by Hill’s coefficients42. Changes in the cation/anion selectivities of the channels are thought to be determined by the binding anesthetics in a narrow part of the pore. To establish whether the lipid-mediated effects of the drugs are caused by alterations in the surface or dipole components of the membrane boundary potential, two different concentrations of an electrolyte in bilayer bathing solutions were tested (0.1 and 2 M KCl). To identify the possible actions of local anesthetics on lipid packing, pure bilayers from dioleoylphosphocholine (DOPC) were compared with CHOL enriched membranes. Lipid bilayers comprising anionic phospholipids, dioleoylphosphoserine (DOPS), and raft-mimicking mixture of DOPC, sphingomyelin (SM), and CHOL were also tested.
Results and Discussion
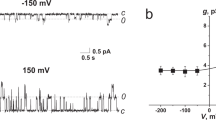
Figure 2a demonstrates the current fluctuations corresponding to the openings and closings of single SRE channels in DOPC membranes bathed in 0.1 M KCl (pH 7.4) in the absence (control) and presence of 10 mM LDC, PLC, MPV, and PRC; 3 mM BZC; and 1 mM TTC. LDC, PLC, MPV, and TTC enhanced the amplitude of the SRE channels, while PRC did not influence this value. BZC significantly reduced the channel conductance. Figure 3a shows the corresponding G(V) curves in the absence and presence of the local anesthetics. LDC and MPV increased G by approximately 15%, while PLC and TTC enhanced G by approximately 30%. Simultaneously, PRC did not change G, while BZC reduces the channel amplitude by approximately 20%. We previously showed that local anesthetics affect the boundary potential of model lipid membranes. Changes in the boundary potential by the positively charged amide-linked anesthetics LDC, PLC, and MPV are due to increases in the surface potential of the bilayer, while TTC increases its dipole potential17. Indeed, the surface potential increment of the DOPC membrane at 10 mM LDC, PLC and MPV was approximately 24 ± 5 mV. The increase in the dipole potential of DOPC bilayers induced by 1 mM TTC equaled 48 ± 14 mV. PRC did not obviously affect the magnitudes of the surface and dipole components of the DOPC membrane boundary potential at concentrations up to 10 mM. Here, we evaluated the changes in dipole potential induced by the addition of 3 mM BZC to the bathing solution, which reduced the dipole potential by 55 ± 8 mV. Comparing the effects of the drugs on the SRE channel current amplitude and electrical potential at the membrane/water interface, the changes in G upon the introduction of anesthetics presumably correspond to the changes in the boundary potential, its dipole or surface components.
Current fluctuations corresponding to the openings and closings of single SRE channels in lipid bilayers in the absence (control) and presence of various local anesthetics: 10 mM LDC, PLC, MPV and PRC; 3 mM BZC; and 1 mM TTC. The membranes were composed of DOPC (a,b) or DOPC:CHOL (67:33 mol%) (c,d) and bathed in 0.1 M (a,c) or 2.0 M KCl (pH 7.4) (b,d). The transmembrane voltage was equal to −150 mV. C – closed state of the channel, O – open state of the pore.
G(V) curves of single SRE channels in the absence (■) and presence of 10 mM LDC (◊), PLC (▼), MPV (Δ) or PRC (▲); 3 mM BZC (*); and 1 mM TTC (●). The membranes were composed of DOPC (a,b), DOPC:CHOL (67:33 mol%) (c,d), DOPS (e) or DOPC:CHOL:SM (47:33:20 mol %) (f) and bathed in 0.1 M (a,c) or 2.0 M KCl (pH 7.4) (b,d,e,f).
To verify the above assumption, G was measured at a high electrolyte concentration in the membrane bathing solution (2 M KCl at pH 7.4), i.e., under the condition of screening the surface potential by electrolyte ions. In this case, only the effects of the membrane dipole potential should be observed. Figure 2b presents the current fluctuations of single SRE channels in DOPC membranes bathed in 2 M KCl (pH 7.4) in the absence and presence of the local anesthetics. TTC increased G, BZC noticeably reduced G, and PRC did not obviously change G. Figure 3b demonstrates the corresponding G(V) curves before and after the addition of the drugs. The observed differences in shapes of the conductance-voltage characteristics of SRE channels in dilute and concentrated electrolyte solutions were discussed in43. TTC increased G by 15%, while BZC reduced the channel amplitude by 30%. The ester-bound anesthetics did not affect the shape of the G(V) curve. Thus, the effects of TTC, PRC, and BZC on G at 0.1 and 2 M KCl are the same and, for this reason, can be attributed to the altered dipole potential. Surprisingly, at 2 M KCl, 10 mM LDC, PLC and MPV pronouncedly reduced the SRE channel conductance at negative voltages. As indicated in the Materials and methods section, negative voltage signs are related to the flow of cations from the trans to the cis side of the experimental chamber. Considering that SRE channels are asymmetric, cone-shaped peptide-lipid pores, with the cis opening being much narrower than the trans opening41, cations of amide-linked anesthetics presumably penetrate the SRE pore from its wider trans mouth, which leads to reductions in G at negative voltages. The electrostatic repulsion between positively charged molecules of amide-bound local anesthetics and SRE prevented the permeation of LDC, PLC and MPV into the channel at low electrolyte concentrations in the membrane bathing solution. Thus, at high electrolyte concentrations with smaller Debye radii, steric blocking of the SRE channel by amide-linked anesthetics might occur.
The blocking of SRE channels by anesthetics should primarily result in decreased channel opening times. To verify this assumption, the dwell times of SRE channels were measured. Table 1 shows that the effects of the amide-bound drugs on the SRE channels at low and high salt concentrations were not the same. These compounds drastically decreased τ at 2 M KCl (up to 20-fold) compared to the slight reduction observed at 0.1 M KCl. The ester-linked anesthetics TTC and BZC reduced τ several fold at both 0.1 and 2 M KCl, while PRC did not obviously change this value.
The slight reductions in SRE channel opening times caused by anesthetics of both types at 0.1 M KCl and by ester-linked compounds at 2 M KCl might be attributed to the generally recognized disordering effect of local anesthetics on the lipid bilayer6,7,8,9,10,12 and/or their influence on a distribution of lateral pressure in a membrane44. CHOL is known to play a key role in controlling membrane fluidity, and the incorporation of CHOL into the membrane leads to the increased ordering of lipid hydrocarbon chains and a reduction in the area per molecule45,46. The current fluctuations of single SRE channels in DOPC membranes enriched with CHOL (33 mol%) and bathed in 0.1 and 2 M KCl in the absence (control) or presence of the drugs are shown in Fig. 2c,d, respectively. Figure 3c,d show the corresponding G(V) curves in the absence and presence of the amide-bound anesthetics and procaine. Table 1 shows that in the absence of anesthetics, the introduction of CHOL into the membrane forming solution increased τ at both 0.1 M and 2 M KCl. As expected, CHOL contributes to the deoligomerization of SRE molecules during pore dismantling. Table 1 shows that the effects of anesthetics on τ in CHOL-free and CHOL-enriched lipid bilayers are similar. Thus, the observed effects of the ester-linked anesthetics at both 0.1 M KCl and 2 M KCl and the amide-bound compounds at 0.1 M KCl on τ are most likely related to membrane disordering. Therefore, significant decreases in τ induced by amide-linked anesthetics might be due to the pore blocking by local anesthetics at 2 M KCl.
It is known that anesthetics show a preference for specific membrane domains, namely the lipid rafts47, and their membrane-fluidizing effects might be influenced by anionic phospholipids48. In order to further examine the assumption that the observed effects of amide-bound drugs on SRE channels do not qualitatively depend on membrane lipid nature, we additionally tested bilayers comprising of pure DOPS (Fig. 4e) and DOPC:CHOL:SM (47:33:20 mol %) (Fig. 4f) at 2 M KCl. Comparing Fig. 4b,d,e,f one can conclude that the effects of amide-linked anesthetics on G are not practically influenced by the membrane lipid composition, in particular, by the presence of anionic lipids or lipid ordered domains enriched with cholesterol and SM. The action of the anesthetics on τ in bilayers comprising of DOPC, DOPS, DOPC:CHOL and DOPC:CHOL:SM are also similar (Table 1). These data are consistent with the hypothesis of direct blocking SRE pore with the anesthetics.
(a) Dependence of the inverse mean dwell times of SRE channels (τon−1) on the concentrations (C) of LDC (■), PLC (▲), and MPV (○). (b) Dependence of the inverse mean dwell times of syringopeptin 22 A (□), syringostatin A (*), syringotoxin B (▲), and CH3-SRE (∆) channels on the concentration of LDC. The straight lines represent the linear approximations of the dependence growth linear regions. The slopes of the lines characterize the numbers of anesthetic molecules interacting with single channels formed by different lipopeptides (m). The membranes were composed of DOPC and bathed in 0.1 M KCl (pH 7.4). The transmembrane voltage was equal to −100 mV.
Using Hyperchem 7.0, we evaluated the mean sizes of the anesthetic molecules, corresponding to their larger linear dimensions. Thus, we approximated the LDC, MPV, PLC, PRC, TTC and BZC molecules by spheres with diameters of 10.3, 7.8, 10.8, 13.4, 16.9, and 10.4 Å (sd = 0.1), respectively. Considering that the mean diameter of the wider lipidic mouth of an SRE channel is approximately 14 Å41, LDC, MPV, PLC, and BZC potentially permeate the SRE pore from the trans side, while PRC and TTC penetration is restricted. The amide-bound drugs have positive charges at neutral pH and can penetrate the SRE pore only at high electrolyte concentrations due to small Debye lengths. BZC is not charged at neutral pH and can reduce G at both 0.1 M and 2 M KCl (Fig. 3a,b, respectively) due to the partial occupation of the pore volume by non-electrolytes.
To evaluate the stoichiometry of blocking the SRE channel with the amide-linked anesthetics, we studied the dependence of τ on the anesthetic concentration (Fig. 4). The dependences are presented in two logarithmic coordinates; in this case, the linear approximation of τ(C) curve growth regions gave the Hill’s coefficients42 for the analysis of anesthetic cooperative binding with the SRE channel. The coefficients were equal to 1 for MPV and PLC, while that for LDC was approximately 2 (Fig. 4a). Thus, the cooperativity of the interaction clearly indicates the existence of specific binding sites for amide-bound anesthetics in SRE channels. Suspecting its aromatic nature, as is the case for voltage-dependent sodium channels, we tested the structurally similar lipopeptides syringotoxin B, syringostatin A, and syringopeptin 22 A and the Asp-methylated form of SRE (CH3-SRE). Unlike SRE, CH3-SRE, and syringopeptin 22 A, syringotoxin B, and syringostatin A do not have any aromatic amino acids in their peptide heads (Fig. 1b). Figure 4b presents the dependence of the inverse mean dwell times of channels formed by CH3-SRE, syringotoxin B, syringostatin A, and syringopeptin 22 A on the LDC concentration in the two logarithmic coordinates. Similar to the SRE pore, two LDC molecules bound single channels formed by all of the tested lipopeptides. Considering that several (about 6) lipopeptide molecules form single conductive unit49 and comparing the structures of the pore-forming compounds, we proposed that dehydroaminobutyric acid residues might be the binding sites because of double bonds in their side chains.
The specific binding of positively charged anesthetics in the narrow, lipopeptide part of the channel should also influence pore selectivity. Figure 5 demonstrates the different cation/anion selectivities of the bilayers treated with SRE in the absence and presence of amide-bound drugs. Before the addition of the anesthetics to the membrane bathing solution at a 10-fold electrolyte gradient through the membrane, the reversal potential was −40 ± 6 mV, while the values of Vrev in the presence of LDC, MPV, and PLC were equal to −4 ± 4, −28 ± 2, and −13 ± 9 mV, respectively. The reversal potential in the absence of the drugs corresponded to the anion transfer number, equaling 0.86 ± 0.07 (Table 1). The similar values of the transfer numbers were obtained in the presence of ester-bound anesthetics. The anion transfer numbers in the presence of 10 mM LDC, MPV, and PLC were 0.53 ± 0.21, 0.75 ± 0.04, and 0.61 ± 0.09, respectively. Thus, the data obtained agree with the assumption that the blocking of SRE channels with amide-bound anesthetics allows their interaction with peptide part similar to those reported for channel proteins1 and unlike the blocking of spontaneous lipid pores with anesthetics50. Figure 6 schematically represents SRE channel blocking with LDC.
Anion/cation selectivities of single SRE channels in DOPC membranes. I(V) curves of bilayers treated with SRE in the absence of the drugs (■) and presence of 10 mM LDC (○), PLC (▲), and MPV (∆) in an asymmetric electrolyte concentration system. The 0.4 M KCl solution (pH 7.4) comprised the cis compartment, while the 4 M KCl solution (pH 7.4) comprised the trans compartment. Inset: the intercepts on the V axis represent the reversal potentials in the absence (Vrev) and presence of LDC (VLDCrev), PLC (VPLCrev), and MPV (VMPVrev).
Conclusions
Based on the above considerations, the amide-linked anesthetics LDC, MPV, and PLC block ion passage through SRE channels at high electrolyte concentrations in the membrane bathing solution. The local anesthetics permeate the SRE pore from its wider trans mouth, which leads to a sharp drop in channel conductance at negative voltages and a decrease in its lifetime by two orders of magnitude. The interaction cooperativity observed with LDC indicates the existence of specific binding sites for amide-bound anesthetics in SRE channels, and possible binding site candidates include dehydroaminobutyric acid residues. The ester-bound anesthetics TTC and BZC affect SRE channel conductance in accordance with changes in the bilayer dipole potential, while alterations in the pore dwell times are related to the disordering effects of anesthetics on lipid bilayers. The action of BZC, which was neutral at the pH used, might include the reduction in channel amplitude due to the permeation of non-electrolytes into the SRE pore.
Materials and Methods
All of the chemicals used in this study were of analytical grade. DOPC, DOPS, SM and CHOL were obtained from Avanti Polar Lipids, Inc. (Pelham, AL). The local anesthetics (BZC and hydrochlorides of LDC, PLC, MPV, PRC, and TTC), KCl, HEPES, and hexadecane were purchased from Sigma Chemical (St. Louis, MO). Water was distilled twice, deionized and degassed.
SRE, syringopeptin 22 A, syringostatin A, and syringotoxin B were isolated and purified as described previously51 and kindly provided by Dr. J.Y. Takemoto (Utah State University, USA). CH3-SRE was synthesized in the lab of Dr. J.Y. Takemoto.
Formation of planar lipid bilayers and registration of single channels
Virtually solvent-free planar lipid bilayers were prepared using a monolayer-opposition technique52 on a 50-μm-diameter aperture in a 10-μm-thick Teflon film separating two (cis and trans) compartments of a Teflon chamber. The aperture was pretreated with hexadecane. Lipid bilayers were made from DOPC, DOPS, and mixtures of DOPC:CHOL (67:33 mol%), and DOPC:CHOL:SM (47:33:20 mol %). Solutions of 0.1 M or 2.0 M KCl were the same in both Teflon chamber compartments and were buffered by 5 mM HEPES-KOH at pH 7.4. After the membrane was completely formed and stabilized, liopeptides from a stock solution (5 mM in water, pH 3.0) were added to the aqueous phase at cis side of the bilayer to obtain a final concentration ranging from 1 to 5 μM for SRE, 0.1 ÷ 0.2 μM for syringopeptin 22 A, 1 ÷ 2 μM for syringostatin A, 3 ÷ 13 μM for syringotoxin B and 4 ÷ 7 μM for CH3-SRE. Ag/AgCl electrodes with 1.5% agarose/2 M KCl bridges were used to apply the transmembrane voltage (V) and measure the transmembrane current (I). “Positive voltage” referred to when the cis side compartment was positive relative to the trans side compartment.
The anesthetics from a 600 mM water stock solution were added to both sides of the membranes at final concentrations in the range from 0.1 to 10 mM for LDC, PLC, MPV; 10 mM for PRC; 3 mM for BZC; and 1 mM for TTC. The choice of the concentrations used for different anesthetics was determined by their buffer/lipid solubility and the dependences of physical properties of lipid bilayers and reconstituted syringomycin channels on the concentration of tested drugs.
Current measurements were carried out using an Axopatch 200B amplifier (Molecular Devices, LLC, Orlean, CA, USA) in the voltage clamp mode. Data were digitized by Digidata 1440 A and analyzed using pClamp 10 (Molecular Devices, LLC, Orlean, CA, USA) and Origin 7.0 (OriginLab Corporation, Northampton, MA, USA). Data acquisition was performed with a 5-kHz sampling frequency and low-pass filtering at 200 Hz. The current tracks were processed through an 8-pole Bessel 100-kHz filter. Single-channel conductance (G) was defined as the ratio between the current flowing through a single SRE channel and the transmembrane voltage. All experiments were performed at room temperature.
The I values and channel dwell times (τ) histograms were constructed for the tested voltages. The relative frequency (n/N) was set as the histogram ordinate, where n was the number of current fluctuations corresponding to a given current or time level and N was the total number of fluctuations. For conductance fluctuation analysis, N ranged from 500 to 2000. Peaks on the I histograms were fitted by the normal density function. For the dwell time histograms, N was equal to 1000–3000, and the distribution was fitted by an exponential density function. The distribution hypotheses were verified using χ2 minimization (P < 0.05).
Estimation of changes in the membrane dipole potential upon BZC adsorption
The steady-state membrane conductance induced by K+-nonactin was modulated via the two-sided addition of BZC to the membrane bathing solution up to a final concentration of 3 mM. Lipid bilayers were made from pure DOPC, and 0.1 M solutions were buffered using 5 mM HEPES–KOH at pH 7.4. Considering that BZC is neutral at pH 7.4, changes in the electrical potential at the membrane/water boundary should be attributed to only changes in the membrane dipole potential (∆φd), and this value can be evaluated assuming that membrane conductance is related to φd by the Boltzmann distribution as follows18:
where Gm and Gm0 are the steady-state membrane conductances induced by K+-nonactin in the presence and absence of the anesthetic, respectively, and e, k, and T have their standard meanings.
Measurement of SRE channel cation/anion selectivity
The transport numbers of K+ (t+) and Cl− (t− = 1 − t+) was assessed by measuring zero current (reversal) potential (Vrev) under 10-fold salt concentration gradient and by using the general expression53
where γ1, γ2, C1 and C2 indicate activity coefficients and KCl concentrations in the cis and trans compartments, respectively. To measure the selectivity in the presence and absence of the drugs SRE treated membranes of similar macroscopic currents were tested.
Computational optimization of the anesthetic molecules
Calculations of the geometric parameters of the LA molecules were performed by HyperChem 7.0 (Hypercube, Inc., Gainesville, FL, USA) using the semi-empirical method established by Hartree-Fock-Rutaan based on STO-3G. Using this method, the error in the bond lengths was 0.003 nm, and that in the angles was 4°. Furthermore, the local anesthetics were approximated as spheres with diameters corresponding to the maximum linear dimensions of the optimized molecules.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Hille, B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J. Gen. Physiol. 69, 497–515 (1977).
Ragsdale, D. S., McPhee, J. C., Scheuer, T. & Catterall, W. A. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 265, 1724–1728, https://doi.org/10.1126/science.8085162 (1994).
Wright, S. N., Wang, S.-Y., Xiao, Y.-F. & Wang, G. K. State-dependent cocaine block of sodium channel isoforms, chimeras, and channels coexpressed with the β1 subunit. Biophys. J. 76, 233–245, https://doi.org/10.1016/S0006-3495(99)77192-4 (1999).
Yarov-Yarovoy, V. et al. Molecular determinants of voltage-dependent gating and binding of pore-blocking drugs in transmembrane segment IIIS6 of the Na(+) channel α subunit. J. Biol. Chem. 276, 20–27, https://doi.org/10.1074/jbc.M006992200 (2001).
Seeman, P. The membrane actions of anesthetics and tranquilizers. Pharmacol. Rev. 24, 585–655 (1972).
Singer, M. A. Interaction of dibucaine and propanol with phospholipid bilayer membranes-effect of alterations in fatty acyl composition. Biochem. Pharmacol. 26, 51–57, https://doi.org/10.1016/0006-2952(77)90129-0 (1977).
Smith, C. P., Auger, M. & Jarrell, H. C. Molecular details on anesthetic–lipid interaction. Ann. N. Y. Acad. Sci. 625, 668–684, https://doi.org/10.1111/j.1749-6632.1991.tb33901.x (1991).
Lee, A. G. Model for action of local anesthetics. Nature. 262, 545–548 (1976).
Kopeikina, L. T., Kamper, E. F., Siafaka, I. & Stavridis, J. Modulation of synaptosomal plasma membrane-bound enzyme activity through the perturbation of plasma membrane lipid structure by bupivacaine. Anesth. Analg. 85, 1337–1343 (1997).
Yun, I. et al. Amphiphilic effects of local anesthetics on rotational mobility in neuronal and model membranes. Biochim. Biophys. Acta. 1564, 123–132, https://doi.org/10.1016/S0005-2736(02)00409-1 (2002).
Janoff, A. S., Pringle, M. J. & Miller, K. W. Correlation of general anesthetic potency with solubility in membranes. Biochim. Biophys. Acta. 649, 125–128, https://doi.org/10.1016/0005-2736(81)90017-1 (1981).
Hattori, M., Dohi, S., Nozaki, M., Niwa, M. & Shimonaka, H. The inhibitory effects of local anesthetics on superoxide generation of neutrophils correlate with their partition coefficients. Anesth. Analg. 84, 405–412 (1997).
Fraceto, L. F., Spisni, A., Schreier, S. & de Paula, E. Differential effects of uncharged aminoamide local anesthetics on phospholipid bilayers, as monitored by 1H-NMR measurements. Biophys. Chem. 115, 11–18, https://doi.org/10.1016/j.bpc.2004.12.003 (2005).
Hata, T., Sakamoto, T., Matsuki, H. & Kaneshina, S. Partition coefficients of charged and uncharged local anesthetics into dipalmitoylphosphatidylcholine bilayer membrane: estimation from pH dependence on the depression of phase transition temperatures. Colloid. Surf. B: Biointer. 22, 77–84, https://doi.org/10.1016/S0927-7765(01)00160-6 (2000).
Hata, T., Matsuki, H. & Kaneshina, S. Effect of local anesthetics on the bilayer membrane of dipalmitoylphosphatidylcholine: interdigitation of lipid bilayer and vesicle-micelle transition. Biophys. Chem. 87, 25–36, https://doi.org/10.1016/S0301-4622(00)00175-7 (2000).
Auger, M. et al. Effects of the local anesthetic tetracaine on the structural and dynamic properties of lipids in model membranes: a high-pressure Fourier transform infrared study. Biochem. 27, 6086–6093, https://doi.org/10.1021/bi00416a038 (1988).
Efimova, S. S., Zakharova, A. A., Schagina, L. V. & Ostroumova, O. S. Local anesthetics affect gramicidin A channels via membrane electrostatic potentials. J. Membr. Biol. 249, 781–787, https://doi.org/10.1007/s00232-016-9926-x (2016).
Andersen, O. S., Finkelstein, A., Katz, I. & Cass, A. Effect of phloretin on the permeability of thin lipid membranes. J. Gen. Physiol. 67, 749–771, https://doi.org/10.1085/jgp.67.6.749 (1976).
Pohl, E. E., Krylov, A. V., Block, M. & Pohl, P. Changes of the membrane potential profile induced by verapamil and propranolol. Biochim. Biophys. Acta. 1373, 170–178, https://doi.org/10.1016/S0005-2736(98)00098-4 (1998).
Peterson, U. et al. Origin of membrane dipole potential: contribution of the phospholipid fatty acid chains. Chem. Phys. Lipids. 117, 19–27, https://doi.org/10.1016/S0009-3084(02)00013-0 (2002).
Ermakov, Y. A. & Sokolov, V. S. Boundary potentials of bilayer lipid membranes: methods and interpretations. Planar Lipid Bilayers (BLMs) and their applications. 7, 109–141 (2003).
Demchenko, A. P. & Yesylevskyy, S. O. Nanoscopic description of biomembrane electrostatics: results of molecular dynamics simulations and fluorescence probing. Chem. Phys. Lipids 160, 63–84, https://doi.org/10.1016/j.chemphyslip.2009.05.002 (2009).
Starke-Peterkovic, T. & Clarke, R. J. Effect of headgroup on the dipole potential of phospholipid vesicles. Eur. Biophys. J. 39, 103–110, https://doi.org/10.1007/s00249-008-0392-y (2009).
Rokitskaya, T. I., Antonenko, Y. N. & Kotova, E. A. Effect of the dipole potential of a bilayer lipid membrane on gramicidin channel dissociation kinetics. Biophys. J. 73, 850–854, https://doi.org/10.1016/S0006-3495(97)78117-7 (1997).
Rokitskaya, T. I., Kotova, E. A. & Antonenko, Y. N. Membrane dipole potential modulates proton conductance through gramicidin channel: movement of negative ionic defects inside the channel. Biophys J. 82, 865–873, https://doi.org/10.1016/S0006-3495(02)75448-9 (2002).
Luchian, T. & Mereuta, L. Phlorizin- and 6-ketocholestanol-mediated antagonistic modulation of alamethicin activity in phospholipid planar membranes. Langmuir. 22, 8452–8457, https://doi.org/10.1021/la0613777 (2006).
Ostroumova, O. S., Kaulin, Y. A., Gurnev, P. A. & Schagina, L. V. Effect of agents modifying the membrane dipole potential on properties of syringomycin E channels. Langmuir. 23, 6889–6892, https://doi.org/10.1021/la7005452 (2007).
Asandei, A., Mereuta, L. & Luchian, T. Influence of membrane potentials upon reversible protonation of acidic residues from the OmpF eyelet. Biophys. Chem. 135, 32–40, https://doi.org/10.1016/j.bpc.2008.02.018 (2008).
Mereuta, L., Luchian, T., Park, Y. & Hahm, K. S. Single-molecule investigation of the interactions between reconstituted planar lipid membranes and an analogue of the HP(2–20) antimicrobial peptide. Biochem. Biophys. Res. Commun. 373, 467–472, https://doi.org/10.1016/j.bbrc.2008.04.046 (2008).
Asandei, A. & Luchian, T. Ion selectivity, transport properties and dynamics of amphotericin B channels studied over a wide range of acidity changes. Colloids Surf. B Biointerfaces. 67, 99–106, https://doi.org/10.1016/j.colsurfb.2008.08.006 (2008).
Apetrei, A., Mereuta, L. & Luchian, T. The RH 421 styryl dye induced, pore model-dependent modulation of antimicrobial peptides activity in reconstituted planar membranes. Biochim. Biophys. Acta. 1790, 809–816, https://doi.org/10.1016/j.bbagen.2009.04.002 (2009).
Ostroumova, O. S., Malev, V. V., Ilin, M. G. & Schagina, L. V. Surfactin activity depends on the membrane dipole potential. Langmuir. 26, 15092–15097, https://doi.org/10.1021/la102691y (2010).
Mereuta, L., Asandei, A. & Luchian, T. Meet me on the other side: trans-bilayer modulation of a model voltage-gated ion channel activity by membrane electrostatics asymmetry. PLoS One 6, e25276, https://doi.org/10.1371/journal.pone.0025276 (2011).
Ostroumova, O. S., Efimova, S. S. & Schagina, L. V. Probing amphotericin B single channel activity by membrane dipole modifiers. PLoS One 7, e30261, https://doi.org/10.1371/journal.pone.0030261 (2012).
Efimova, S. S., Schagina, L. V. & Ostroumova, O. S. Channel-forming activity of cecropins in lipid bilayers: effect of agents modifying the membrane dipole potential. Langmuir. 30, 7884–92, https://doi.org/10.1021/la501549v (2014).
Shimooka, T., Shibata, A. & Terada, H. The local anesthetic tetracaine destabilizes membrane structure by interaction with polar headgroups of phospholipids. Biochim. Biophys. Acta. 1104, 123–132, https://doi.org/10.1016/0005-2736(92)90039-O (1992).
Yokoyama, S. Correlation between pharmacological potency and micellar surface potential of local anesthetic. Toxicol. Lett. 100–101, 365–368, https://doi.org/10.1016/S0378-4274(98)00208-2 (1998).
Hianik, T. et al. The electrostriction, surface potential and capacitance relaxation of bilayer lipid membranes induced by tetracaine. Bioelectrochem. Bioenerg. 46, 1–5, https://doi.org/10.1016/S0302-4598(98)00119-6 (1998).
HoЁgberg, C.-J. & Lyubartsev, A. P. Effect of local anesthetic lidocaine on electrostatic properties of a lipid bilayer. Biophys. J. 94, 525–531, https://doi.org/10.1529/biophysj.107.104208 (2008).
Malev, V. V. et al. Syringomycin E channel: a lipidic pore stabilized by lipopeptide? Biophys. J. 82, 1985–1994, https://doi.org/10.1016/S0006-3495(02)75547-1 (2002).
Ostroumova, O. S., Gurnev, P. A., Schagina, L. V. & Bezrukov, S. M. Asymmetry of syringomycin E channel studied by polymer partitioning. FEBS Letters. 581, 804–808, https://doi.org/10.1016/j.febslet.2007.01.063 (2007).
Stefan, M. I. & Novere, N. L. Cooperative binding. PLoS Comput. Biol. 9, e1003106, https://doi.org/10.1371/journal.pcbi.1003106 (2013).
Malev, V. V. et al. Spatial charge distribution effects in the conductance of syringomycin E ion channels formed in lipid bilayers. Biol. Membrany (in Russian). 18, 145–153 (2001).
Weinrich, M., Rostovtseva, T. K. & Bezrukov, S. M. Lipid-dependent effects of halothane on gramicidin channel kinetics: a new role for lipid packing stress. Biochemistry. 48, 5501–5503, https://doi.org/10.1021/bi900494y (2009).
Róg, T., Pasenkiewicz-Gierula, M., Vattulainen, I. & Karttunen, M. Ordering effects of cholesterol and its analogues. Biochim. Biophys. Acta. 1788, 97–121, https://doi.org/10.1016/j.bbamem.2008.08.022 (2009).
Ohvo-Rekilä, H., Ramstedt, B., Leppimäki, P. & Slotte, J. P. Cholesterol interactions with phospholipids in membranes. Progress in Lipid Research. 41, 66–97, https://doi.org/10.1016/S0163-7827(01)00020-0 (2002).
Bandeiras, C., Serro, A. P., Luzyanin, K., Fernandes, A. & Saramago, B. Anesthetics interacting with lipid rafts. Eur. J. Pharm. Sci. 48, 153–165, https://doi.org/10.1016/j.ejps.2012.10.023 (2013).
Tsuchiya, H., Ueno, T., Mizogami, M. & Takakura, K. Local anesthetics structure-dependently interact with anionic phospholipid membranes to modify the fluidity. Chem. Biol. Interact. 183, 19–24, https://doi.org/10.1016/j.cbi.2009.10.006 (2010).
Feigin, A. M., Takemoto, J. Y., Wangspa, R., Teeter, J. H. & Brand, J. G. Properties of voltage-gated ion channels formed by syringomycin E in planar lipid bilayers. J. Membr. Biol. 149, 41–47, https://doi.org/10.1007/s002329900005 (1996).
Blicher, A., Wodzinska, K., Fidorra, M., Winterhalter, M. & Heimburg, T. The temperature dependence of lipid membrane permeability, its quantized nature, and the influence of anesthetics. Biophys. J. 96, 4581–4591, https://doi.org/10.1016/j.bpj.2009.01.062 (2009).
Bidwai, A. P. & Takemoto, J. Y. Bacterial phytotoxin, syringomycin, induces a protein kinase-mediated phosphorylation of red beet plasma membrane polypeptides. Proc. Natl. Acad. Sci. USA 84, 6755–6759 (1987).
Montal, M. & Muller, P. Formation of bimolecular membranes from lipid monolayers and study of their electrical properties. Proc. Nat. Acad. Sci. USA 65, 3561–3566 (1972).
Morf, W. E. Calculation of liquid-junction potentials and membrane potentials on the basis of the Planck theory. Anal. Chem. 49, 810–813, https://doi.org/10.1021/ac50014a035 (1977).
Acknowledgements
The work was supported by the Russian Science Foundation (#14-14-00565-P).
Author information
Authors and Affiliations
Contributions
O.O. designed the research and contributed to the acquisition of funding. A.Z. and S.E. performed the experiments and analyzed the results. A.Z. and O.O. wrote the manuscript. L.S. and V.M. contributed to manuscript draft and data management.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zakharova, A.A., Efimova, S.S., Schagina, L.V. et al. Blocking ion channels induced by antifungal lipopeptide syringomycin E with amide-linked local anesthetics. Sci Rep 8, 11543 (2018). https://doi.org/10.1038/s41598-018-30077-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30077-6
This article is cited by
-
Fengycin induces ion channels in lipid bilayers mimicking target fungal cell membranes
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.