Abstract
Elevation of the levels of reactive oxygen species (ROS) is a major tissue-degenerative phenomenon involved in aging and aging-related diseases. The detailed mechanisms underlying aging-related ROS generation remain unclear. Presently, the expression of microRNA (miR)-142-5p was significantly upregulated in bone marrow mesenchymal stem cells (BMMSCs) of aged mice. Overexpression of miR-142 and subsequent observation revealed that miR-142 involved ROS accumulation through the disruption of selective autophagy for peroxisomes (pexophagy). Mechanistically, attenuation of acetyltransferase Ep300 triggered the upregulation of miR-142 in aged BMMSCs, and miR-142 targeted endothelial PAS domain protein 1 (Epas1) was identified as a regulatory protein of pexophagy. These findings support a novel molecular mechanism relating aging-associated ROS generation and organelle degradation in BMMSCs, and suggest a potential therapeutic target for aging-associated disorders that are accompanied by stem cell degeneration.
Similar content being viewed by others
Introduction
Aging worsens functions of human tissues and organs at multiple levels, causing a gradual reduction in the ability to resist stress, damage, and various related diseases. Cellular senescence is considered an important aging hallmark and the direct reason for the above mentioned changes1,2. In recent years, accumulating evidence has indicated that reactive oxygen species (ROS), which include superoxide anion and hydroxyl radicals, generated from both intrinsic and extrinsic events induce cell damage and senescence during aging. There are numerous studies, which report that ROS and oxidative damage increase with age3,4, that reducing oxidative damage extends the lifespan of various model organisms, and increased production of ROS shortens their lifespan5. ROS contribute to cellular senescence onset and progression by damaging mitochondrial DNA (mtDNA) and modifying the telomerase reverse transcriptase (TERT) enzyme6, histones, and DNA by acting in interconnected epigenetic phases7,8. Furthermore, high ROS levels provoke p53 activation, which induces p53-mediated apoptosis and cell senescence9. Although all cells in an organism can be affected by the accumulation of ROS, the effects of ROS on stem cells are particularly important for understanding the processes of aging and its related diseases10,11. Accumulation of oxidative damage in stem cells can lead to loss of stemness, cell transformation, tumorigenesis, or tissue injury11. Thus, elucidating the molecular mechanisms underlying ROS accumulation in stem cells is important to develop therapies for inhibiting the underlying cause of aging-related tissue dysfunction or diseases.
Degenerated cellular organelles are a major source of ROS. Oxygen is consumed in various metabolic reactions in different intracellular locations, with mitochondria, ER, and peroxisomes being the major sites; thus, dysfunction of these organelles directly leads to the generation of a large amount of ROS12. Among these, the peroxisome is a very important source of ROS, which is mainly produced through metabolic pathways, including fatty acid β-oxidation, photorespiration, nucleic acid, and polyamine catabolism13,14,15. It has been estimated that about 35% of all H2O2 formed in the rat liver is derived from its peroxisomes. The number, morphology, and size of peroxisomes are dynamically regulated in response to environmental and developmental cues16. Selective autophagy for peroxisomes, called pexophagy, is a quality control mechanism to maintain the proper function of peroxisomes15. However, the decline of autophagic activity and accumulation of damaged macromolecules and organelles are well known characteristics of aged cells17,18,19. Importantly, increases in peroxisome number,20 along with reduction of peroxisomal enzymes20,21 during aging, has also been reported. Although partial mechanisms inducing the decreased expression of genes associated with autophagy have been discovered22, the mechanisms underlying the impairment of autophagic function by aging remain poorly understood.
Recently, short strand noncoding RNAs called miRNAs have been found to be involved in the regulation of autophagy23. During aging, it has been reported that certain miRNAs are involved in aging-associated degenerative changes, including inhibition of autophagy and promotion of ROS generation24,25,26.
Here we identified miR-142-5p (miR-142) as a prominent miRNA in aged BMMSCs and found that miR-142 induced increasing numbers of peroxisomes and cellular ROS levels by inhibiting pexophagy through the suppression of Epas1 expression.
Results
To identify miRNAs upregulated in aged BMMSCs, we performed miRNA-sequencing for PαS-double positive cells collected from the bone-marrow (BM) of young and aged mice. It was revealed that 32 miRNAs exhibited a log2 fold change of >3 in the aged BMMSCs. Among these, we chose miRNAs showing a mean value of relative expression >1,000 and focused on miR-142 since it showed the largest difference in expression levels between young and aged BMMSCs (Table S1), and its expression level was higher than ubiquitously expressed miRNA in undifferentiated BMMSCs such as miR-125b27, miR-20428, and let7c29. Quantitative RT-PCR (qRT-PCR) showed the upregulation of miR-142 in the BM and in BMMSCs collected from aged mice (Figs. 1 and S1).
Next, we examined if miR-142 was involved in ROS generation in BMMSCs. When BMMSCs were transfected with a miR-142 mimic, ROS levels increased (Fig. 2A). To determine the reason for the increased ROS levels, we performed electron-microscopic observation of BMMSCs transfected with the miR-142 mimic. Using TEM, we found that the number of peroxisomes increased in BMMSCs containing the miR-142 mimic (Fig. 2B,C). From these results, we hypothesized that miR-142 induced ROS generation via induction of peroxisome accumulation.
miR-142 promotes ROS generation and peroxisome accumulation in BMMSCs in vitro. (A) ROS detection by the CellRox system in BMMSCs transfected with the miR-142 mimic. (B) Transmission electron microscopic (TEM) image of BMMSCs transfected with the miR-142 mimic. Left panel is TEM of BMMSCs expressing scrambled sequences, and right panel shows TEM of BMMSCs containing the miR-142 mimic. White arrows show peroxisomes. Scale bar = 3 μm. (C) Number of peroxisomes per one cell slide (N = 10). Asterisk represents a significant difference compared with the control cells transfected with scrambled RNA at P < 0.05.
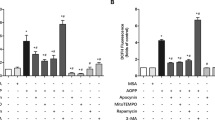
As a regulation mechanism of peroxisomal abundance, we focused on pexophagy, which is one of the most important regulatory mechanisms of peroxisomes. To detect peroxisome and pexophagic activity, we produced transgenic cells expressing peroxisome-targeted DsRed (DsRed-PTS1) and LC3B-GFP reflecting autophagy. Under normal conditions, approximately 33% of DsRed-positive peroxisomes overlapped with GFP fluorescence, indicating that these peroxisomes underwent pexophagy. When the cells were observed 1 h after rapamycin treatment, the abundance of GFP/DsRed-double-positive particles increased, while the DsRed single positive particles slightly decreased (Fig. S2). When the cells were treated with Wy-14643, which is a selective agonist of peroxisome proliferator-activated receptor-α30, the abundance of DsRed positive particles significantly increased (Fig. S2). Based on these results, we concluded that our system was useful for detecting both peroxisomes and pexophagy. By transfecting the miR-142 mimic, the abundance of DsRed-positive particles representing peroxisomes significantly increased. However, GFP signals that represented autophagy were suppressed in the cells (Fig. 3A,B). To determine whether the pexophagy activity was affected by miR-142, we produced a pexophagy reporter that is a GFP and PTS1-tagged tandem monomeric DsRed (RFP). The reporter protein locates to the peroxisomes by the PTS1 peroxisome targeting signal, and when an autophagosome targets the peroxisome, the GFP domain of the reporter is digested by lysosomes and the DsRed fragment, which is relatively stable, remained31. Under autophagy-induced culture conditions, a clear band showing the DsRed domain was detected. Conversely, the DsRed fragments were decreased by transfection of miR-142 mimic (Fig. 3C)
Selective autophagy for peroxisome is suppressed by miR-142. (A) Fluorescent imaging of pexophagy in BMMSCs transfected with the miR-142 mimic. (B) Fluorescent imaging-based quantification of peroxisome and pexophagy in BMMSCs transfected with the miR-142 mimic (N = 6). Asterisk represents a significant difference compared with the control at P < 0.05. (C) Detection of pexophagy activity using the PTS1-tagged GFP-DsRed tandem reporter. The GFP-DsRed is cleaved and the GFP is digested by lysosomal enzymes to yield the DsRed fragment. Decreased intensity of the DsRed band indicates the attenuation of pexophagy activity. αRFP (long exp) indicates the band obtained by prolonged exposure with the anti-RFP antibody (αRFP).
To elucidate the mechanism of the miR-142-mediated pexophagy regulation, we performed target prediction using miRDB32 and DIANA-Tarbase v 7.033. From the results of the prediction analysis and previous studies34, we hypothesized that miR-142 affects peroxisome abundance through suppression of Epas1, which was an important regulator of pexophagy, in aged BMMSCs. To demonstrate that Epas1 plays an important role in pexophagy during aging, we observed the expression level of Epas1 in young and aged BMMSCs, and then performed siRNA-mediated suppression of Epas1 to evaluate pexophagy activity using the DsRed-PTS1/LC3-GFP system in BMMSCs. In the aged BMMSCs, Epas1 expression was suppressed compared with young BMMSCs (Fig. 4A). Treatment with siRNA against Epas1 (siEpas1) resulted in approximately 85% suppression of Epas1 mRNA compared with control treated with a scrambled siRNA sequence and ROS generation (Figs. 4B and S3). Western blot (WB) analysis showed that decreased Epas1 expression also occurred at the protein level with siRNA treatment (Fig. 4C). The decreased amount of Epas1 affected the pexophagy-related genes Pex10 and Pex14. Consistent with previous studies34, peroxisomal abundance increased and LC3-GFP-positive peroxisomes showing pexophagy decreased under siEpas1 conditions (Figs. 4D and S3). Consistently, the GFP-DsRed tandem reporter assay also shows that the reduction of Epas1 expression suppressed pexophagy activity (Fig. 4E).
Epas1 is involved in regulation of pexophagy in BMMSCs. (A) Expression level of Epas1 mRNA in young and aged PαS fractions. Asterisk represents a significant difference between young and aged samples at P < 0.05. (B) Downregulation of Epas1 and the pexophagy-related genes Nbr1, Pex10 and Pex14 by siRNA (siEpas1) treatment in the BMMSCs (N = 6). SCR = BMMSCs treated with scrambled control RNA. Asterisk represents a significant difference compared with the control at P < 0.05. (C) WB analysis of Epas1 expression in the siEpas1-treated BMMSCs. (D) Fluorescent imaging-based quantification of peroxisomes and pexophagy in the BMMSCs transfected with siEpas1 (N = 6). Asterisk represents a significant difference compared with the control at P < 0.05. (E) Determination of pexophagy activity with the PTS1-tagged GFP-DsRed tandem reporter in BMMSCs transfected with siEpas1. αRFP (long exp) indicates the band obtained by prolonged exposure with the anti-RFP antibody (αRFP).
We investigated if miR-142 downregulated Epas1 expression in BMMSCs. As expected, the expression level of Epas1 was suppressed in BMMSCs transfected with the miR-142 mimic (Fig. 5A). WB analysis also showed decreased expression of Epas1 in BMMSCs transfected with the miR-142 mimic (Fig. 5B). To confirm if miR-142 targets Epas1, we constructed a luciferase (Luc) expression plasmid containing the 3′ UTR sequence of Epas1 and compared the expression levels between wild-type Luc and Luc-Epas1 3′ UTR in BMMSCs transfected with the miR-142 mimic. In normal BMMSCs, the expression levels of Luc were not different either with or without Epas1 3′ UTR. In contrast, expression of Luc-Epas1 3′ UTR was significantly suppressed in BMMSCs transfected with the miR-142 mimic (Fig. 5C). To further determine the molecular relationship between miR-142, Epas1, and pexophagy, we performed a compensation experiment using an overexpression plasmid for an active mutant of Epas1 (acEpas1), which is stable even under normoxia and lacks a 3′ UTR35. Consistent with our hypothesis, the introduction of acEpas1 restored pexophagy activity and suppressed the miR-142-induced peroxisomal accumulation (Fig. 5D,E), indicating that the miR-142-mediated suppression of pexophagy occurred via regulation of Epas1. To determine whether the suppression of pexophagy by miR-142 was canceled by overexpression of acEpas1, we performed the GFP-DsRed tandem reporter assay and observed that pexophagy activity was recovered by acEpas1 expression (Fig. 5F).
miR-142 downregulates Epas1. (A) qRT-PCR of Epas1 expression in BMMSCs treated with the miR-142 mimic (N = 6). Asterisk represents a significant difference compared with the control at P < 0.05. (B) WB analysis of Epas1 expression in BMMSCs treated with scrambled control RNA (control) or with the miR-142 mimic. (C) Luc assay with the Luc-Epas1 3′ UTR construct and the miR-142 mimic (N = 3). Asterisk represents a significant difference compared with the control at P < 0.05. (D) Fluorescent imaging showing the results of the compensation experiment with the active form of Epas1 (acEpas1) in BMMSCs treated with the miR-142 mimic. (E) Fluorescent imaging-based quantification of peroxisomes and pexophagy in BMMSCs transfected with the miR-142 mimic, and both the miR-142 mimic and the acEpas1 encoding plasmid (N = 6). Asterisk represents a significant difference compared with the control at P < 0.05. (F) Determination of pexophagy activity with the PTS1-tagged GFP-DsRed tandem reporter in BMMSCs transfected with the miR-142 mimic and/or the acEpas1 encoding plasmid. αRFP (long exp) indicates the band obtained by prolonged the exposure with anti-RFP antibody (αRFP).
Next, we examined the molecular mechanisms responsible for upregulation of miR-142 in aged tissues. It has been demonstrated that the expression level of miR-142 is suppressed by acetyltransferase Ep30036. Ep300 is a protein that undergoes aging-associated downregulation37. We found that the expression level of Ep300 was lower in aged BM and BMMSCs than in young mice (Figs. 6A and S1). From these data, we hypothesized that suppression of Ep300 increased expression of miR-142 in aged BMMSCs. To validate this hypothesis, we suppressed expression of Ep300 with a siRNA in BMMSCs and observed the expression levels of miR-142 and Epas1. When the BMMSCs were treated with siRNA against Ep300 (siEp300), a significant decrease in Ep300 (Fig. 6B,C), an increase in miR-142, and a decrease in Epas1 expression were observed (Fig. 6D). In the siEP300 treated cells, amounts of ROS levels, accumulation of peroxisomes, and amounts of pexophagy were examined. Transfection of siEP300 increased intra-cellular ROS levels (Fig. 6E). The effect of siEp300 and miR-142 was additive; both the fluorescence imaging assay with the DsRed-PTS1/LC3-GFP system and the GFP-DsRed tandem reporter assay showed that co-transfection of the miR-142 mimic and siEP300 increased suppression of pexophagy compared with the single transfection of miR-142 (Fig. 6F,G).
Downregulation of Ep300 leads to overexpression of miR-142 in BMMSCs. (A) qRT-PCR of Ep300 in PαS-BMMSCs collected from young and aged mice (N = 6). Asterisk represents a significant difference compared with the PαS-BMMSCs from young mice at P < 0.05. (B) qRT-PCR of Ep300 in BMMSCs treated with scrambled control RNA (control) or with siRNA against Ep300 (siEp300) (N = 3). Asterisk represents a significant difference compared with the control at P < 0.05. (C) WB analysis of Ep300 expression in BMMSCs treated with scrambled control RNA (control) or with siEp300. (D) qRT-PCR of miR-142 and Epas1 in BMMSCs treated with siEp300 (N = 3). Asterisk represents a significant difference compared with the control at P < 0.05. (E) ROS detection by the CellROX system in BMMSCs transfected with siEp300. (F) Fluorescent imaging-based quantification of peroxisomes and pexophagy in BMMSCs transfected with the miR-142 mimic and siEp300 (N = 6). Asterisk represents a significant difference compared with the control at P < 0.05. (G) Determination of pexophagy activity with the PTS1-tagged GFP-DsRed tandem reporter in BMMSCs transfected with the miR-142 mimic and/or siEp300. αRFP (long exp) indicates the band obtained by prolonged exposure with the anti-RFP antibody (αRFP).
From these results, we concluded that miR-142 received suppressive regulation by Ep300 and that disruption of Ep300 during aging could be a trigger for the upregulation of miR-142 expression and the subsequent disruption of pexophagy regulation and ROS generation in aged cells.
Discussion
Accumulated ROS disturbs the maintenance and proliferative ability of stem cells38 by activating cell cycle suppressors39 or inducing differentiation commitment40,41. Furthermore, elevated ROS levels in MSCs reduce their engraftment potential and induce apoptosis after transplantation26,42. Thus, elucidating the molecular mechanisms underlying ROS accumulation and the development of therapeutic ROS modulation are essential to address aging and aging-related diseases. As a molecule involved in ROS accumulation in aged stem cells, we focused on the aging-associated miRNAs and performed miRNA sequencing. The results revealed that miR-142 was significantly upregulated in aged BMMSCs.
Consistent with our observation, Park et al., also found that the expression level of miR-142-5p increases in dendritic cells derived from aged bone marrow43. However, Zang et al. reported that the expression level of miR-142 in serum decreases during the aging process44. Tissue or cell lineage specificity could be responsible for the discrepancy. For example, Zang et al. observed decreased expression of miR-29b in serum obtained from aged individuals. However, miR-29b is upregulated in aged brain45, aorta46, and cochlear tissues47. These findings highlight that care is needed in the selection of miR-142 as a marker for detection of tissue/organ aging or as a common molecule inducing stem cell aging in different tissues.
In the cultured BMMSCs transfected with miR-142, an increased ROS level was detected with the CellROX assay. Previously, several research groups have demonstrated the involvement of miRNA in ROS generation. For example, miR-210, which is upregulated during hypoxia, induces accumulation of ROS and apoptosis48 by suppressing mitochondrial activity49. miR-14250, miR-37751 and miR-200c52 are also reported to be involved in ROS generation by targeting anti-oxidant genes. However, suppression of antioxidants such as Nrf2, Sod1, Foxo1, and Foxo3 by miR-142 was not significant in the present study (Fig. S4). In a previous study, we addressed a mechanism of the aging-associated miRNA (Ag-miRNA) induced ROS generation and revealed that miR-155 inhibits expression of antioxidant related genes and increased ROS levels by debilitating tolerance against oxidation stresses. We hypothesized that miR-142 induced ROS accumulation through a considerably different mechanism from the previous models since overexpression of miR-142 further exacerbated the miR-155-induced ROS accumulation (Fig. S5). Interestingly, TEM observation clearly revealed that the number of peroxisomes increased in BMMSCs transfected with the miR-142 mimic. This phenomenon was reproducible even when unpassaged primary BMMSCs with short culture period (120 h) were used (Fig. S6).
Under normal physiological conditions, the amount of peroxisomes is regulated by a balance between biogenesis and turnover. Especially, the selective autophagy of peroxisomes—pexophagy—is an important process responsible for the maintenance of peroxisomal quality and quantity53. Senescent cells contain approximately twice as many peroxisomes immunolabeled with the peroxisomal membrane protein 70 kDa (PMP70) or Pex14p20, as compared to early passage cells. Similarly, peroxisomes proliferate in the human retinal epithelia of aged individuals54. These findings indicate that the turnover of peroxisomes is disrupted in aged cells/tissues, and could be a cause of ROS generation that occurs with aging. In our study with a transgenic BMMSC line stably expressing LC3-GFP/DsRed-PTS1, we hypothesized that miR-142 suppressed pexophagy and led to increased numbers of peroxisomes. To date, it has been posited that a ubiquitin-binding autophagic receptor Nbr1 is necessary and sufficient for pexophagy activity34,55. On the other hand, miR-142 did not seem to affect the gene expression of Nbr1 at least in our study. Compared with the direct suppression of Nbr1 by siRNA, in which a three-fold increase in peroxisomes compared with control was detected, the effect of miR-142 overexpression for peroxisome accumulation was weak. However, the function of miR-142 was additive to siNbr1 treatment (Figs. S7 and S8). These findings indicate that miR-142 regulates pexophagy by targeting an upstream or independent molecule from Nbr1. We investigated the direct targets of miR-142 using target prediction with open software, and focused on Epas1 (Hypoxia inducible factor 2A), which is a master regulator of the adaptive response to hypoxia. Walter et al. reported that hypoxia inducible factor (HIF) 2A ensures the efficient depletion of the peroxisome pool by simultaneously inducing pexophagy34. As a possible reason for the regulation of pexophagy by Epas1, peroxisomal function may be highly dependent on oxygen concentration56. Thus HIF signaling that is used to adapt to low oxygen conditions could be a suitable molecule to regulate the numbers of peroxisomes and their oxygen consumption. Although the aging-dependent expression changes of Epas1 in BMMSCs are still unclear, significant downregulation of Epas1 in aged PαS BMMSCs were observed in the present study. Evidence supporting the idea that Epas1 was reduced in aged BM cells was also found in a previous gene expression profile57. These data prompted our hypothesis that Epas1 could be a promising candidate mediating miR-142 expression and the dysregulation of pexophagy in aged BMMSCs. Consistent with a previous report34, suppression of Epas1 resulted in deterioration of pexophagy and accumulation of peroxisomes. Furthermore, consistent with our expectation, Epas1 expression was suppressed in BMMSCs transfected with the miR-142 mimic. However, overexpression of Epas1 counteracted the miR-142 induced proliferation of peroxisomes. From these results, we concluded that miR-142 suppressed pexophagy via regulation of Epas1.
As a factor triggering the upregulation of miR-142, we focused on Ep300. It has been reported that miR-142 is downregulated by acetyltransferase Ep30036. Importantly, Ep300 appears to be downregulated with aging37 and with increasing doubling times in cultured cells58. Furthermore, it has also been reported that inhibition of Ep300 can induce aging phenotypes, such as senescence associated β-galactosidase expression58 and accumulation of DNA damage59. Consistent with our expectation, suppression of Ep300 led to increased expression of miR-142, accumulation of peroxisomes, and accumulation of ROS. These data support the conclusion that there is a molecular pathway that is responsible for inducing ROS generation in aged BMMSCs. In the first step of this pathway, reduction of Ep300, which occurs during aging or cellular senescence processes, leads to upregulation of miR-142. The increased miR-142 targets Epas1, and the reduced level of Epas1 results in disruption of the normal maintenance of peroxisomes (Fig. 7) . From the perspective of a therapeutic application, miR-142 could be an attractive target for miRNA-targeting therapy, since miR-142 is highly expressed in Alzheimer’s disease60, multiple sclerosis61, and osteoporosis62, in addition to aged tissues. These findings indicated that the inhibition of miR-142 using a specific inhibitor could be a potential therapeutic approach for some chronic diseases and various aging-associated cell/tissue degenerations.
Schematic representation of miR142 regulation and function of the disruption of pexophagy and ROS generation. In normal (young) cells, the amount and quality of peroxisomes are maintained by pexophagy. In aged cells, the expression level of Ep300 that possesses a repressor molecule against miR-142 is downregulated. The upregulated miR-142 targets Epas1 and downregulation of pexophagic activity is induced.
Obviously, it is impossible to explain the aging-associated deterioration of peroxisomal maintenance and ROS generation only by the above scheme. We think there are at least three limitations. First, reduction of Ep300 and Epas1 may possibly generate ROS without mediation of miR-142. For example, Sun et al., reported that acetylation of Nrf2 by CBP/Ep300 involves maximum binding of Nrf2 to specific ARE-containing promoters63. This suggests that the reduction of Ep300 could lead to the wasting of anti-oxidation-related gene expression. Furthermore, several studies showed that depletion of Epas1 expression can reduce the expression of genes related to anti-oxidant systems, such as Sod1, catalase, and heme oxygenase 1 (Hmox1)64,65. Second, miR-142 expression can be enhanced not only by reduction of Ep300 but also by stimulation with enhancer molecules. The inflammatory cytokines, IL-4 and IL-13, can trigger miR-142 expression66. It is well known that aging tissues are in an inflammation-like condition and express various inflammatory molecules. Therefore, it is highly possible that these cytokine signals and Ep300 downregulation simultaneously enhance expression of miR-142 in the actual tissues where aging-associated degeneration occurs. Third, we should pay attention to the roles of miR-142 in normal development and tissue homeostasis. In general, miRNAs have multiple target genes. Thus, understanding its original role is very complicated. For example, even miR-21, which is one of the well-studied pathogenic miRNAs, is important for normal development67,68; it has been reported that miR-142 is critical for hematopoiesis69 and the control of adaptive growth in cardiomyocytes70. Thus, further detailed studies of the targets and functions of miR-142 in normal tissue homeostasis are essential. This understanding may enable the use of miR-142 as a marker and target for therapeutic control.
Materials and Methods
Ethical statement
All procedures involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Kindai University. All experiments using animals were performed in accordance with institutional guidelines and regulations. IACUC approved project No. is KAME-26-043.
Isolation of the MSC from BM tissues (BMMSCs)
Four-week- and 1.5-year-old C57BL/6N male mice were used for the experiment as young and aged model. Bone marrow tissues were prepared as previously reported71. Long bones were collected from the hind limbs of the euthanized mice. The bone marrow was flushed out with α-MEM twice, and the residual long bones were cut into small pieces around 2–3 mm3 and treated with collagenase type II for 15 min. Then, dissociated tissues were washed twice with PBS and reacted with anti-PDGFRα (17-1401-81, eBioscience, San Diego, CA, USA), anti-Sca1 (61-5981-82, Thermo Fisher Scientific, Waltham, MA, USA), anti-CD45 (35-0451-U500, TONBO bioscience, San Diego, CA, USA), and anti-Ter119 (35-5921-U500, TONBO bioscience) for FACS isolation of the MSCs. As a control, cells were reacted with isotype IgG conjugated with each florescent dye (eBioscience). The PDGFRα+/Sca1+/CD45−/Ter119− population was sorted using a FACS Aria II (BD Biosciences, Franklin Lakes, NJ, USA).
Detection of ROS in the BMMSCs from young and aged mice
BMMSCs were prepared as described above. ROS levels were analyzed using the CellROX Green Reagent (Thermo Fisher Scientific) and a FACS CantoTM II (BD Biosciences).
miRNA sequence
Libraries were generated from 10–20 ng of RNA, which was prepared from the FACS-sorted PDGFRα+/Sca1+/CD45−/Ter119− cells with the miRNeasy Micro Kit (Qiagen, Hilden, Germany), using the SMARTer smRNA-seq Kit (TAKARA Bio Inc., Shiga, Japan). The next-generation single-read sequencing was performed using the sequencer NextSeq500 (Illumina, San Diego, CA, USA) using the single-end 50 nt high output sequencing mode. The trimmed reads were mapped to the reference genome of mouse (GRCm38.p5) by STAR (version 2.6.1a) program. The sequence quality was assessed with FastQC software.
Preparation of mouse BMMSCs
BMMSCs were isolated as previously reported26. BMMSCs were cultured in α-MEM (Wako, Tokyo, Japan) containing 10% fetal bovine serum (Hyclone, Logan, UT, USA) under 5% CO2 and 5% O2 at 37 °C.
Fluorescent observation of pexophagy
A cDNA encoding the monomeric derivative of DsRed fluorescent protein was cloned into the piggybac cDNA expression vector with the PTS1 (serine-lysine-leucine) sequence72 using the In-Fusion HD Cloning Kit (TAKARA Bio Inc., Shiga, Japan).
The LC3-GFP cDNA was amplified from the pEGFP-LC3 plasmid (Addgene #21073) using the Tks Gflex DNA polymerase (TAKARA Bio Inc.) and cloned into the piggybac cDNA expression vector. PTS1-DsRed and pEGFP-LC3 was introduced using a CUY21 electroporator (NEPA Gene, Tokyo, Japan). Fluorescent images were captured using a BZ-X710 all-in-one fluorescent microscope (KEYENCE Corporation, Osaka, Japan) and analyzed integrated values of fluorescent brightness for each channel by an image analysis software BZ-X Analyzer Ver 1.3 (KEYENCE Corporation). To determine whether each fluorescent protein localized to peroxisomes and autophagosomes, a chemical activator of peroxisome proliferation, Wy1464330 and that of autophagy, Rapamycin was used73. DsRed/GFP double-positive dots were counted as peroxisomes processed by autophagy (pexophagy).
Pexophagy activity detection assay
The EGFP sequence was amplified using Tks Gflex DNA polymerase and subcloned into the Nco I restriction enzyme site with a GGC short linker using the In-Fusion HD Cloning system. To enhance pexophagy activity, the BMMSCs were cultured in the amino acid-free DMEM for autophagy induction (Fujifilm Holdings Corporation, Tokyo, Japan) for 1 h before sampling. The full length and digested reporter molecules were detected using anti-RFP antibody (αRFP, M204-3, MBL, Nagoya, Japan) by WB as detailed below.
RT-PCR for miRNA
RNA preparation and cDNA production were performed using the TRI Reagent® (Molecular Research Center, Inc., Cincinnati, OH, USA) and the miRNA cDNA Synthesis Kit with the Poly(A) Polymerase Tailing Kit (Applied Biological Materials Inc., Rochmond, BC, USA). Quantitative RT-PCR was performed using BrightGreen Express 2X qPCR MasterMix-No Dye (Applied Biological Materials Inc., BC, Canada) with a Thermal Cycler Dice® Real Time System at 95 °C for 30 s followed by 40 cycles at 95 °C for 5 s and 60 °C for 15 s. Expression quantity was evaluated by the ΔΔCt method (ΔΔCt = ΔCtsample − ΔCtcontrol). U6 snRNA was used as a control gene for normalization.
RT-PCR for mRNA
RNA isolation and subsequent reverse-transcription were performed using TRI Reagent® and the PrimeScript® RT Master Mix Kit (TAKARA Bio Inc.). Quantitative real-time PCR was performed using Perfect real-time SYBR green II (TAKARA Bio Inc.) and a Thermal Cycler Dice® Real Time System. Expression quantity was evaluated by the ΔΔCt method using Gapdh as a control gene for normalization. To prevent contamination of genomic DNA, primers were designed to span at least one intron. Primer sequences are listed in Supplementary Table S2.
WB analysis
All samples were lysed in SDS buffer, electroporated and blotted onto a PVDF membrane (Hybond-P; GE Healthcare Japan, Tokyo, Japan). The PVDF membranes were then treated with Block Ace for 1 hour (Dainippon Sumitomo Pharma, Osaka, Japan) and reacted with primary antibodies overnight at 4 °C. Chemiluminescence detection was performed with horseradish peroxidase (HRP)-conjugated secondary antibodies and Immunostar® LD (Wako) reagents. Antibodies are listed in Supplementary Table S3. The raw WB data are shown in Supplementary Fig. S9.
Transfection of the mimic RNA and siRNA into mouse BMMSCs
Mimic RNA of miR-142 (mmu-miR142a-5p mimic, GeneDesign, Inc., Osaka, Japan) and siRNA against Epas1 or Ep300 were transfected using the Lipofectamine™ RNAiMAX Transfection Reagent (Thermo Fisher Scientific). Sequences of the siRNAs used are shown in Supplementary Table S4.
Transmission electron microscopy (TEM) observation
BMMSCs were fixed in 2% glutaraldehyde in 50 mM PIPES buffer at room temperature for 2.5 h, then post-fixed, following rinsing, in 1% OsO4 for 1 h. Following dehydration in ethanol, the cell pellets were embedded in epoxy resin, cut into 70 nm sections, stained with uranyl acetate and lead citrate, and viewed with a HT7700 Automated TEM (Hitachi High Technologies, Inc., Tokyo, Japan) at 100 kV.
Statistical analysis
Significant differences were detected with the Tukey-Kramer HSD test when the experiment had >three different groups. The data from two different groups were analyzed with a Student’s t-test, as appropriate. Caliculations were performed using JMP software version 10.0.0 (SAS Institute, Cary, NC, USA). Differences were assessed with a two-sided test with an α level of 0.05. The numbers of replicates (N) indicate biological replicates from independent experiments.
References
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217, https://doi.org/10.1016/j.cell.2013.05.039 (2013).
Cruickshanks, H. A. et al. Senescent cells harbour features of the cancer epigenome. Nat. Cell Biol. 15, 1495–1506, https://doi.org/10.1038/ncb2879 (2013).
Stadtman, E. R. Protein oxidation and aging. Sci. 257, 1220–1224 (1992).
Cui, H., Kong, Y. & Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal. Transduct. 2012, 646354, https://doi.org/10.1155/2012/646354 (2012).
Wickens, A. P. Ageing and the free radical theory. Respir. Physiol. 128, 379–391 (2001).
van Deursen, J. M. The role of senescent cells in ageing. Nat. 509, 439–446, https://doi.org/10.1038/nature13193 (2014).
D’Aquila, P., Bellizzi, D. & Passarino, G. Mitochondria in health, aging and diseases: the epigenetic perspective. Biogerontology 16, 569–585, https://doi.org/10.1007/s10522-015-9562-3 (2015).
Afanas’ev, I. New nucleophilic mechanisms of ros-dependent epigenetic modifications: comparison of aging and cancer. Aging Dis. 5, 52–62, https://doi.org/10.14336/AD.2014.050052 (2014).
Ahmed, E. K., Rogowska-Wrzesinska, A., Roepstorff, P., Bulteau, A. L. & Friguet, B. Protein modification and replicative senescence of WI-38 human embryonic fibroblasts. Aging Cell 9, 252–272, https://doi.org/10.1111/j.1474-9726.2010.00555.x (2010).
Kobayashi, C. I. & Suda, T. Regulation of reactive oxygen species in stem cells and cancer stem cells. J. Cell Physiol. 227, 421–430, https://doi.org/10.1002/jcp.22764 (2012).
Shyh-Chang, N., Daley, G. Q. & Cantley, L. C. Stem cell metabolism in tissue development and aging. Dev. 140, 2535–2547, https://doi.org/10.1242/dev.091777 (2013).
Moldovan, L. & Moldovan, N. I. Oxygen free radicals and redox biology of organelles. Histochem. Cell Biol. 122, 395–412, https://doi.org/10.1007/s00418-004-0676-y (2004).
Deb, R. & Nagotu, S. Versatility of peroxisomes: An evolving concept. Tissue Cell 49, 209–226, https://doi.org/10.1016/j.tice.2017.03.002 (2017).
Cho, D. H., Kim, Y. S., Jo, D. S., Choe, S. K. & Jo, E. K. Pexophagy: Molecular Mechanisms and Implications for Health and Diseases. Mol. Cell 41, 55–64, https://doi.org/10.14348/molcells.2018.2245 (2018).
Kumar, S., Kawalek, A. & van der Klei, I. J. Peroxisomal quality control mechanisms. Curr. Opin. Microbiol. 22, 30–37, https://doi.org/10.1016/j.mib.2014.09.009 (2014).
Heiland, I. & Erdmann, R. Biogenesis of peroxisomes. Topogenesis of the peroxisomal membrane and matrix proteins. FEBS J. 272, 2362–2372, https://doi.org/10.1111/j.1742-4658.2005.04690.x (2005).
Terman, A. & Brunk, U. T. Myocyte aging and mitochondrial turnover. Exp. Gerontol. 39, 701–705, https://doi.org/10.1016/j.exger.2004.01.005 (2004).
Martinez-Lopez, N., Athonvarangkul, D. & Singh, R. Autophagy and aging. Adv. Exp. Med. Biol. 847, 73–87, https://doi.org/10.1007/978-1-4939-2404-2_3 (2015).
Cuervo, A. M. & Macian, F. Autophagy and the immune function in aging. Curr. Opin. Immunol. 29, 97–104, https://doi.org/10.1016/j.coi.2014.05.006 (2014).
Legakis, J. E. et al. Peroxisome senescence in human fibroblasts. Mol. Biol. Cell 13, 4243–4255, https://doi.org/10.1091/mbc.e02-06-0322 (2002).
Perichon, R., Bourre, J. M., Kelly, J. F. & Roth, G. S. The role of peroxisomes in aging. Cell Mol. Life Sci. 54, 641–652, https://doi.org/10.1007/s000180050192 (1998).
Escobar, K. A., Cole, N. H., Mermier, C. M. & VanDusseldorp, T. A. Autophagy and aging: Maintaining the proteome through exercise and caloric restriction. Aging Cell, e12876, https://doi.org/10.1111/acel.12876 (2018).
Xu, J., Wang, Y., Tan, X. & Jing, H. MicroRNAs in autophagy and their emerging roles in crosstalk with apoptosis. Autophagy 8, 873–882, https://doi.org/10.4161/auto.19629 (2012).
Harries, L. W. MicroRNAs as Mediators of the Ageing Process. Genes. 5, 656–670, https://doi.org/10.3390/genes5030656 (2014).
Deshpande, S. et al. Reduced Autophagy by a microRNA-mediated Signaling Cascade in Diabetes-induced Renal Glomerular Hypertrophy. Sci. Rep. 8, 6954, https://doi.org/10.1038/s41598-018-25295-x (2018).
Onodera, Y. et al. miR-155 induces ROS generation through downregulation of antioxidation-related genes in mesenchymal stem cells. Aging Cell 16, 1369–1380, https://doi.org/10.1111/acel.12680 (2017).
Mizuno, Y. et al. miR-125b inhibits osteoblastic differentiation by down-regulation of cell proliferation. Biochem. Biophys. Res. Commun. 368, 267–272, https://doi.org/10.1016/j.bbrc.2008.01.073 (2008).
Huang, J., Zhao, L., Xing, L. & Chen, D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cell 28, 357–364, https://doi.org/10.1002/stem.288 (2010).
Chen, T. S. et al. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 38, 215–224, https://doi.org/10.1093/nar/gkp857 (2010).
Oruqaj, G. et al. Compromised peroxisomes in idiopathic pulmonary fibrosis, a vicious cycle inducing a higher fibrotic response via TGF-beta signaling. Proc. Natl Acad. Sci. USA 112, E2048–2057, https://doi.org/10.1073/pnas.1415111112 (2015).
Chino, H., Hatta, T., Natsume, T. & Mizushima, N. Intrinsically Disordered Protein TEX264 Mediates ER-phagy. Mol. Cell 74, 909–921 e906, https://doi.org/10.1016/j.molcel.2019.03.033 (2019).
Wong, N. & Wang, X. miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 43, D146–152, https://doi.org/10.1093/nar/gku1104 (2015).
Vergoulis, T. et al. TarBase 6.0: capturing the exponential growth of miRNA targets with experimental support. Nucleic Acids Res. 40, D222–229, https://doi.org/10.1093/nar/gkr1161 (2012).
Walter, K. M. et al. Hif-2alpha promotes degradation of mammalian peroxisomes by selective autophagy. Cell Metab. 20, 882–897, https://doi.org/10.1016/j.cmet.2014.09.017 (2014).
Hu, C. J., Wang, L. Y., Chodosh, L. A., Keith, B. & Simon, M. C. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol. Cell Biol. 23, 9361–9374 (2003).
Sharma, S. et al. Repression of miR-142 by p300 and MAPK is required for survival signalling via gp130 during adaptive hypertrophy. EMBO Mol. Med. 4, 617–632, https://doi.org/10.1002/emmm.201200234 (2012).
Li, Q., Xiao, H. & Isobe, K. Histone acetyltransferase activities of cAMP-regulated enhancer-binding protein and p300 in tissues of fetal, young, and old mice. J. Gerontol. A Biol. Sci. Med. Sci 57, B93–98 (2002).
Ito, K. et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 12, 446–451, https://doi.org/10.1038/nm1388 (2006).
Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nat. 431, 997–1002, https://doi.org/10.1038/nature02989 (2004).
Lin, C. H., Li, N. T., Cheng, H. S. & Yen, M. L. Oxidative stress induces imbalance of adipogenic/osteoblastic lineage commitment in mesenchymal stem cells through decreasing SIRT1 functions. J. Cell Mol. Med. 22, 786–796, https://doi.org/10.1111/jcmm.13356 (2018).
Denu, R. A. & Hematti, P. Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxid. Med. Cell Longev. 2016, 2989076, https://doi.org/10.1155/2016/2989076 (2016).
Rodrigues, M., Turner, O., Stolz, D., Griffith, L. G. & Wells, A. Production of reactive oxygen species by multipotent stromal cells/mesenchymal stem cells upon exposure to fas ligand. Cell Transpl. 21, 2171–2187, https://doi.org/10.3727/096368912X639035 (2012).
Park, S., Kang, S., Min, K. H., Woo Hwang, K. & Min, H. Age-associated changes in microRNA expression in bone marrow derived dendritic cells. Immunol. Invest. 42, 179–190, https://doi.org/10.3109/08820139.2012.717328 (2013).
Zhang, H. et al. Investigation of microRNA expression in human serum during the aging process. J. Gerontol. A Biol. Sci. Med. Sci 70, 102–109, https://doi.org/10.1093/gerona/glu145 (2015).
Fenn, A. M. et al. Increased micro-RNA 29b in the aged brain correlates with the reduction of insulin-like growth factor-1 and fractalkine ligand. Neurobiol. Aging 34, 2748–2758, https://doi.org/10.1016/j.neurobiolaging.2013.06.007 (2013).
Boon, R. A. et al. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ. Res. 109, 1115–1119, https://doi.org/10.1161/CIRCRESAHA.111.255737 (2011).
Xue, T. et al. miR-29b overexpression induces cochlear hair cell apoptosis through the regulation of SIRT1/PGC-1alpha signaling: Implications for age-related hearing loss. Int. J. Mol. Med. 38, 1387–1394, https://doi.org/10.3892/ijmm.2016.2735 (2016).
Chan, S. Y. et al. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 10, 273–284, https://doi.org/10.1016/j.cmet.2009.08.015 (2009).
Chen, Z., Li, Y., Zhang, H., Huang, P. & Luthra, R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 29, 4362–4368, https://doi.org/10.1038/onc.2010.193 (2010).
Wang, N. et al. Down-regulation of microRNA-142-5p attenuates oxygen-glucose deprivation and reoxygenation-induced neuron injury through up-regulating Nrf2/ARE signaling pathway. Biomed. Pharmacother. 89, 1187–1195, https://doi.org/10.1016/j.biopha.2017.03.011 (2017).
Wang, Q. et al. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 22, 4126–4135, https://doi.org/10.1096/fj.08-112326 (2008).
Carlomosti, F. et al. Oxidative Stress-Induced miR-200c Disrupts the Regulatory Loop Among SIRT1, FOXO1, and eNOS. Antioxid. Redox Signal. 27, 328–344, https://doi.org/10.1089/ars.2016.6643 (2017).
Anding, A. L. & Baehrecke, E. H. Cleaning House: Selective Autophagy of Organelles. Dev. Cell 41, 10–22, https://doi.org/10.1016/j.devcel.2017.02.016 (2017).
Feher, J. et al. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol. Aging 27, 983–993, https://doi.org/10.1016/j.neurobiolaging.2005.05.012 (2006).
Deosaran, E. et al. NBR1 acts as an autophagy receptor for peroxisomes. J. Cell Sci. 126, 939–952, https://doi.org/10.1242/jcs.114819 (2013).
De Duve, C. & Baudhuin, P. Peroxisomes (microbodies and related particles). Physiol. Rev. 46, 323–357, https://doi.org/10.1152/physrev.1966.46.2.323 (1966).
Liu, L. F., Shen, W. J., Ueno, M., Patel, S. & Kraemer, F. B. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics 12, 212, https://doi.org/10.1186/1471-2164-12-212 (2011).
Bandyopadhyay, D. et al. Down-regulation of p300/CBP histone acetyltransferase activates a senescence checkpoint in human melanocytes. Cancer Res. 62, 6231–6239 (2002).
Yan, G. et al. Selective inhibition of p300 HAT blocks cell cycle progression, induces cellular senescence, and inhibits the DNA damage response in melanoma cells. J. Invest. Dermatol. 133, 2444–2452, https://doi.org/10.1038/jid.2013.187 (2013).
Song, J. & Kim, Y. K. Identification of the Role of miR-142-5p in Alzheimer’s Disease by Comparative Bioinformatics and Cellular Analysis. Front. Mol. Neurosci. 10, 227, https://doi.org/10.3389/fnmol.2017.00227 (2017).
Talebi, F. et al. MicroRNA-142 regulates inflammation and T cell differentiation in an animal model of multiple sclerosis. J. Neuroinflammation 14, 55, https://doi.org/10.1186/s12974-017-0832-7 (2017).
Teng, Z. et al. miR-142-5p in Bone Marrow-Derived Mesenchymal Stem Cells Promotes Osteoporosis Involving Targeting Adhesion Molecule VCAM-1 and Inhibiting Cell Migration. Biomed. Res. Int. 2018, 3274641, https://doi.org/10.1155/2018/3274641 (2018).
Sun, Z., Chin, Y. E. & Zhang, D. D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol. Cell Biol. 29, 2658–2672, https://doi.org/10.1128/MCB.01639-08 (2009).
Bertout, J. A., Patel, S. A. & Simon, M. C. The impact of O2 availability on human cancer. Nat. Rev. Cancer 8, 967–975, https://doi.org/10.1038/nrc2540 (2008).
Majmundar, A. J., Wong, W. J. & Simon, M. C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309, https://doi.org/10.1016/j.molcel.2010.09.022 (2010).
Su, S. et al. miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program. Nat. Commun. 6, 8523, https://doi.org/10.1038/ncomms9523 (2015).
Zhu, S. et al. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 18, 350–359, https://doi.org/10.1038/cr.2008.24 (2008).
Cheng, Y. & Zhang, C. MicroRNA-21 in cardiovascular disease. J. Cardiovasc. Transl. Res. 3, 251–255, https://doi.org/10.1007/s12265-010-9169-7 (2010).
Chapnik, E. et al. miR-142 orchestrates a network of actin cytoskeleton regulators during megakaryopoiesis. Elife 3, e01964, https://doi.org/10.7554/eLife.01964 (2014).
Shrestha, A. et al. Generation and Validation of miR-142 Knock Out Mice. PLoS One 10, e0136913, https://doi.org/10.1371/journal.pone.0136913 (2015).
Zhu, H. et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat. Protoc. 5, 550–560, https://doi.org/10.1038/nprot.2009.238 (2010).
Wiemer, E. A., Wenzel, T., Deerinck, T. J., Ellisman, M. H. & Subramani, S. Visualization of the peroxisomal compartment in living mammalian cells: dynamic behavior and association with microtubules. J. Cell Biol. 136, 71–80, https://doi.org/10.1083/jcb.136.1.71 (1997).
Edwards, S. R. & Wandless, T. J. The rapamycin-binding domain of the protein kinase mammalian target of rapamycin is a destabilizing domain. J. Biol. Chem. 282, 13395–13401, https://doi.org/10.1074/jbc.M700498200 (2007).
Author information
Authors and Affiliations
Contributions
T. Teramura and K.F. were responsible for conceptualization. Y.O. and T. Teramura designed the studies. K.H., Y.O., T.M., T. Tsujimoto, T. Takehara and S.N. performed biological experiments. Y.O. and T. Takehara provided data analysis and interpretation. K.H. and T. Teramura. wrote the manuscript. All authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Houri, K., Mori, T., Onodera, Y. et al. miR-142 induces accumulation of reactive oxygen species (ROS) by inhibiting pexophagy in aged bone marrow mesenchymal stem cells. Sci Rep 10, 3735 (2020). https://doi.org/10.1038/s41598-020-60346-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60346-2
This article is cited by
-
MicroRNA expression and oxidative stress markers in pectoral muscle of broiler chickens fed diets supplemented with phytobiotics composition
Scientific Reports (2024)
-
Non-coding RNAs in photoaging-related mechanisms: a new paradigm in skin health
Biogerontology (2022)
-
Integrated miRNA-mRNA transcriptomic analysis of hepatopancreas reveals molecular strategies in Chinese mitten crab (Eriocheir sinensis) under acute nitrite stress
Aquaculture International (2021)
-
Protein quality control of cell stemness
Cell Regeneration (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.