Abstract
Arboreal and flying frugivorous animals represent primary dispersers in the Neotropics. Studies suggest a possible compensation for the loss of large species by smaller ones with expanding rampant anthropogenic pressures and declining populations of larger frugivores. However, studies on seed dispersal by frugivores vertebrates generally focus on the diurnal, terrestrial, canopy, and flying species, with the nocturnal canopy ones being less studied. Setting camera traps high in the canopy of fruiting nutmeg trees revealed for the first time the high frequency of the kinkajou (Potos flavus, Schreber, 1774, Procyonidae), an overlooked nocturnal frugivore species (Order Carnivora) in the Guianas. The diversity of the fruit species consumed by the kinkajou calls for considering it as an important seed disperser. The overlap of the size of seeds dispersed by frugivores observed in nutmeg trees suggests that the small (2–5 kg) kinkajou may compensate for the loss of large (5–10 kg) frugivorous vertebrates in the canopy. Camera traps visualise how the kinkajou is adapted to forage in the nutmeg tree crown and grab the fruit. Such information is vital for conservation because compensation of seed dispersal by small frugivores is crucial in increasing anthropogenic stressors.
Similar content being viewed by others
Introduction
Frugivore vertebrates eat fruit and disperse plant diaspores, defecating, regurgitating, or spitting out seeds beyond the vicinity of the parent tree, where it is less advantageous to develop as a seedling (Janzen-Connell model), given the higher risks of competition and mortality by pathogens and other predators1,2,3. Therefore, seed dispersal by fruit-eaters plays a crucial role in shaping the seed-seedling shadow, animal-mediated seed dispersal being a primary driving mechanism of tropical forest dynamics and maintaining diversity in tropical forests4,5,6,7.
Studies on seed dispersal by vertebrates generally focus on the diurnal, terrestrial, canopy, and flying frugivore species that are easy to observe from the ground using binoculars. For instance, in neotropical forests, large body-sized primates and birds represent the primary groups responsible for dispersing small-to-large seeds8,9,10,11,12,13,14,15. However, hunters also prefer these animals, sold for meat, medicine, game trophies, and pets16,17. Studies often focus on bats regarding nocturnal frugivore species, highlighting their crucial function for small-seeded plant dispersal during the early stages of succession18,19. Other nocturnal canopy frugivores are less studied, even though, being a diverse assemblage of vertebrates, they may play an important role in the dispersal for many plants according to their food preferences20. For example, a recent study on nocturnal neotropical oilbirds (Steatornis caripensis, von Humbold 1817) showed their ability to disperse large seeds (29 mm in width) up to 10 km on average, suggesting that they may perform similar ecological roles to some of the extinct megafauna, despite the oilbirds’ smaller size21. Nocturnal frugivores have also received some further attention. They are acknowledged as seed dispersers in various tropical rainforests, such as the Viverrid palm civets (Paradoxurus hermaphroditus, Pallas 1977) and binturong (Arctictis binturong, Raffles 1821) in Asia22,23, and the Procyonid kinkajou (Potos flavus, Shrebber 1774) in the neotropics20,24,25. Although more research considers nocturnal frugivores, there is still a gap in knowledge regarding their role in seed dispersal because of the difficulty of studying them. It can be highlighted by the recent discovery of a new olingo species (Bassaricyon spp.)26, another arboreal Procyonid in Ecuador, and the potential existence of different species within the genus Potos27. However, the development of safer single-rope tree climbing28 and remote camera trapping29 offers new opportunities to observe and analyse their behavioural ecology in treetops. Indeed, Gregory et al. (2017) recorded that 94% of canopy activity in natural branches happened at night30.
Nutmeg tree species (Myristicaceae) form a pantropical family that heavily relies on animals for their seed dispersal, thus are excellent ecological models for the study of animal-plant interactions31. In neotropical biomes, primates and toucans are the primary consumers and dispersers of nutmeg tree seeds8,9,11,12,32,33,34. Facing an increase in anthropogenic pressure27, populations of large arboreal frugivores in tropical forests are threatened35,36,37, leading to the downsizing of frugivore communities38,39. Hence, it is expected the seed dispersal process to be altered, resulting in a decline in animal-dispersed tree species diversity40,41. Nevertheless, studies suggest the existence of possible compensation for the disappearance of large mammalian frugivores with replacement by smaller ones, among them kinkajous in disturbed forest habitats8,42,43. Although kinkajous rarely forage in fruiting nutmeg trees in a study in Central America34,44, they are known to consume and disperse nutmeg tree seeds in the Amazonian forests of the Guianas45,46. Observations in neotropical nutmeg (Virola spp.) tree species45 showed that seed-dispersal for nutmeg trees is not disrupted when large diurnal, arboreal frugivores are missing8. Given that kinkajou has a body mass (2–5 kg) similar to that of the diurnal frugivores brown capuchin (Sapajus apella, Linnaeus 1758)20 and a dietary diversity overlapping that of the black spider monkey (Ateles paniscus, Linnaeus 1758), we assume that kinkajous are significant seed dispersers 24. Here, we hypothesised that kinkajous might compensate for the loss of other larger arboreal frugivores among plant species that share a diversified array of consumers.
To test this hypothesis, we used camera traps, which are an effective method for studying arboreal fauna29,47,48,49. The cost of detecting nocturnal animals such as kinkajous is reduced by half when using camera traps instead of line transect survey50. In previous studies, frugivores were observed in nutmeg trees from the ground12. Others followed animals using arboreal camera traps studies in trees using easy and safe climbing gear 29,47,50. We, therefore, set up camera traps directly in two well-known Virola species (height between 30 and 40 m) in the forest near the newly-constructed National 2 Road (Fig. 1). We sampled 12 trees during the fruiting peak season (December-January): Virola kwatae, Sabatier 1997 (7 trees) and V. michelii, Heckel 1898 (5 trees). Through analysis of camera trap images, this study aimed to assess the occurrence of the frugivore kinkajou in Virola trees compared to other diurnal and nocturnal counterparts. Because the varying capacities of foraging animals to disperse seeds creates selective pressures on seed size51, we supplement our results with a literature-review-based analysis of the average seed size dispersed by small- to medium- and large-bodied frugivores.
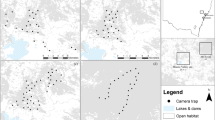
Study trees in the forested areas (FA) surrounding the ecological corridors along the National 2 Road between Régina and Saint-Georges in French Guiana (see further description in Coutant et al. 202257). The map was edited on ArcMap software version 10.6.1 (https://www.esri.com/).
Results
The camera trap survey was for 1320 trap nights (55 days × 12 trees × 2 camera traps), and we observed 24 identified species and unidentified species grouped per order visiting Virola trees (Fig. S1). Fruiting was staggered, with early and late-fruiting individual trees (Tab. S1). The analysis of 162,885 images on 11 fruiting trees and one flowering tree produced 587 independent events with high variability between trees (Fig. S1, S2, Tab. S1). Photographs 30 min apart were considered independent events, as usually defined in literature51,52,53,54. We recorded nine frugivorous species known to be regular Virola seed dispersers (Fig. 2, Tab. S2, Fig. S3). On the one hand, all known frugivores visited the single early-fruiting V. michellii tree (tree #3), which had 29% (N = 171) of all independent events. Contrastingly, no frugivores visited the late-fruiting V. michellii (tree #2) and the non-fruiting V. kwatae tree (tree #6) (Fig. 2, Tab. S2). The kinkajou was the most frequent fruit-eating species overall, representing 48% (N = 280) of the events. The guan (Penelope marail, Statius Muller 1776) ranked second (15%, N = 89 independent events), including 80 events in the same tree (tree #3, Fig. 2, Tab. S2). Howler monkey (Alouatta macconnelli, Linnaeus 1766) and brown capuchin (Sapajus apella) each represented 4% of events, while the five species of toucans combined accounted for 6% of events, the channel-billed toucan (Ramphastos vitellinus, M.H.K. Lichtenstein 1823) being the primary (50%) species in nutmeg trees.
Number of independent events by tree for the nine main frugivores recorded as visiting the canopy of nutmeg trees between 12/01/19 and 01/24/20 in National 2 Road in French Guiana. Trees were grouped by their fruiting statues, either “early”, “intermediate”, or “late”. Trees 2 and 6 are not represented because no frugivores were recorded in their crown.
Kinkajou activity peaked between 00:00 and 05:30 (Fig. 3a). Diurnal frugivores showed two activity peaks during the day, except for A. macconnelli, primarily active around 15:00 (Fig. 3b). Sapajus apella had a maximum activity around 08:00 and 17:30 (Fig. 3b). Penelope marail and toucans showed a similar pattern of activity, but P. marail was predominantly active at dawn and dusk, whereas toucans were active around 09:00 and 14:00 (Fig. 3c).
Night activity of (a) kinkajou (Potos flavus), and daily activity of (b) primates (Alouatta macconnelli and Sapajus apella) and (c) birds (Penelope marail and Ramphastidae, toucans) in Virola spp trees near National 2 Road between 12/01/19 and 01/24/20. Small bars (x-axis) represent recorded events: the greater the number of events observed at a given time, the greater the density (y-axis).
Based on estimates from the literature, the overall distribution of average seed width dispersed by the frugivores that visit Virola trees shows similar patterns among species and groups (Fig. 4). Dunn tests corroborate these observations and highlight trends among toucans to disperse seeds significantly smaller (median width and length = 5.77 × 8.5 mm) than other larger vertebrate species (details in Tab. S3, S4, S5, S6, S7, and Fig. S4). The arboreal vertebrate species show similar patterns in the distribution of dispersed seed dimensions (Fig. 4, S4) with similar median seed width (A. paniscus = 9 mm; P. flavus = 10 mm; A. macconnelli = 9 mm; S. apella = 8 mm) and similar median seed length (A. paniscus = 15 mm; P. flavus = 15.5 mm; A. macconnelli = 15.5 mm; S. apella = 14.5 mm).
Density plot of average seed width (mm) dispersed by major neotropical arboreal frugivores. Data are obtained from literature based on studies in French Guiana, except for data on Ramphastidae spp., which were compiled from studies in the Atlantic Forest. Solid black line = average Virola michelii seed width. Dashed line = average Virola kwatae seed width. A.m. = Alouatta macconnelli; A.p. = Ateles paniscus; C.p. = Caluromys philander; P.f. = Potos flavus; P.m. = Penelope marail; R.r. = Rupicola rupicola; R. spp. = Ramphastidae spp.; S.a. = Sapajus apella; S.m. = Saguinus midas. Data for Virola seed size are from Ratiarison & Forget (2013)12.
Discussion
Kinkajou (Potos flavus) frequently visited the two species of Virola nutmeg trees during the peak fruiting season at the study site (see Tab. S2, Fig. S5). Our study shows for the first time that kinkajous are very common in nutmeg trees. At the same time, the primary consumer (i.e., black spider monkey, Ateles paniscus) is nearly absent in the study forest.
The analysis of the activity of kinkajous in trees suggests that fruiting nutmeg trees are often visited when fewer alternative fruit resources are available in the early rainy season, a period with low fruit production55,56. The observed occurrence of kinkajous in fruiting Virola trees maybe related to a deficit of the larger diurnal, arboreal consumer, i.e. the spider monkey, at the study area. In other forests, such as the protected Nouragues Nature Reserve25, spider monkeys are the most common diurnal visitors of fruiting Virola trees between October and March12. When spider monkeys are scarce, there is thus a greater abundance of ripe, dehiscent fruit that may reach maturity and dehisce in the canopy during daily hours, exposing red aril43. The consumed part is then easily accessible to other diurnal and nocturnal consumers, i.e. birds and carnivores, that would otherwise be unable to open fruit when spider monkeys are present. Confirmation that kinkajou visitation rates are directly impacted by the presence of spider monkeys requires a camera trap study where both species are present (ongoing studies).
Consequently, fruit surveys on the ground had shown a more significant proportion of Virola fruit with single valve husks at disturbed vs control forests57. However, the difference in seed removal rates across sites suggested that secondary consumers played an important role as alternative seed dispersers (e.g., toucans), eventually compensating for the disappearance of the primary seed dispersers57. This study shows that the nocturnal frugivore kinkajou complements the effects of birds as seed dispersers of Virola seeds. During the first hours after sunset, kinkajou travel from its diurnal shelter to feed on Virola fruit (Fig. 3a) and return before dawn to their daily sleeping roost located further away from the feeding trees46. This behaviour has never been documented since studies of frugivory traditionally focus on diurnal frugivores.
The review of the size of seeds dispersed by the main frugivores observed and known to forage in nutmeg trees showed the significant overlap between frugivores. This result supports the hypothesis that smaller animal species might compensate for the absence of large ones. In particular, the pattern of seed size highlights the role of kinkajous, howler monkeys, and brown capuchins as potential replacement species for spider monkeys in human-affected forest areas. Moreover, studies have shown that kinkajous disperse seeds on average 200 m away and up to 300 m from parent trees46. In comparison, howler monkeys and brown capuchins disperse seeds up to 390 m and 225 m, respectively on average58, within more extensive home ranges than kinkajous59,60. On the other hand, spider monkeys seem to disperse seeds between 150 and 250 m, depending on sites58.
Although these observations support the possible compensatory role of these three species, in the absence of spider monkeys for seed dispersal, howler monkeys and brown capuchins remain preferred hunting targets36. In addition, howler monkeys and brown capuchins also only shared 35 to 59% overlap in fruit feeding species with spider monkeys61 and presented a more diversified diet composed of leaves and arthropods59,60 at that time, making them less dependent on the availability of ripe fruits. Contrastingly, kinkajous are barely hunted and remain abundant in many forest areas62. As a frugivore generalist and opportunist, kinkajous can eat similar plants as black spider monkeys with a similar digestive retention time63 in the Guianas, as observed for the Central American spider monkey in Panama24. Like neotropical primates, kinkajous are adapted to forage into the canopy using their prehensile tail64. It allows them to move on small branches65 where they can reach and grasp fruit using their forepaws, teeth, snout or nose66,67. All previous facts support the hypothesis that kinkajous may play a significant role in compensating seed dispersal of nutmeg trees and, more generally, for a fraction of the fruits usually consumed by spider monkeys.
These observations highlight the importance of studying the role of kinkajous and other nocturnal species such as olingos (Brassaricyon gabbii, Allen 1876) in seed dispersal. Olingos are understudied as they were traditionally confused with kinkajous because of their similar appearance. However, a study in Panama suggested that olingos share some ecological and behavioural traits with kinkajous and may disperse ingested seeds68. Since almost no studies have focused on their potential role for seed dispersal, there is thus a clear need to increase research on this group to better understand their ecological importance, especially in disturbed forests. Similarly, little is known about other diurnal frugivorous carnivores such as the coati (Nasua nasua, Linnaeus 1766), which could also play a key role in seed dispersal in fragmented forests where it can persist69, as well as the tayra (Eira barbara, Linnaeus 1758) that could also be involved in supporting seed dispersal processes as it also consumes fruits70.
In this study, another arboreal, nocturnal frugivore was recorded once: Caluromys philander (Linnaeus 1758). This marsupial consumes 25 different fruit species in the forests of Cabassou, French Guiana, including three nutmegs species20. At the Piste de Saint-Élie, French-Guiana, C. philander was not eating any Virola fruits. Still, it seems to share a large part of its diet composed of flowers and small-seeded fruits during the dry and wet season, respectively, with kinkajous45. In addition, our seed-size review demonstrated its capability to disperse seeds slightly smaller than those of kinkajous (not significant, Tab. S4,S6, S7). Even though C. philander was only observed once here, its presence in a wide range of habitats in French-Guiana71 without being threatened by hunting suggests that it could play an important role in dispersing small seeds72, as indicated for small rodents73.
Seed size can be a fundamental threshold level for seed dispersal, especially for birds. This review highlighted a significant trend for toucans and the Guianan Cock-of-the-rock (Rupicola rupicola, Linnaeus 1766) to consume and disperse smaller seeds. Although Cock-of-the-rock was not observed in the study forest, this smaller bird is known to disperse large seeds (Tab. S7), including species consumed by spider monkeys, toucans, and kinkajous (P-M. Forget, pers. obs). Larger birds swallow these seeds, such as the guan (Penelope marail). The gizzard of this species is only slightly muscular, allowing the seed to pass through the whole digestive tract without being crushed74,75. Conclusion on toucans need to be tempered and could be explained by a possible site effect. Indeed, Mata Atlantica data were used to describe seed size consumed by toucans. However, seeds from Mata Atlantica are possibly smaller than in French Guiana, which could bias our results. This highlights the clear need for studies to evaluate their role in seed dispersal compensation.
This study points out the importance of smaller vertebrates for seed dispersal and explores a new approach for studying arboreal frugivores with camera traps. Beyond the difficulty of finding the most suitable location for setting up cameras to maximise the chance of capturing animals, implementing this methodology involves very tedious work that requires time, climbing skills, specific equipment to access the upper branches in the tree crown, and the presence of at least two people capable of climbing to ensure safety28. During this study, approximately two trees were equipped/unequipped at a height over 30 m per day by two climbers. The number of climbs is a limitation that complicates the ability to increase the number of fruit trees and sites covering a large area, i.e., that of the home range of frugivores to be inventoried far from forest access points. In addition, even when fixed directly to the trunk or the main branches, unavoidable swinging movements of the crown can cause many triggers of non-target stimuli49, especially during the day when the wind blows. Saturation of the memory card follows, plus battery draining faster than expected, making it impossible to maintain traps in place for more than two months without a checkup. It also underestimates frugivorous bird frequency that perches on smaller branches away from the trunk. For instance, toucans were recorded only with 33 independent events while they are known to be a regular consumer of Virola fruit and little affected by hunting in this area8,9,43. Future studies should consider setting up supplementary cameras in adjacent trees to observe frugivorous birds as Zhu et al. (2021)76 did and succeeded in recording many of their interactions with fruits.
Despite these constraints, arboreal camera traps allowed for continuous, non-invasive observation of animals in the canopy, both day and night, providing a wealth of information (diversity, activities, and behaviour) about the frugivore guild of Virola spp. in the area. Specifically, this method effectively studied nocturnal frugivores, such as kinkajous, in Virola spp. and many other staple fruit resources for arboreal mammals and birds (e.g., Tetragastris spp.13,44). The effectiveness of this method is consistent with the finding of a study from Bowler et al. (2017)47 who were also able to obtain a large number of images of kinkajous without ever observing it during counts and transect observations. Schipper et al. (2007)77 showed that standard flash photography in camera traps leads to avoidance behaviour in kinkajou. Here, the use of red infrared moving sensor camera traps suggested by Schipper et al. (2007)77 minimised disruption to the foraging behaviour of the animals, as evidenced by a large number of kinkajous captured. It is also the case for primates who look at the lens, sometimes touching it (S. apella), but do not linger on it and do not seem bothered by camera traps when feeding. Therefore, it favours using these devices to characterise arboreal fauna. This method is currently promising for studies in the canopy29,40,47,48,49,51.
During this study, thanks to many recorded events for kinkajous (N = 280, during 1320 trap nights), we showed how arboreal camera trapping protocols in targeted fruiting trees could complement the classical radio-tracking of animals foraging in their home range. Firstly, camera traps can help survey several independent populations of frugivores in various habitats and feeding trees. In contrast, radio-tracking is often restricted to fewer individuals in their respective home ranges. Secondly, one can further document interactions with other frugivores species in the canopy within-tree and across plant species during their daily or nocturnal activities. And finally, camera traps record all behavioural attitudes of animals, whether they are moving along the trunk or within the branches of the canopy, handling and consuming fruit, swallowing seeds, resting, or simply visiting the treetops. These observations are simply impossible from the ground at night.
Cameras might, therefore, successfully be implemented in addition to line transects47, eco-acoustics9, or radio-tracking10,42 to obtain the best overview of the diversity and behavioural ecology of the arboreal and flying fauna. Camera traps revealed the high frequency of an overlooked nocturnal species for the first time, suggesting its potential role in compensating the loss of other large frugivorous vertebrates such as black spider monkeys for nutmeg trees seed dispersal. Camera traps also allowed visualising how frugivore species foraging within the canopy reach ripe fruit and how they behave, highlighting the advantage of primates, which can pick, open, and peel fruit. Kinkajous handle the dehiscent fruit, and swallow seed and aril. They may pull the twig with the open fruit, and swallow the arilled seed. Then, they later defecate cleaned seeds in their droppings, similarly to primates such as spider monkeys, capuchins and howler monkeys (Forget, P-M, pers. obs.; dual observation along with Didier Julien-Laferrière in February 1986 at Piste de Saint-Elie). Such information is vital for conservation because compensation for seed dispersal by small frugivores is crucial in the context of increasing anthropogenic disturbance.
To conclude, the extension of protocols such as this one to other fruiting trees is essential to measure the impact of human disturbance on the ecology and dynamics of tropical forests. Fruit-eating species compensation seems to be mainly dependent on the diet of the smallest frugivores, as pointed out by Boissier et al. (2020)8, who observed a significant impact of anthropisation on the dispersal of Sapotaceae. In contrast, it was more moderate for trees of the families Myristicaceae and Burserasae, whose fruits are mainly consumed by mammals and birds13,78. It is also expected to see fruiting plants with the largest seeds most affected by the loss of larger frugivores, as just a few large frugivores (A. paniscus) can consume and disperse these seeds, whereas other species such as kinkajous are too small to consume these seeds (see Fig. 4, S4). The use of camera traps in anthropised areas appears necessary to characterise the canopy fauna more completely by including all nocturnal species likely to participate in seed dispersal processes. Identifying these species is a crucial point that would allow a better understanding of the sharing of resources between diurnal and nocturnal species and a better understanding of seed dispersal compensation processes made possible by the functional redundancy of the ecosystem.
Material and methods
Study site
The study was conducted in French Guiana (Fig. 1). The climate is equatorial and characterised by turns between a wet season from December to July and a dry season from August to November. Peak fruiting period occurs between March and May55,56. The study site was based in an anthropised forest in Eastern French Guiana (Fig. 1). More precisely, sampled trees were located near (< 1 km) the ecological corridors implemented along the National 2 Road (RN2 in french) that facilitate animal passage on the ground or in the canopy (Fig. 1). Because the road follows the hilly topography of the forest, these corridors are at elevations ranging from 30 to 90 m.
Study tree species
Virola kwatae and V. michelii (Myristicaceae) are two dioecious trees of the canopy in the Guiana Shield with an associated frugivores community well studied in the Nouragues Nature Reserve12,79,80 and a disturbed forest8,43. Virola kwatae is more likely to be found on hills and forests on steep slopes80, while V. michelii is more common and can be found in high densities in secondary areas20. Both species fruit synchronously between November and March before the forest fruiting peak between March and May55,79,80. Fruiting duration is ca. two months and 3–4 months in Virola michelii and V. kwatae, respectively78,79. They produce a dehiscent capsule-like fruit with two valves protecting a seed covered by a red lipid-rich aril exposed when the fruit reaches maturity12,79,80. Seeds of V. kwatae are larger (seed size average: 2.8 × 1.8 cm) than seeds of V. michelii (seed size average: 2.0 × 1.4 cm). In this study, 12 trees were sampled in five forest areas (FA) located 1000 m from the road and corridors (Fig. 1b). The first forest area (FA01) is the closest to Saint-Georges with the lowest elevation (30–50 m), and the last forest area (FA07) is the farthest and is slightly higher (70–90 m).
Camera trapping protocol
Three models of Reconyx® camera traps were used: HC600 Hyperfire, XR6 Ultrafire, and Hyperfire2. The red infrared moving sensor and the absence of a white flash during shooting do not disturb the behaviour of nocturnal animals77. We set the HC600 and Hyperfire2 traps to take five photographs upon movement detection, while the XR6 Ultrafire was programmed to take three pictures and one video. We activated the video function for experimental purposes to supplement the information gathered from the photographs by documenting the behaviour of the foraging animals. We placed 34 camera traps (including 21 HC600 Hyperfire, 8 Hyperfire2, and 5 XR6 Ultrafire) in the crowns of 11 fruiting trees (6 Virola kwatae and 5 V. michelii trees). In addition, cameras were set in one non-fruiting tree (V. kwatae) to test for a possible attractive effect of the fruits on the other fertile trees. Except for two trees with two camera traps, all others had three traps in their canopy on the trunk and main branches, all between 30 and 40 m high. We carried out a camera trap survey between 11/25/19 and 12/01/19, while removal took place between 1/24/20 and 1/28/20. Upon removal of the traps, the fruiting status of the trees was characterised and categorised as follows:
- A: early fruiting, many fruits on the ground and few fruits in the canopy;
- B: intermediate fruiting, many fruits on the ground and in the canopy;
- C: late fruiting, few fruits on the ground, and most fruits in the canopy;
- D: no fruiting.
Camera trapping analysis
Following the protocol of Niedballa et al. (2016)81, data (images) were first extracted and organised using the camtrapR® package on Rstudio® Version 1.1.463. Digikam® software tagged images displaying an animal to add the identified species name to the image's metadata. In some cases, the individuals photographed did not allow for species identification, so we tagged the pictures with the common name of the animal observed (e.g., sloth, large marsupial, large rodent). Photographs that did not identify a particular animal (blurred image, only part of the organism photographed) were classified as unidentified species. Each identification was then used to determine the independent events obtained. An event is defined by recording an individual by a camera trap. It is considered independent of another at least 30 min apart, as is usually expressed in literature51,52,53,54. To avoid pseudo-replicates, counting events was performed by considering traps in the same tree as a single trap. If an individual of a species is recorded by two camera traps of the same tree within a 30-min interval, then only one event is counted. Only one event is counted if more than one individual is observed in the image. Because not all trees had the same number of camera traps, we analysed only the pictures collected by two traps on each tree to ensure consistent sampling effort among the 12 sites. The two traps that provided the most images were chosen to select them. To standardise the period of data recording on each tree, only images obtained between 01/12/19 starting at 18:00 and 24/01/20 until 06:00 were analysed, a period of 55 days. The overlap® package82 was used to produce activity plots of the main frugivores. Graphs were centred either at noon or midnight depending on their activity (e.g., kinkajou is a nocturnal frugivore, so its figure is centred on midnight) to stay consistent with their behaviour.
Seed size analysis
The average length and width of seeds consumed and dispersed by neotropical frugivores were compiled from studies conducted in French Guiana. The major frugivore species were selected even though we did not observe them in this study. Almost no data existed for toucans in French Guiana. However, the species of toucans from Mata Atlantica are very similar to those from French Guiana because they are either the same species (e.g., Ramphastos vitellinus) or species with comparable sizes and body mass (e.g., Selenidera maculirostris (160 g) in Mata Atlantica vs Selenidera piperivora (150 g) the Guianas). Therefore, we used a dataset for the group Ramphastidae from Mata Atlantica 83,84 to compare seed size among frugivores visiting Virola trees. Data for Ramphastos vitellinus, Pteroglossus aracari, and Selenidera maculirostris were selected and combined to form the toucans’ group. As normality and homoscedasticity were not verified (tested with Shapiro and Levene test), testing for differences in average seed size consumed by the 9 main arboreal frugivores was done using Kruskall-Wallis tests from the package rstatix85 on Rstudio® Version 1.1.463. Then, to identify which paired of frugivores were different, Dunn tests with a Bonferroni correction were done.
All the methods were performed in accordance with relevant guidelines and regulations. All analyses and figures were done and edited on Rstudio® Version 1.1.463.
References
Clark, D. A. & Clark, D. B. Spacing dynamics of a Tropical rain forest tree: Evaluation of the Janzen-Connell model. Am. Nat. 124, 769–788 (1984).
Comita, L. S. et al. Testing predictions of the Janzen-Connell hypothesis: A meta-analysis of experimental evidence for distance- and dendity-dependent seed and seedling survival. J. Ecol. 102, 845–856 (2014).
Jansen, P. A. & Forget, P.-M. Scatterhoarding rodents and tree regeneration. in Nouragues (eds. Bongers, F., Charles-Dominique, P., Forget, P.-M. & Théry, M.) vol. 80 pp. 275–288 (Springer Netherlands, 2001).
Janzen, D. H. Herbivores and the number of tree species in Tropical forests. Am. Nat. 104, 501–528 (1970).
Hammond, D. S. Tropical forests of the Guiana shield: ancient forests in a modern world. (CABI Publishing, 2005).
Forget, P.-M. et al. Frugivores and seed dispersal (1985–2010); the ‘seeds’ dispersed, established and matured. Acta Oecologica 37, 517–520 (2011).
Levey, D. J., Silva, W. R. & Galetti, M. Seed dispersal and frugivory: Ecology, evolution and conservation. (CABI Publishing, 2002).
Boissier, O., Feer, F., Henry, P. & Forget, P. Modifications of the rain forest frugivore community are associated with reduced seed removal at the community level. Ecol. Appl. 30, (2020).
Ducrettet, M. et al. Monitoring canopy bird activity in disturbed landscapes with automatic recorders: A case study in the tropics. Biol. Conserv. 245, 108574 (2020).
Holbrook, K. M. Home range and movement patterns of toucans: Implications for seed dispersal. Biotropica 43, 357–364 (2011).
Holbrook, K. M. & Loiselle, B. A. Dispersal in a Neotropical tree, Virola flexuosa (Myristicaceae): Does hunting of large vertebrates limit seed removal?. Ecology 90, 1449–1455 (2009).
Ratiarison, S. & Forget, P.-M. The role of frugivores in determining seed removal and dispersal in the neotropical nutmeg. Trop. Conserv. Sci. 6, 690–704 (2013).
Ratiarison, S. & Forget, P.-M. Frugivores and seed removal at Tetragastris altissima (Burseraceae) in a fragmented forested landscape of French Guiana. J. Trop. Ecol. 21, 501–508 (2005).
Stevenson, P. R., Link, A., González-Caro, S. & Torres-Jiménez, M. F. Frugivory in canopy plants in a western Amazonian forest: Dispersal systems, phylogenetic ensembles and keystone plants. PLoS ONE 10, e0140751 (2015).
Todeschini, F., de Toledo, J. J., Rosalino, L. M. & Hilário, R. R. Niche differentiation mechanisms among canopy frugivores and zoochoric trees in the northeastern extreme of the Amazon. Acta Amaz 50, 263–272 (2020).
Wilkie, D. S., Bennett, E. L., Peres, C. A. & Cunningham, A. A. The empty forest revisited. Ann. N. Y. Acad. Sci. 1223, 120–128 (2011).
Shanee, N. Trends in local wildlife hunting, trade and control in the Tropical Andes Biodiversity Hotspot, northeastern Peru. Endanger. Species Res. 19, 177–186 (2012).
Muscarella, R. & Fleming, T. H. The role of frugivorous bats in Tropical forest succession. Biol. Rev. 82, 573–590 (2007).
Willig, M. R. et al. Phyllostomid bats of lowland Amazonia: Effects of habitat alteration on abundance. Biotropica 39, 737–746 (2007).
Charles-Dominique, P. et al. Les mammifères frugivores arboricoles nocturnes d’une forêt guyanaise: Inter-relation plantes-animaux. Rev. Ecol. Terre Vie 35, (1981).
Stevenson, P. R., Cardona, L., Cárdenas, S. & Link, A. Oilbirds disperse large seeds at longer distance than extinct megafauna. Sci. Rep. 11, 420 (2021).
Colon, C. P. & Campos-Arceiz, A. The impact of gut passage by Binturongs (Arctictus binturong) on seed germination. Raffles Bull. Zool. 61, 417–421 (2013).
Nakashima, Y., Inoue, E., Inoue-Murayama, M. & Abd. Sukor, J. R. Functional uniqueness of a small carnivore as seed dispersal agents: A case study of the common palm civets in the Tabin Wildlife Reserve, Sabah, Malaysia. Oecologia 164, 721–730 (2010).
Kays, R. W. Food preferences of kinkajous (Potos flavus): A frugivorous carnivore. J. Mammal. 80, 589–599 (1999).
Julien-Laferrière, D. Frugivory and Seed Dispersal by Kinkajous. in Nouragues: Dynamics and Plant-Animal Interactions in a Neotropical Rainforest (eds. Bongers, F., Charles-Dominique, P., Forget, P.-M. & Théry, M.) 217–226 (Springer, 2001).
Helgen, K. M. et al. Taxonomic revision of the olingos (Bassaricyon), with description of a new species, the Olinguito. ZooKeys 324, 1–83 (2013).
Nascimento, F. F. et al. The evolutionary history and genetic diversity of kinkajous, Potos flavus (Carnivora, Procyonidae). J. Mamm. Evol. 24, 439–451 (2017).
Picart, L. et al. The CAFOTROP method: An improved rope-climbing method for access and movement in the canopy to study biodiversity. Ecotropica 20, 45–52 (2014).
Moore, J. F. et al. The potential and practice of arboreal camera trapping. Methods Ecol. Evol. 12, 1768–1779 (2021).
Gregory, T., Carrasco-Rueda, F., Alonso, A., Kolowski, J. & Deichmann, J. L. Natural canopy bridges effectively mitigate Tropical forest fragmentation for arboreal mammals. Sci. Rep. 7, 3892 (2017).
Queenborough, S. A. & Forget, P. M. Adding spice to life: A special issue on the Myristicaceae. Trop. Conserv. Sci. 3 (2013).
Farwig, N., Schabo, D. G. & Albrecht, J. Trait-associated loss of frugivores in fragmented forest does not affect seed removal rates. J. Ecol. 105, 20–28 (2017).
Russo, S. E. Responses of dispersal agents to tree and fruit traits in Virola calophylla (Myristicaceae): Implications for selection. Oecologia 136, 80–87 (2003).
Howe, H. F. & Vande Kerckhove, G. A. Removal of wild nutmeg (Virola Surinamensis) crops by birds. Ecology 62, 1093–1106 (1981).
Laurance, W. F. et al. Averting biodiversity collapse in Tropical forest protected areas. Nature 489, 290–294 (2012).
de Thoisy, B., Renoux, F. & Julliot, C. Hunting in northern French Guiana and its impact on primate communities. Oryx 39, 149–157 (2005).
de Thoisy, B. et al. Rapid evaluation of threats to biodiversity: Human footprint score and large vertebrate species responses in French Guiana. Biodivers. Conserv. 19, 1567–1584 (2010).
Peres, C. A. & Dolman, P. M. Density compensation in Neotropical primate communities: Evidence from 56 hunted and nonhunted Amazonian forests of varying productivity. Oecologia 122, 175–189 (2000).
Hansen, D. M. & Galetti, M. The forgotten megafauna. Science 324, 42–43 (2009).
Whitworth, A. et al. Human disturbance impacts on rainforest mammals are most notable in the canopy, especially for larger-bodied species. Divers. Distrib. 25, 1166–1178 (2019).
Harrison, R. D. et al. Consequences of defaunation for a Tropical tree community. Ecol. Lett. 16, 687–694 (2013).
Terborgh, J. et al. Tree recruitment in an empty forest. Ecology 89, 1757–1768 (2008).
Boissier, O., Bouiges, A., Mendoza, I., Feer, F. & Forget, P.-M. Rapid assessment of seed removal and frugivore activity as a tool for monitoring the health status of Tropical forests. Biotropica 46, 633–641 (2014).
Howe, H. F. Fruit production and animal activity at two Tropical trees. Ecol. Trop. For. Seas. Rhythms Long-Term Chang. 189–199 (1982).
Julien-Laferriere, D. Foraging strategies and food partitioning in the Neotropical frugivorous mammals Caluromys philander and Potos flavus. J. Zool. 247, 71–80 (1999).
Julien-Laferriere, D. Radio-tracking observations on ranging and foraging patterns by kinkajous (Potos flavus) in French Guiana. J. Trop. Ecol. 9, 19–32 (1993).
Bowler, M. T., Tobler, M. W., Endress, B. A., Gilmore, M. P. & Anderson, M. J. Estimating mammalian species richness and occupancy in Tropical forest canopies with arboreal camera traps. Remote Sens. Ecol. Conserv. 3, 146–157 (2017).
Debruille, A., Kayser, P., Veron, G., Vergniol, M. & Perrigon, M. Improving the detection rate of binturongs (Arctictis binturong) in Palawan Island, Philippines, through the use of arboreal camera-trapping. Mammalia 84, 563–567 (2020).
Gregory, T., Carrasco Rueda, F., Deichmann, J., Kolowski, J. & Alonso, A. Arboreal camera trapping: taking a proven method to new heights. Methods Ecol. Evol. 5, 443–451 (2014).
Whitworth, A., Braunholtz, L. D., Huarcaya, R. P., MacLeod, R. & Beirne, C. Out on a limb: Arboreal camera traps as an emerging methodology for inventorying elusive rainforest mammals. Trop. Conserv. Sci. 9, 675–698 (2016).
Laughlin, M. M., Martin, J. G. & Olson, E. R. Arboreal camera trapping reveals seasonal behaviors of Peromyscus spp. in Pinus strobus canopies. 14 (2020).
Thorn, M., Scott, D. M., Green, M., Bateman, P. W. & Cameron, E. Z. Estimating brown hyaena occupancy using baited camera traps. South Afr. J. Wildl. Res. 39, 1–10 (2009).
Si, X., Kays, R. & Ding, P. How long is enough to detect terrestrial animals? Estimating the minimum trapping effort on camera traps. PeerJ 2, e374 (2014).
Olson, E. R. et al. Arboreal camera trapping for the Critically Endangered greater bamboo lemur Prolemur simus. Oryx 46, 593–597 (2012).
Mendoza, I. et al. Inter-annual variability of fruit timing and quantity at Nouragues (French Guiana): Insights from hierarchical Bayesian analyses. Biotropica 50, 431–441 (2018).
Sabatier, D. Saisonnalité et déterminisme du pic de fructification en forêt guyanaise. Rev. Ecol. Terre Vie 40, 289–320 (1985).
Coutant, O. et al. Roads disrupt frugivory and seed removal in tropical animal-dispersed plants in French Guiana. Front. Ecol. Evol. 10, 805376 (2022)
Chapman, C. A. & Russo, S. E. Primate seed dispersal: Linking behavioral ecology with forest community structure. in Primates in Perspective 510–525 (Oxford University Press, 2006).
Zhang, S.-Y. Activity and ranging patterns in relation to fruit utilization by brown capuchins (Cebus apella) in French Guiana. Int. J. Primatol. 16, 489–507 (1995).
Julliot, C. Seed dispersal by red howling monkeys (Alouatta seniculus) in the Tropical rain forest of French Guiana. Int. J. Primatol. 17, 239–258 (1996).
Guillotin, M., Dubost, G. & Sabatier, D. Food choice and food competition among the three major primate species of French Guiana. J. Zool. 233, 551–579 (1994).
Coutant, O. et al. Amazonian mammal monitoring using aquatic environmental DNA. Mol. Ecol. Resour. 21, 1875–1888 (2021).
Lambert, J. E., Fellner, V., McKenney, E. & Hartstone-Rose, A. Binturong (Arctictis binturong) and Kinkajou (Potos flavus) Digestive Strategy: Implications for Interpreting Frugivory in Carnivora and Primates. PLoS ONE 9, e105415 (2014).
Youlatos, D. Osteological correlates of tail prehensility in carnivorans. J. Zool. 259, 423–430 (2003).
Lemelin, P. & Cartmill, M. The effect of substrate size on the locomotion and gait patterns of the kinkajou (Potos flavus) - Lemelin - 2010 - Journal of Experimental Zoology Part A: Ecological Genetics and Physiology - Wiley Online Library. J. Exp. Zool. 313A, 157–168 (2010).
McClearn, D. Locomotion, posture, and feeding behavior of kinkajous, coatis, and raccoons. J. Mammal. 73, 245–261 (1992).
Rensch, B. & Dücker, G. Manipulierfähigkeit eines Wickelbären bei längeren Handlungsketten. Z. Für Tierpsychol. 26, 104–112 (1969).
Kays, R. W. The behavior and ecology of olingos (Bassaricyon gabbii) and their competition with kinkajous (Potos flavus) in central Panama. 64, 1–10 (2000).
Alves-Costa, C. P. & Eterovick, P. C. Seed dispersal services by coatis (Nasua nasua, Procyonidae) and their redundancy with other frugivores in southeastern Brazil. Acta Oecologica 32, 77–92 (2007).
Bonaccorso, F. J., Glanz, W. E. & Sandford, C. M. Feeding assemblages of mammals at fruiting Dipteryx panamensis (Papilionaceae) trees in Panama: Seed predation, dispersal, and parasitism. Rev. Biol. Trop. 28, 61–72 (1980).
Julien-Laferrière, D. Organisation du peuplement de marsupiaux en Guyane française. Rev. Ecol. Terre Vie 46, 125–144 (1991).
Atramentowicz, M. The opportunistic frugivory of three Diphelphid marsupials of French Guiana. Rev. Ecol. Terre Vie 43, 47–57 (1988).
Carreira, D. C. et al. Small vertebrates are key elements in the frugivory networks of a hyperdiverse Tropical forest. Sci. Rep. 10, 10594 (2020).
Erard, C., Théry, M. & Sabatier, D. Fruit characters in the diet of syntopic large frugivorous forest bird species in French Guiana. Rev. Ecol. Terre Vie 62, 323–350 (2007).
Théry, M., Erard, C. & Sabatier, D. Les fruits dans le régime alimentaire de Penelope marail (Aves, Cracidae) en forêt guyanaise: Frufivorie stricte et sélective? Rev. Ecol. Terre Vie 47, (1992).
Zhu, C. et al. Arboreal camera trapping: a reliable tool to monitor plant-frugivore interactions in the trees on large scales. Remote Sens. Ecol. Conserv.
Schipper, J. Camera-trap avoidance by kinkajous Potos flavus: rethinking the “non-invasive” paradigm. 36, 5 (2007).
Ratiarison, S. Frugivorie dans la canopée de la forêt guyanaise : conséquences pour la pluie de graines. (Paris 6, 2003).
Sabatier, D. Fructification et dissémination en forêt guyanaise : l’exemple de quelques espèces ligneuses. (Université de Montpellier, 1983).
Sabatier, D. Description et biologie d’une nouvelle espèce de Virola (Myristicaceae) de Guyane. Adansonia 19, 273–278 (1997).
Niedballa, J., Sollmann, R., Courtiol, A. & Wilting, A. camtrapR: An R package for efficient camera trap data management. Methods Ecol. Evol. 7, 1457–1462 (2016).
Ridout, M. S. & Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 14, 322–337 (2009).
Bello, C. et al. Atlantic frugivory: a plant–frugivore interaction data set for the Atlantic forest. Ecology 98, 1729–1729 (2017).
Galetti, M., Laps, R. & Pizo, M. A. Frugivory by toucans (Ramphastidae) at two altitudes in the Atlantic forest of Brazil. Biotropica 32, 842–850 (2000).
Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package version 0.7.0. https://CRAN.R-project.org/package=rstatix (2021).
Acknowledgements
We thank Labex DRIIHM-OHM Oyapock, Fondation pour la Recherche pour la Biodiversité, UMR 7179 CNRS-MNHN, Association CAFOTROP, Hévéa, Petzl foundation and the Society Les Amis du Muséum for the Financial support. We thank Drs Tremaine Gregory, Jennifer Moore, Roland Kays, and two anonymous reviewers for their helpful and constructive comments on the manuscript. We thank all helpers on fieldwork that made this study possible.
Author information
Authors and Affiliations
Contributions
M.S. Writing, analysis, figures editing; O.C. Review, editing, and supervision; B.B. Tree climbing and canopy access as technical help; L.P. Tree climbing and canopy access as technical help; E.G. Study design, fieldwork, review, and editing; P-M.F. Study design, fieldwork, writing, review, editing and supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Séguigne, M., Coutant, O., Bouton, B. et al. Arboreal camera trap reveals the frequent occurrence of a frugivore-carnivore in neotropical nutmeg trees. Sci Rep 12, 7513 (2022). https://doi.org/10.1038/s41598-022-11568-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11568-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.