Abstract
Salmonella Typhi, a bacteria, is responsible for typhoid fever, a potentially dangerous infection. Typhoid fever affects a large number of people each year, estimated to be between 11 and 20 million, resulting in a high mortality rate of 128,000 to 161,000 deaths. This research investigates a robust numerical analytic strategy for typhoid fever that takes infection protection into consideration and incorporates fuzzy parameters. The use of fuzzy parameters acknowledges the variation in parameter values among individuals in the population, which leads to uncertainties. Because of their diverse histories, different age groups within this community may exhibit distinct customs, habits, and levels of resistance. Fuzzy theory appears as the most appropriate instrument for dealing with these uncertainty. With this in mind, a model of typhoid fever featuring fuzzy parameters is thoroughly examined. Two numerical techniques are developed within a fuzzy framework to address this model. We employ the non-standard finite difference (NSFD) scheme, which ensures the preservation of essential properties like dynamic consistency and positivity. Additionally, we conduct numerical simulations to illustrate the practical applicability of the developed technique. In contrast to many classical methods commonly found in the literature, the proposed approach exhibits unconditional convergence, solidifying its status as a dependable tool for investigating the dynamics of typhoid disease.
Similar content being viewed by others
Introduction
Typhoid is a result of typhus, a condition with identical symptoms. This endemic illness is brought on by the extremely pathogenic bacteria Salmonella typhi. This bacterium was disseminated by polluted water and other carriers. Typhoid is characterized by a persistent fever, a very poor appetite, vomiting, a very bad headache, and exhaustion. The incubation period for typhoid is 7 to 14 days. The patient’s intestine, which is where the germ naturally dwells, serves as its home. There is an increase in the number of mononuclear phagocytic cells in the blood. The patient’s blood culture is a key factor in determining how to treat typhoid. Chloramphenicol is ingested if the strain is amoxicillin sensitive. The oral dose of ciprofloxacin or norfloxacin is used to eradicate the issue in the asymptomatic carrier. Due to multi-drug resistance bacteria, antibiotic treatment has grown more challenging globally. In many nations, eliminating the disease will only be possible with the provision of clean, safe, and sanitary living circumstances, wholesome food, and the aforementioned medical services. These actions may lessen or eradicate the condition, though it is difficult to reach this goal. Following the implementation of health education initiatives that alter behavior toward illness prevention and treatment, the public can be made more aware. Every year, typhoid affects millions of people throughout the world. Typhoid is currently treated with oral and injectable vaccines, however, these two are insufficient to eradicate the illness. The length of the sickness can be shortened if a drug-resistant strain is used to treat the infected person1,2.
The dynamics of infectious diseases have been studied and explained using a variety of mathematical models3. To investigate the spread of infection at various endemicity levels, Cvjetanovic et al. built a mathematical model for typhoid disease. The model was used to predict the likely impact of typhoid fever prevention strategies, such as mass vaccination campaigns and sanitation programs, on a chosen population in terms of illness prevention as well as in terms of relative costs and benefits4. In order to examine the dynamics of typhoid fever sickness while including infection resistance, Nthiiri et al. developed a mathematical model. Using the next-generation matrix technique, the model’s stable states are identified and the reproduction number is calculated. The model’s stability is analyzed to identify the factors that contribute to the disease’s spread within a specific community5. The dynamics of the typhoid fever model were investigated, as well as the existence and uniqueness of the solution, by Peter et al. For the model, stability analysis is also carried out6. Bakach et al. reviewed some mathematical models of typhoid7. Karunditu et al. formulated a mathematical model of typhoid fever incorporating unprotected humans. The local and global stability of equilibrium points is also studied8. A mathematical model for the transmission of typhoid was developed by Nyaberi and Musaili, and it examines the effects of treatment on the dynamics of the illness9. Birger et al. studied mathematical models of typhoid transmission by considering FQNS and multidrug resistance separately. The effect of vaccination was predicted on the basis of forecasts of vaccine coverage10. A mathematical model for typhoid fever spread in a population is formulated. The equilibrium points of the model and their stabilities are investigated11. By utilizing several optimal control strategies, Wameko et al. established a mathematical model to look into the dynamics of typhoid disease. Typhoid disinformation among the population is reduced when the three control techniques are quickly implemented, as demonstrated12.
Due to population-wide variations in susceptibility, exposure, infectivity, and recovery, the parameters employed in epidemic models are imprecise. If different age groups, population groups, and resistance patterns are taken into account, differences may result. To take into consideration these varying degrees of persons, more realistic models are required. Mishra et al. claim that due to the high level of uncertainty, epidemic systems, particularly those involving infectious diseases, require a new approach13. Fuzzy sets and fuzzy logic have been extensively utilized to tackle real-world problems across diverse domains, encompassing medicine, engineering, economics, and numerous other fields where human decision-making plays a pivotal role in assessment and logical reasoning14,15,16,17,18, just to mention a few. Moreover, scholars have harnessed this theoretical framework in epidemiology as well. Incorporating fuzzy theory and treating the transmission coefficient as a fuzzy set, Barros et al. suggested a SI model19. Fuzzy logic was used by Ortega et al. to predict issues with infectious disease epidemiology. A rabies model in dogs with incomplete vaccinations was discussed20.
Mondal et al. developed an SIS model for investigating the plague using the fuzzy set theory21. Das and Pal developed a SIR model and studied it mathematically and numerically22. Sadhukhan et al. conducted research on harvesting optimization in a food chain model in a fuzzy environment23. To capture the dynamics of coronavirus illness, Li et al. developed a fuzzy SEIR model supplemented by confidence index theory24. Abdy et al. presented an SIR model that incorporated fuzzy parameters to depict the dynamics of COVID-1925. Furthermore, Allehiany et al. explored a fuzzy SIR model employing Euler, RK-4, and NSFD methods26. The NSFD approach, first described by Micken27, has been used by a number of researchers for solving systems of differential equations28,29,30, to name a few. Adak and Jana investigated an SIS model involving treatment control with the utilization of fuzzy numbers31.
The existing mathematical models of typhoid are insufficient for the advancement of fuzzy numerical and mathematical procedures. We investigated a typhoid model with fuzzy parameters with this in mind. We can cope with the difficulties of uncertainty quantification in mathematical disease modeling by using fuzzy theory. As a result, the introduction of fuzzy parameters aids in our ability to comprehend the dynamics of typhoid transmission. Even the biological factors employed in mathematical models are not always constant because each community changes as the environment change. The majority of the issues linked to the rise in the earth’s average temperature are caused by global warming. The rate at which the virus spreads throughout the population is also impacted by temperature changes. Fuzzy mathematical models are more insightful than crisp models in this regard. The creation, use, and analysis of first order explicit numerical techniques in fuzzy non-standard finite difference situations are novel aspects of the current work.
The rest of this study is organized as follows: The formulation of the fuzzy model is discussed in the following section. Within the same section, we discuss equilibrium analysis, stability analysis, and the fuzzy basic reproduction number. Following that, in the numerical modeling section, we elaborate on the creation of the forward Euler scheme and NSFD schemes for the examined model. This section also includes an assessment of the NSFD scheme’s stability and consistency. The next part displays numerical simulations involving the developed techniques. Finally, in the final section, we summarize concluding observations and outline future research directions.
Typhoid fever model with fuzzy parameters
We examined the mathematical model previously discussed by Arif et al.2
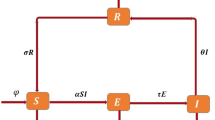
Here, \(T_1\) represents the proportion of humans who are protected, \(T_2\) represents the proportion of humans who are susceptible, \(T_3\) signifies the fraction of humans who are currently infected, and \(T_4\) indicates the fraction of humans receiving treatment. The rate at which humans receiving treatment transitions from the fraction of infected humans is denoted as \(\beta\). The variable \(\alpha\) represents the rate at which individuals enroll in the protected human compartment against typhoid, while \((1-\alpha )\) denotes the rate of individuals who remain susceptible to the virus, \(\delta\) signifies the rate at which individuals experience a transient phase due to typhoid fever, and \(\theta\) reflects the per capita rate at which susceptible individuals contract typhoid fever infection. Lastly, \(\mu\) represents the natural rate of human death and birth. The depiction of the communication dynamics in the typhoid fever model is presented in Fig. 1.
The fuzzy representation of the model mentioned above can be expressed as follows:
We assume that susceptible humans contract typhoid fever infection at a per capita rate denoted by \(\theta (\Omega )\) and the fraction of treated humans stemming from infected individuals, \(\beta (\Omega )\), are represented as fuzzy numbers, contingent upon the individual’s virus load. The variable \(\theta (\Omega )\) can be defined as19
The \(\theta (\Omega )\) reaches its peak when \(\Omega\) is at its maximum value, and it becomes insignificant when \(\Omega\) is at its minimum. \(\Omega _{min}\) represents the minimum virus load required for disease transmission, and disease transmission is at its highest when \(\Omega\) equals \(\Omega _M\), reaching a value of 1. Similarly, \(\beta (\Omega )\) can be defined as32
where \(\beta _0>0\) is the minimum treatment rate.
Equilibrium analysis
The analyzed model possesses a disease-free equilibrium point (DFE) and two endemic equilibrium points (EE).
- Case 1.:
-
If \(\Omega <\Omega _{min}\) and \(\beta (\Omega )>0\) then \(\theta (\Omega )=0\) and we obtain,
$$\begin{aligned} T^0(T_1^0, T_2^0, T_3^0, T_4^0)=\bigg (\frac{\alpha \mu }{\gamma +\mu }, \frac{(\gamma +\mu )(1-\alpha )+\gamma \alpha }{\gamma +\mu }, 0, 0\bigg ). \end{aligned}$$In this scenario, the human population remains free from typhoid, and this state is referred to as the DFE. From a biological perspective, typhoid disease is considered eradicated when the disease concentration within the population falls below the minimum threshold required for its sustained existence.
- Case 2.:
-
If \(\Omega _{min}\le \Omega \le \Omega _M\) and \(\beta (\Omega )>0\) then \(\theta (\Omega )=\frac{\Omega -\Omega _{min}}{\Omega _M-\Omega _{min}}\) and we obtain,
$$\begin{aligned} T^*=(T_1^*, T_2^*, T_3^*, T_4^*), \end{aligned}$$where
$$\begin{aligned} T_1^*& = \frac{\alpha \mu }{\gamma +\mu },\\ T_2^*& = \frac{\delta +\beta (\Omega )+\mu }{\theta (\Omega )},\\ T_3^*& = \frac{(\gamma +\mu )(1-\alpha )\mu \theta (\Omega )+\gamma \alpha \mu \theta (\Omega )-\mu (\delta +\beta (\Omega )+\mu )(\gamma +\mu )}{\theta (\Omega )(\gamma +\mu )(\delta +\beta (\Omega )+\mu )}, \end{aligned}$$and
$$\begin{aligned} T_4^*=\frac{\beta (\Omega )\bigg ((\gamma +\mu )(1-\alpha )\mu \theta (\Omega )+\gamma \alpha \mu \theta (\Omega )-\mu (\delta +\beta (\Omega )+\mu )(\gamma +\mu )\bigg )}{\theta (\Omega )(\gamma +\mu )(\delta +\beta (\Omega )+\mu )}. \end{aligned}$$ - Case 3.:
-
If \(\Omega _M<\Omega\) and \(\beta (\Omega )>0\) then \(\theta (\Omega )=1\), and we obtain
$$\begin{aligned} T^{**}=(T_1^{**}, T_2^{**}, T_3^{**}, T_4^{**}), \end{aligned}$$where
$$\begin{aligned} T_1^{**}& = \frac{\alpha \mu }{\gamma +\mu },\\ T_2^{**}& = \frac{\delta +\beta (\Omega )+\mu }{\theta (\Omega )},\\ T_3^{**}& = \frac{(\gamma +\mu )(1-\alpha )\mu \theta (\Omega )+\gamma \alpha \mu \theta (\Omega )-\mu (\delta +\beta (\Omega )+\mu )(\gamma +\mu )}{\theta (\Omega )(\gamma +\mu )(\delta +\beta (\Omega )+\mu )}, \end{aligned}$$and
$$\begin{aligned} T_4^{**}=\frac{\beta (\Omega )\bigg ((\gamma +\mu )(1-\alpha )\mu \theta (\Omega )+\gamma \alpha \mu \theta (\Omega )-\mu (\delta +\beta (\Omega )+\mu )(\gamma +\mu )\bigg )}{\theta (\Omega )(\gamma +\mu )(\delta +\beta (\Omega )+\mu )}. \end{aligned}$$
The points \(T^*\) and \(T^{**}\) represent situations where the prevalence of typhoid disease exceeds the minimum threshold necessary for its propagation, resulting in the persistence of typhoid within the human population.
Stability analysis
To check the stability, let us assume the following system:
and
The Jacobian of the system (11)–(14) can be represented as:
The Jacobian at the DFE is
The local asymptotic stability of the steady-state is confirmed when the absolute eigenvalues of the Jacobian matrix mentioned earlier having negative real parts. Analyzing the Jacobian matrix, we find that the eigenvalues are as follows: \(\lambda _1= -(\gamma +\mu )\), \(\lambda _2 = \lambda _4= - \mu\), and \(\lambda _3 =- (\delta +\beta (\Omega )+\mu )\). The fact that all of these eigenvalues having negative real parts validates the desired result.
Fuzzy basic reproductive number \(R_t^f\)
Using the next-generation matrix technique, we calculated the reproductive number, denoted as \(R_t\).
Verma et al.33 examined different scenarios by manipulating the parameters and, in each case, determined the reproduction number to assess whether the virus spread was effectively managed.
Now, \(R_t\), being a function of the typhoid disease, can be analyzed as follows34:
- Case 1.:
-
If \(\Omega <\Omega _{min}\) and \(\beta (\Omega )>0\) then \(\theta (\Omega )=0\) and \(R_t(\Omega )=0\).
- Case 2.:
-
If \(\Omega _{min}\le \Omega \le \Omega _M\) and \(\beta (\Omega )>0\) then \(\theta (\Omega )=\frac{\Omega -\Omega _{min}}{\Omega _M-\Omega _{min}}\) and \(R_t(\Omega )=\frac{\theta (\Omega )(\gamma +\mu -\alpha \mu )}{(\gamma +\mu )(\delta +\beta (\Omega )+\mu )}\).
- Case 3.:
-
If \(\Omega _M<\Omega\) and \(\beta (\Omega )>0\) then \(\theta (\Omega )=1\) and \(R_t(\Omega )=\frac{\gamma +\mu -\alpha \mu }{(\gamma +\mu )(\delta +\beta (\Omega )+\mu )}\). The disease-dependent function \(R_t(\Omega )\) correlates positively with the disease parameter \(\Omega\), and its definition includes a fuzzy variable. As a result, the expected value of \(R_t(\Omega )\) is well defined, and its representation can be written as a triangular fuzzy number, as follows:
$$\begin{aligned} R_t(\Omega )=\bigg (0, \frac{\theta (\Omega )(\gamma +\mu -\alpha \mu )}{(\gamma +\mu )(\delta +\beta (\Omega )+\mu )}, \frac{\gamma +\mu -\alpha \mu }{(\gamma +\mu )(\delta +\beta (\Omega )+\mu )}\bigg ). \end{aligned}$$(16)Now, \(R_t^f\) can be found as
$$\begin{aligned} R_t^f=E[R_t (\Omega )], \end{aligned}$$(17)and therefore,
$$\begin{aligned} R_t^f=\frac{(\gamma +\mu -\alpha \mu )(2\theta (\Omega )+1)}{4(\gamma +\mu (\delta +\beta (\Omega )+\mu )}. \end{aligned}$$(18)
Numerical modeling
Forward Euler scheme
The Forward Euler scheme is a well-known explicit first-order numerical approach for solving ordinary differential equations. It is computationally efficient and provides a rapid estimation of the behavior of solutions over time.
- Case 1.:
-
If \(\Omega <\Omega _{min}\) and \(\beta (\Omega )>0\) then \(\theta (\Omega )=0\) and
$$\begin{aligned} T_1^{n+1}& = T_1^n+h[\alpha \mu -(\gamma +\mu ) T_1^n ],\end{aligned}$$(23)$$\begin{aligned} T_2^{n+1}& = T_2^n+h[(1-\alpha )\mu +\gamma T_1^n-\mu T_2^n ], \end{aligned}$$(24)$$\begin{aligned} T_3^{n+1}& = T_3^n-h[(\delta +\beta (\Omega )-\mu ) T_3^n ], \end{aligned}$$(25)$$\begin{aligned} T_4^{n+1}& = T_4^n+h[\beta (\Omega )) T_3^n-\mu T_4^n ].\end{aligned}$$(26) - Case 2.:
-
If \(\Omega _{min}\le \Omega \le \Omega _M\) and \(\beta (\Omega )>0\) then \(\theta (\Omega )=\frac{\Omega -\Omega _{min}}{\Omega _M-\Omega _{min}}\) and the Euler scheme becomes
$$\begin{aligned} T_1^{n+1}& = T_1^n+h[\alpha \mu -(\gamma +\mu ) T_1^n ],\end{aligned}$$(27)$$\begin{aligned} T_2^{n+1}& = T_2^n+h[(1-\alpha )\mu +\gamma T_1^n-\theta (\Omega ) T_2^n T_3^n-\mu T_2^n ], \end{aligned}$$(28)$$\begin{aligned} T_3^{n+1}& = T_3^n+h[\theta (\Omega ) T_2^n T_3^n-(\delta +\beta (\Omega )+\mu ) T_3^n ], \end{aligned}$$(29)$$\begin{aligned} T_4^{n+1}& = T_4^n+h[\beta (\Omega )) T_3^n-\mu T_4^n ].\end{aligned}$$(30) - Case 3.:
-
If \(\Omega _M<\Omega\) and \(\beta (\Omega )>0\) then \(\theta (\Omega )=1\) and
$$\begin{aligned} T_1^{n+1}& = T_1^n+h[\alpha \mu -(\gamma +\mu ) T_1^n ],\end{aligned}$$(31)$$\begin{aligned} T_2^{n+1}& = T_2^n+h[(1-\alpha )\mu +\gamma T_1^n- T_2^n T_3^n-\mu T_2^n ], \end{aligned}$$(32)$$\begin{aligned} T_3^{n+1}& = T_3^n+h[ T_2^n T_3^n-(\delta +\beta (\Omega )+\mu ) T_3^n ], \end{aligned}$$(33)$$\begin{aligned} T_4^{n+1}& = T_4^n+h[\beta (\Omega )) T_3^n-\mu T_4^n ].\end{aligned}$$(34)
Non-standard finite difference (NSFD) scheme
The NSFD scheme is a class of numerical methods for approximating solutions to differential equations. These approaches differ from traditional finite difference methods in their approach to discretizing the domain and approximating derivatives. It has the potential to improve accuracy in the solution of differential equations. The NSFD numerical model is formulated based on the NSFD theory introduced by Mickens35.
- Case 1.:
-
If \(\Omega <\Omega _{min}\) and \(\beta (\Omega )>0\) then \(\theta (\Omega )=0\) and the NSFD scheme becomes
$$\begin{aligned} T_1^{n+1}& = \frac{T_1^n+h\alpha \mu }{1+h(\gamma +\mu )},\end{aligned}$$(39)$$\begin{aligned} T_2^{n+1}& = \frac{T_2^n+h(1-\alpha )\mu +\gamma T_1^n)}{1+h\mu },\end{aligned}$$(40)$$\begin{aligned} T_3^{n+1}& = \frac{T_3^n}{1+h(\delta +\beta (\Omega )+\mu )}, \end{aligned}$$(41)$$\begin{aligned} T_4^{n+1}& = \frac{T_4^n+h\beta (\Omega ) T_3^n}{1+h\mu }.\end{aligned}$$(42) - Case 2.:
-
If \(\Omega _{min}\le \Omega \le \Omega _M\) and \(\beta (\Omega )>0\) then \(\theta (\Omega )=\frac{\Omega -\Omega _{min}}{\Omega _M-\Omega _{min}}\) and the NSFD Scheme becomes
$$\begin{aligned} T_1^{n+1}& = \frac{T_1^n+h\alpha \mu }{1+h(\gamma +\mu )},\end{aligned}$$(43)$$\begin{aligned} T_2^{n+1}& = \frac{T_2^n+h(1-\alpha )\mu +\gamma T_1^n)}{1+h(\theta (\Omega )T_3^n +\mu )},\end{aligned}$$(44)$$\begin{aligned} T_3^{n+1}& = \frac{T_3^n+h\theta (\Omega ) T_2^n T_3^n}{1+h(\delta +\beta (\Omega )+\mu )}, \end{aligned}$$(45)$$\begin{aligned} T_4^{n+1}& = \frac{T_4^n+h\beta (\Omega ) T_3^n}{1+h\mu }.\end{aligned}$$(46) - Case 3.:
-
If \(\Omega _M<\Omega\) and \(\beta (\Omega )>0\) then \(\theta (\Omega )=1\) and the scheme becomes
$$\begin{aligned} T_1^{n+1}& = \frac{T_1^n+h\alpha \mu }{1+h(\gamma +\mu )},\end{aligned}$$(47)$$\begin{aligned} T_2^{n+1}& = \frac{T_2^n+h(1-\alpha )\mu +\gamma T_1^n)}{1+h(T_3^n +\mu )},\end{aligned}$$(48)$$\begin{aligned} T_3^{n+1}& = \frac{T_3^n+h T_2^n T_3^n}{1+h(\delta +\beta (\Omega )+\mu )}, \end{aligned}$$(49)$$\begin{aligned} T_4^{n+1}& = \frac{T_4^n+h\beta (\Omega ) T_3^n}{1+h\mu }.\end{aligned}$$(50)
Consistency analysis
To check the consistency of the proposed scheme, we start by taking Eq. (35), and we have
Taking into account the Taylor’s series expansion for \(T_1^{n+1}\),
Substituting the value of \(T_1^{n+1}\) in Eq. (51), we get
Taking \(h\rightarrow 0\), we get
or
From Eq. (36), we have
Taking into account the Taylor’s series expansion for \(T_2^{n+1}\),
Substituting the value of \(T_2^{n+1}\) in Eq. (56), we get
Taking \(h\rightarrow 0\), we get
From Eq. (37), we have
Similarly, taking into account the Taylor’s series expansion for \(T_3^{n+1}\),
Substituting the value of \(T_3^{n+1}\) in Eq. (61), we get
Simplifying Eq. (63) and taking \(h\rightarrow 0\), we get
or
Similarly, we can get
by applying Taylor’s series on Eq. (38). Hence, we can conclude that our proposed scheme exhibits first-order consistency.
Stability of the NSFD scheme
To study the stability analysis, let us assume the following system:
The Jacobian matrix corresponding to the system (67)–(70) is
Jacobian at the DFE is
Eigenvalues of the above Jacobian matrix are \(\lambda _1=\frac{1}{1+h(\gamma +\mu )}<1\), \(\lambda _2=\lambda _4=\frac{1}{1+h\mu }<1\) and \(\lambda _3=\frac{1}{1+h(\delta +\beta (\Omega )+\mu )}<1\). Because all eigenvalues are less than one, this validates the desired outcome that the NSFD scheme is stable at the DFE36. NSFD schemes do not constitute a singular category of numerical methods. Numerous researchers have expanded upon the Mickens theory. For instance, Gurski37 introduced a straightforward mathematical framework for NSFD schemes, specifically tailored for small systems of nonlinear differential equations. This approach leverages conventional techniques used in approximating differential equations, including the incorporation of artificial viscosity and the implementation of a predictor-corrector scheme. In their work, they examined both the NSFD scheme proposed by Mickens and the one developed by Erdogan and Ozis for first-order equations.
Numerical simulations
In this section, we present the simulation results of the performance of Euler method illustrated in Fig. 2a–f, and the simulation results obtained using the NSFD method illustrated in Fig. 3a–f.
The graphical representations of the performance of Euler method are illustrated at various step sizes in Fig. 2a–f. Initially, at a small step size \(h=1\), the method exhibits stability, positivity, and convergence. However, as the step size is slightly increased to \(h=5\), the method begins to oscillate and generates negative values in all three cases. In models like this, negative values are not meaningful since all compartments represent populations, and negativity is not feasible. Consequently, it can be deduced that this method is not a dependable tool for describing such models. In Fig. 3a–f, we present the simulation results obtained using the NSFD method. This time, the method demonstrates stability, both at smaller and larger step sizes, and an increase in the step size does not adversely affect its convergence and positivity, which are crucial characteristics for modeling disease dynamics. The method consistently displays stable and convergent behavior across all three cases, underscoring its reliability for studying disease dynamics in such conditions. It is worth noting that many classical numerical schemes and their fuzzy counterparts tend to lose their convergence and positivity and struggle to maintain stability as the step size increases. In contrast, the proposed method proves to be more efficient and capable of addressing such issues. We provide the convergence analysis for proposed NSFD scheme analytically and then verify it numerically in numerical simulations section. It can be noted here that the proposed NSFD scheme gives unconditional convergence and remains consistent with the continuous dynamical system. Figure 3a–f show the convergence of NSFD scheme to true equilibria, retaining all the essential features of continuous model unlike the Euler scheme which fails to do so (see Fig. 2a–f).
Conclusions
In this study, we have considered a typhoid model with fuzzy parameters. We assumed that the infection does not transmit equally among the individual of the populations. Similarly, the treatment rate is also not the same for each individual. As a function of the virus concentrations, we treated the typhoid transmission rate \(\theta (\Omega )\) and the treatment rate \(\beta (\Omega )\) as fuzzy variables. In deterministic models, these parameters are fixed and independent of the viral load. As a result, it may be said that the fuzzy model is more adaptable and balanced than the crisp system. Fuzzy theory is used to address uncertainty quantification difficulties in mathematical disease modeling. We examined it for various virus loads because fuzzy variables are functions of virus load that depend on virus levels. In light of this, we addressed the studied model’s fuzzy equilibrium points while taking the population’s virus levels into account. We established that the disease-free equilibrium point is reached if the virus concentration is lower than the minimal concentration necessary for disease transmission in the population. When the population’s viral levels exceeded the bare minimum needed for disease transmission, we reached the endemic equilibrium points. For various viral concentrations, the basic reproduction rate is examined. We employed the expected value of a fuzzy number to ascertain the fuzzy basic reproduction number. Two numerical schemes are developed for the approximate solution of the studied model. The developed schemes are analyzed for different amounts of virus. The suggested numerical algorithms must maintain the positive nature of the solutions of such dynamic population models. The Euler method preserved this for only small values of the step sizes and generated negative values by increasing the step size. On the other hand, the NSFD preserved this for all large values of the step size too for different amounts of virus. Additionally, the convergence and consistency of the NSFD scheme are analyzed, demonstrating that the suggested approach is unconditionally convergent and consistent of order 1. The creation, application, and analysis of a non-standard finite difference technique for the numerical analysis of a typhoid illness model with fuzzy parameters are the main foci of the current work. Future developments may include fuzzy stochastic, fuzzy delayed, or fuzzy fractional dynamic senses. Age-structured fuzzy epidemic models could potentially be modeled using the NSFD modeling theory. This research focuses mostly on using triangular fuzzy numbers as membership functions. In the future, we may investigate the use of various fuzzy numbers as potential membership functions, such as trapezoidal, pentagonal, hexagonal, and so on.
Data availability
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
References
Cai, L.-M. & Li, X.-Z. Global analysis of a vector-host epidemic model with nonlinear incidences. Appl. Math. Comput. 217, 3531–3541 (2010).
Arif, M. S. et al. A reliable stochastic numerical analysis for typhoid fever incorporating with protection against infection. Comput. Mater. Continua 59, 787–804 (2019).
Alalhareth, F. K., Atta, U., Ali, A. H., Ahmad, A. & Alharbi, M. H. Analysis of leptospirosis transmission dynamics with environmental effects and bifurcation using fractional-order derivative. Alex. Eng. J. 80, 372–382 (2023).
Cvjetanović, B., Grab, B. & Uemura, K. Epidemiological model of typhoid fever and its use in the planning and evaluation of antityphoid immunization and sanitation programmes. Bull. World Health Organ. 45, 53–75 (1971).
Nthiiri, J. K. et al. Mathematical modelling of typhoid fever disease incorporating protection against infection. Br. J. Math. Comput. Sci. 14, 1–10 (2016).
Peter, O., Ibrahim, M., Akinduko, O. & Rabiu, M. Mathematical model for the control of typhoid fever. IOSR J. Math 13, 60–66 (2017).
Bakach, I., Just, M. R., Gambhir, M. & Fung, I.C.-H. Typhoid transmission: A historical perspective on mathematical model development. Trans. R. Soc. Trop. Med. Hyg. 109, 679–689 (2015).
Karunditu, J. W., Kimathi, G. & Osman, S. Mathematical modeling of typhoid fever disease incorporating unprotected humans in the spread dynamics. J. Adv. Math. Comput. Sci. 32, 1–11 (2019).
Nyaberi, H. O. & Musaili, J. S. Mathematical modeling of the impact of treatment on the dynamics of typhoid. J. Egypt. Math. Soc. 29, 15 (2021).
Birger, R. et al. Estimating the effect of vaccination on antimicrobial-resistant typhoid fever in 73 countries supported by Gavi: A mathematical modelling study. Lancet Infect. Dis. 22, 679–691 (2022).
Joshua, E. & ETUKUDO, U. Mathematical model of the spread of typhoid fever. World J. Appl. Sci. Technol. 3, 10–12 (2011).
Wameko, M., Koya, P. & Wodajo, A. Mathematical model for transmission dynamics of typhoid fever with optimal control strategies. Int. J. Ind. Math. 12, 283–296 (2020).
Mishra, B. K. & Prajapati, A. Spread of malicious objects in computer network: A fuzzy approach. Appl. Appl. Math. Int. J. 8, 684–700 (2013).
Liao, T. W. A fuzzy multicriteria decision-making method for material selection. J. Manuf. Syst. 15, 1–12 (1996).
Bates, J. H. & Young, M. P. Applying fuzzy logic to medical decision making in the intensive care unit. Am. J. Respir. Crit. Care Med. 167, 948–952 (2003).
Fenton, N. & Wang, W. Risk and confidence analysis for fuzzy multicriteria decision making. Knowl.-Based Syst. 19, 430–437 (2006).
Yeh, C.-H. & Chang, Y.-H. Modeling subjective evaluation for fuzzy group multicriteria decision making. Eur. J. Oper. Res. 194, 464–473 (2009).
Saeed, M., Ali, U., Ali, J. & Dayan, F. Fuzzy soft relative method and its application in decision making problem: Fuzzy soft relative method and its application in decision making problem. Proc. Pak. Acad. Sci. A Phys. Comput. Sci. 57, 21–30 (2020).
Barros, L. D., Leite, M. F. & Bassanezi, R. The SI epidemiological models with a fuzzy transmission parameter. Comput. Math. Appl. 45, 1619–1628 (2003).
Ortega, N. R. S., Sallum, P. C. & Massad, E. Fuzzy dynamical systems in epidemic modelling. Kybernetes 29, 201–218 (2000).
Mondal, P. K., Jana, S., Haldar, P. & Kar, T. K. Dynamical behavior of an epidemic model in a fuzzy transmission. Int. J. Uncertain. Fuzziness Knowl.-Based Syst. 23, 651–665 (2015).
Das, A. & Pal, M. A mathematical study of an imprecise sir epidemic model with treatment control. J. Appl. Math. Comput. 56, 477–500 (2018).
Sadhukhan, D., Sahoo, L., Mondal, B. & Maiti, M. Food chain model with optimal harvesting in fuzzy environment. J. Appl. Math. Comput. 34, 1–18 (2010).
Li, C., Huang, J., Chen, Y.-H. & Zhao, H. A fuzzy susceptible-exposed-infected-recovered model based on the confidence index. Int. J. Fuzzy Syst. 23, 907–917 (2021).
Abdy, M., Side, S., Annas, S., Nur, W. & Sanusi, W. An sir epidemic model for COVID-19 spread with fuzzy parameter: The case of Indonesia. Adv. Differ. Equ. 2021, 1–17 (2021).
Allehiany, F. et al. Bio-inspired numerical analysis of COVID-19 with fuzzy parameters. Comput. Mater. Continua 72, 3213–3229 (2022).
Mickens, R. E. Dynamic consistency: A fundamental principle for constructing nonstandard finite difference schemes for differential equations. J. Differ. Equ. Appl. 11, 645–653 (2005).
Cresson, J. & Pierret, F. Non standard finite difference scheme preserving dynamical properties. J. Comput. Appl. Math. 303, 15–30 (2016).
Naveed, M. et al. Mathematical analysis of novel coronavirus (2019-ncov) delay pandemic model. Comput. Mater. Continua 64, 1401–1414 (2020).
Shatanawi, W. et al. An effective numerical method for the solution of a stochastic coronavirus (2019-ncovid) pandemic model. Comput. Mater. Continua 66, 1121–1137 (2021).
Adak, S. & Jana, S. Dynamical behavior of an epidemic model with fuzzy transmission and fuzzy treatment control. J. Appl. Math. Comput. 68, 1929–1948 (2022).
Verma, R., Tiwari, S. & Upadhyay, R. K. Dynamical behaviors of fuzzy sir epidemic model. In Proceedings of the Conference of the European Society for Fuzzy Logic and Technology, 482–492 (Springer, 2017).
Verma, H., Mishra, V. N. & Mathur, P. Effectiveness of lock down to curtail the spread of corona virus: A mathematical model. ISA Trans. 124, 124–134 (2022).
Mangongo, Y. T., Bukweli, J.-D.K. & Kampempe, J. D. B. Fuzzy global stability analysis of the dynamics of malaria with fuzzy transmission and recovery rates. Am. J. Oper. Res. 11, 257–282 (2021).
Mickens, R. E. Numerical integration of population models satisfying conservation laws: NSFD methods. J. Biol. Dyn. 1, 427–436 (2007).
Ratnam, K. V., Rao, P. R. S. & Shirisha, G. Stability preserving NSFD scheme for a cooperative and supportive network. Int. J. Dyn. Control 9, 1576–1588 (2021).
Gurski, K. F. A simple construction of nonstandard finite-difference schemes for small nonlinear systems applied to sir models. Comput. Math. Appl. 66, 2165–2177 (2013).
Author information
Authors and Affiliations
Contributions
Conceptualization, F.D. and N.A. Data curation, M.R. and A.R. Formal analysis, A.H.A. and A.R. Funding acquisition, N.A. and A.H.A. Investigation, N.A. and A.H.A. Methodology, F.D. and N.A. Project administration, N.A. and A.H.A. Resources, M.R. and A.R. Software, A.H.A. Supervision, N.A. Validation, M.R. and A.R. Writing—review and editing, F.D., N.A. and A.H.A. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dayan, F., Ahmed, N., Ali, A.H. et al. Numerical investigation of a typhoid disease model in fuzzy environment. Sci Rep 13, 21993 (2023). https://doi.org/10.1038/s41598-023-48405-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48405-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.