Abstract
To evaluate the agreement and repeatability of an automated topography-based method for non-invasive break-up time (NIBUT) analyses in comparison with two other NIBUT procedures, the fluorescein procedure (fBUT), and with the manual assessment with the same device. In the first experiment, a semi-randomised crossover study was performed on forty-three participants (23.1 ± 2.1 years). NIBUT measurements were collected in a randomised order, in both eyes of participants with EasyTear View + (Easytear, Rovereto), Polaris, and Sirius + (CSO, Firenze). Then a fBUT was collected. The overall measurement procedure was repeated in a further session (retest) on the same day. In a second experiment, a retrospective randomised crossover study was performed on eighty-five NIBUT videos previously recorded by the Sirius+. Two observers assessed manually the videos and the NIBUTs were compared with the automatic ones. In the first experiment, ANOVA showed a significant difference between the four measures in both eyes (p < 0.001). Significant differences were found in the paired comparisons between each NIBUT procedure and fBUT (Wicoxon; p < 0.05). Sirius+ resulted in agreement only with Polaris in the left eye. Correlations between all NIBUT procedures resulted in statistical significance in both eyes. All procedures showed very good test-rest reliability. In the second experiment, a significant correlation between automated and manual NIBUT was found, but also a significant statistical difference between the two measurements, although clinically negligible (0.3 s). The investigated NIBUT devices perform differently from each other (and from fBUT), so they cannot be considered interchangeable. The automated measure of NIBUT with Sirius+ has a negligible clinical difference compared to manual assessment on the same device.
Similar content being viewed by others
Introduction
The tear film is a thin structure (about 2.0–5.5 µm thick1,2), extremely sophisticated in functioning and composition with a crucial role in maintaining ocular surface physiology. The assessment of tear film is of paramount importance in diagnosing dry eye disease (DED)3, One aspect of the tear film which is crucial to investigate for DED diagnosis is its stability3,4,5. Many factors determine the stability of tear film such as a sufficient and balanced production of the main components, which have to be spread efficiently on the ocular surface by the blinking system5. According to the three-layered model of the tear film, the stability is maintained by the prevention of evaporation by the outer lipid layer, the increase of volume and lubricity by the aqueous layer, and the reduction of hydrophobicity of the corneal epithelium by the inner mucin layer.
The lack of stability can be measured by the tear break-up time (TBUT) as the interval of time that elapses between the end of a complete blink and the appearance of the first break in the tear film3,4. The first procedure of TBUT, also known as fluorescein BUT (fBUT), was introduced by Norn in 19696, who proposed to instill sodium fluorescein dye in the tears to detect breaks by using a biomicroscope and cobalt blue light. The “magic” number 10 s would indicate the cut off between normal and abnormal tear film6. Notwithstanding the clinical fortune of fBUT, which became the most common test for tear film assessment7,8,9, it has been largely recognised for its poor reliability10,11, (mainly linked to fluorescein invasiveness)12 There have been proposed variations in the fBUT procedure to improve reliability such as a reduction and control of the amount of sodium fluorescein used13,14,15, or performing multiple measures16 in different occasions17, etc. However, the best way to measure the stability of the tear film should be to use a non-invasive approach3,18 that should avoid altering the the tear film (e.g., by increasing the temperature and/or causing reflex blinking with the illumination systems, instilling fluorescein, forcing blinking, etc.)3,4. The non-invasive break-up time (NIBUT) is determined as the interval of time that elapses between the end of a complete blink and the appearance of a discontinuity or break in the image of a mire or a grid pattern (keratometer mire or Placido disc) reflected on the anterior tear film surface4,19. This approach gives also the possibility to detect changes in the tear film that anticipate a real break-up: for example, a distortion of the grid patterns can be interpreted as a thinning of the film, which can be exploited to measure the tear thinning time20. The NIBUT procedure has become widespread19,21,22,23, and nowadays it has been implemented in modern corneal topography systems24,25,26,27,28,29,30,31,32,33. In these instruments, NIBUT is measured in an automatic way by algorithms that can assess the break-up from the video acquired by videokeratography34. However, differences in Placido disc (e.g., number and size of rings), background illumination, and algorithms might cause differences in results. In this view, the present study, arranged in two experiments, was aimed to evaluate the agreement and repeatability of a recently developed topography-based NIBUT in comparison with two long-standing manual NIBUT procedures and fBUT, as well as the agreement between the NIBUT achieved by the automatic algorithm and a manual assessment.
Methods
First experiment: agreement and repeatability of different BUT measurement procedures
Participants
To evaluate the sample size needed for the study, a priori analysis was performed by the G*Power software (version 3.1.9.4) on preliminary NIBUT and fBUT data measured with the same instruments and procedures used in this study and achieved at the Research Centre (hereinafter referred to as Lab) where the experiment was carried out. Through distribution data (mean and SD) and correlation between them, an effect size of 0.40 was worked out. Considering the need to verify the difference between the means of two repeated test (NIBUT vs fBUT), the analysis type was set on matched pairs t-test (two-sided). Fixing an α error and 1-β (power) at 0.05 and 0.80 respectively, the resulting sample size was N = 41.
Thus, forty-three participants (age: 23.1 ± 2.1 years; range 18.1–29.3 years; sixteen males and twenty-seven females) were enrolled in the study on a voluntary basis. The inclusion criteria are reported in Table 1. Eventual dry eye symptoms were monitored by Ocular Surface Disease Index (OSDI) questionnaire (average score: 10.7 ± 10.0; range: 0.0–39.6).
All participants gave written informed consent, and all procedures were conformed to the Declaration of Helsinki and were approved by the Board of Optics and Optometry of the University of Milano-Bicocca (February, 11th, 2019).
Instruments
Three different devices were used to collect NIBUT data. Two devices, the EasyTear View + (Easytear, Rovereto, Italy) and the Polaris (CSO, Florence, Italy), have a similar structure with a cylindrical internal light source and a diffuser that allow to project diffuse cold light (white LED). The insertion of specific grids inside the internal cylinder light source of the instrument allows the projection of concentric rings onto the tear film (Fig. 1), thus the possibility to detect irregularities of the reflected image. Both instruments were mounted on a digital slit lamp (HR Elite, CSO, Florence, Italy) that allows video recording. The third device, the Sirius+ (CSO, Florence, Italy), is a Placido disc topographer integrated with a Scheimpflug tomographer (Fig. 1). The algorithm integrated in the dedicated software (Phoenix v.4.0, CSO, Florence, Italy) splits the Placido disc’s ring projection into a pre-set number of circular sectors (tiles) with the same area. For each sector, the algorithm keeps a trace of the changes (disruption of the projected ring) in each sector’s structure as time passes by. Only changes that persist until the end of the recording are considered as break-up, whereas a change that is restored to its original shape by the end of the recording is considered a false positive due to possible artifacts (e.g. small elements moving into the tear film layer). Disruptions of the projected ring that are visible since the beginning of the recording, such as eyelash shadow, are excluded from the processing. The algorithm can provide the first break-up regardless the sectors or the break-up map; the first break-ups are displayed topographically for each sector.
Procedure
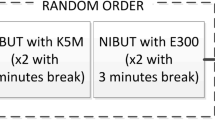
All measurements were performed in the same Lab following the procedure reported in Fig. 2. The same researcher performed all NIBUT measurements by employing the three devices in a randomised order, with an interval between the different procedures of minimum 10 min to wash out any potential tear film destabilization due to the previous measurements35,36. For each instrument, three NIBUT measurements were achieved in a row for each eye.
EasyTear View+ and Polaris measurements were video recorded with the digital slit lamp. As for the Sirius+, the standard length of NIBUT video recording by the software was extended to 50 s to allow the detection of long break-up times. After the NIBUT measurements, the same researcher performed a standard fBUT three times in a row for each eye. fBUT was carried out always at the end due to its invasiveness compared to the NIBUT measurements. The fBUT was performed by fluorescein sodium strips (I-DEW FLO, Endot, UK) used according to Pult & Riede-Pult procedure14, with slit lamp (HR Elite, CSO, Florence, Italy), blue cobalt and yellow filters. The fBUT was video recorded with the digital slit lamp. Subjects, as for the non-invasive devices, were asked to blink twice and then trying to avoid blinking as long as possible. The fBUT was video recorded with the digital slit lamp. Test–retest reliability was evaluated performing the same series of measurements (according to the order randomly selected for each specific subject) in the same day at least 2 h after the first set of measurements.
EasyTear View+ and Polaris NIBUTs, as well as fBUTs, were evaluated on the recorded videos by a researcher that was masked of the other instruments results. The researcher was instructed to play the videos with the task to detect the very first break-up and therefore was given the possibility to rewind the video to better identify the break-up. As far as concerns the Sirius+, only the first NIBUT (first disruption of the projected rings irrespectively to the sector) was obtained directly by the automatic algorithm analysis.
Data analysis
All the following data analyses were carried out for right and left eye separately37. All data sets did not result normally distributed (Shapiro–Wilk test; p < 0.005), thus non-parametric statistics were used. The agreement among the four BUT assessment procedures was investigated by Friedman’s test, then a matched comparison (Wilcoxon signed-rank test) was performed between each pair of measurements. Bonferroni adjustment was used to correct for multiple comparisons for post-hoc analyses. Spearman coefficient of correlation was calculated for each pair of measurements too.
Intra-observer repeatability was evaluated with the coefficients of precision (CP), repeatability (CR) and variation (CV). CP was calculated as 1.96 * sw (sw is the within-subjects standard deviation for repeated measures). CR was calculated as \(1.96*\sqrt{{S}_{w}^{2}*2}\) that is the value under which it would be the difference between two measurements in the 95% of probability38. CV was calculated as sw divided by the overall sample mean.
Test–retest reliability was evaluated for each procedure (mean of the three measures at test and mean of the three measures at retest) by Intraclass Correlation Coefficient (ICC) based on mean measurement, absolute agreement, two-way mixed effects model39. The 95% confidence interval was calculated. Reliability is considered slight, fair, moderate, substantial and excellent if ICC is comprised between 0.01 and 0.20, 0.21 and 0.40, 0.41 and 0.60, 0.61 and 0.80, and more than 0.80 respectively40. A comparison between test and retest was also performed by matched-pairs Wilcoxon test. The statistical analyses were performed with SPSS version 2.8 (IBM SPSS Statistics, USA).
Second experiment: agreement between manual and automatic NIBUT measured by Sirius+
Sample
The present part of the study did not require a direct enrollment of participants and no ethical issue; therefore, the effect size of the experiment was determined using a post hoc procedure by the G*Power software (G*Power; version 3.1.9.4) for a comparison between means of two distributions by Wilcoxon test. Through distribution data (mean and SD) of automatic and manual NIBUT (first and overall measures) and correlation between them, with a sample size of N = 85, the effect size was worked out. Fixing an α error at 0.05, the power effect (1-β) resulted of 0.97 and 0.60 for the difference between the mean of the automatic measure with the first manual NIBUTs (both observers) and the overall mean of all manual NIBUTs (both observers) respectively.
Thus, eighty-five videos of the NIBUT procedure previously performed with Sirius+ (CSO, Florence, Italy) were selected according to the following criteria:
-
No blinking during the length of the recoding
-
The first break-up, detected by automatic assessment, should occur before 17 s (limiting the study to length compatible with tear film instability in which information about the difference between manual and automatic assessment is more useful)
-
No areas grossly out of focus
-
No missing fixation (due to movements of the eye or head)
-
No gross irregularities of the tear film (e.g., mucus, air bubbles, etc.).
Procedure
A flow diagram of the study design is represented in Fig. 3. Two observers with different clinical experience were chosen to evaluate the videos and investigate a possible influence of the experience on the manual (subjective) assessment of NIBUT. Observer 1 was a researcher and an eye care practitioner with more than 20 years of clinical experience. Observer 2 was a recently graduated optometrist with less than one year of experience in clinical practice. The two observers assessed each single video (played in freeware software on the same laptop) in random order, measuring the NIBUT three times in a row (first session). Before proceeding with the evaluation of the videos, common instructions on what should be identified as 'break-up' were provided to both observers. They were required to play the video and stop it as soon as the first break-up (discontinuity or break in the image of the rings) appears; the break-up time was recorded, and the video was rewound from the beginning to perform the other two measures. Observers repeated the assessment after 15 days (second session). The 85 videos were provided in random order (different from the one used in the first session) and without any information about the measures determined during the first session.
The same 85 videos were analysed by the automatic algorithm, the two observers were masked of the instrument results.
Data analysis
All data (first break-up time) used to assess the agreement between manual and automatic NIBUT measured by Sirius+ did not result normally distributed (Shapiro–Wilk test; p < 0.005).
Comparison between automatic (first break-up time) and manual measurements was performed by Wilcoxon test and Spearman correlation on the first manual measurement (mean of the first measure at the test session by the two observers), on the overall manual measurement (mean of all manual measures in both sessions) and on the mean manual measurement separately for each observer. The same statistical tests were used to compare manual NIBUT of the two observers. Bonferroni adjustment was used to correct for multiple comparisons for post-hoc analyses. Spearman coefficient of correlation was calculated for each pair of measurements too. Friedman ANOVA for repeated measures was used to evaluated differences in the three NIBUT assessments performed by two observers in the two sessions.
Intra-operator repeatability was calculated for each of the two observers using the same coefficients previously described in data analysis of the first experiment.
Test–retest reliability (between the two sessions) was evaluated for each observer (mean of the three measures at test and mean of the three measures at retest) by ICC39, as aforementioned in data analyses of the first experiment, and by matched-pairs Wilcoxon test.
Results
First experiment: agreement and repeatability of different BUT measurement procedures
On the right eye, BUT (average of test and retest ± SD) resulted 12.0 ± 7.6, 12.8 ± 6.8, 14.8 ± 8.0, and 8.7 ± 5.2 s with the EasyTear View+, Polaris, Sirius+, and fluorescein-based procedure, respectively (Fig. 4a). On the left eye, BUT resulted 12.0 ± 8.2, 14.1 ± 9.8, 15.6 ± 7.8, and 8.6 ± 5.0 s with the EasyTear View+, Polaris, Sirius+, and fluorescein-based procedure, respectively (Fig. 4b). Friedmann’s analysis of variance showed a significant difference between the four measures in both eyes (p < 0.001). Post-hoc testing among the four procedures is reported in Table 2 along with correlations. All paired comparisons with fBUT showed significant difference for both eyes. Conversely, all paired comparisons between NIBUT procedures on the right eye were not significantly different, whereas on the left eye the comparisons between EasyTear View+, and the other two NIBUT procedures (Polaris and Sirius+) were significant, but the comparison between Polaris and Sirius+ was not. All correlations among procedures resulted significant (p < 0.001).
To investigate the relationship between invasive and non-invasive procedure, fBUT values were reported as a function of the three NIBUTs (Fig. 5).
Intra-observer repeatability for the four instruments, in the two sessions, was rather poor as it is possible to see from the high values of CP, CR, and CV reported in Table 3.
The results of test–retest are shown in Table 4 that reports the descriptive statistics of BUT, ICC, and p-values of paired comparison. ICC was substantial (between 0.61 and 0.80) for the EasyTear View+ measures on both eyes, for the Polaris in left eye, for the Sirius+ and the fBUT in the right eye. For the Sirius+ and the fBUT on the left eye the ICC was moderate and for the Polaris on the right eye was fair40. No test–retest difference was found for all procedures. Moreover, Bland–Altman plots of the test–retest measurements indicate a good agreement between the first and second measurement without any proportional bias (see Supplementary Figs. S1 and S2 online): all correlations (Spearman Rho) between the mean of test and retest and the difference retest-test were not significant.
Second experiment: agreement between manual and automatic NIBUT measured by Sirius+
The distribution of the automatic NIBUTs resulted (mean ± SD) 6.6 ± 3.6 s (range 1.2–16.9 s). The manual NIBUT was (mean ± SD) 7.7 ± 3.8 s (range 2.0–20.7 s) and 6.9 ± 3.5 s (range 2.0–18.1 s) for the first measurement (only first session) and the overall measurement, respectively. A statistically significant difference was found between the automatic NIBUT and both the first manual and the average manual measurement (Wilcoxon test; p < 0.001). Figure 6 shows the scatterplot between the automatic NIBUT and two manual NIBUTs (first and overall average). Pearson correlation coefficient calculated between automatic and manual NIBUTs resulted 0.89 (p < 0.001), and 0.90 (p < 0.001) for the first and the overall and the overall average manual measurements respectively.
NIBUT data achieved by the two observers in the two sessions are reported in Table 5 along with pair comparisons between the two observers for each measure, and pair comparisons between each manual NIBUT achieved by each observer and automatic NIBUT. All NIBUTs resulted significantly different between the two observers, but all were significantly correlated (all Pearsons correlations resulted higher than 0.85; p < 0.001). Friedman ANOVA for repeated measures showed a reduction in manual NIBUT in the 3 measurements in a row both for observer 1 (p = 0.03) and observer 2 (p < 0.001) in the first session, as well as in the second session (p < 0.001 for both Observers). All manual NIBUTs measured by observer 1 (except the second and third measures in the second session), resulted significantly longer than automatic NIBUT (between 0.3 and 1.6 s), whereas for observer 2 the difference was significant only for the first NIBUT in first session (longer time), the second, third NIBUT in the second session and the average NIBUT in the second session (shorter time). However, all manual NIBUTs achieved by the two observers and the automatic NIBUT resulted strongly correlated (all Spearman Rho higher than 0.83; p < 0.001).
Table 6 shows the statistical coefficients of intra-operator repeatability (among the three measures performed in a row in each session), separately for the two observers in the two sessions. Coefficients show good intra-operator repeatability in both observers.
Table 7 reports the descriptive statistics of manual NIBUTs achieved by the two observers and their average at test and retest, the ICC between test and retest measures, and p values of paired comparison between test and retest (Wilcoxon test). ICC was excellent (over than 0.80)40 for both observers. However, NIBUTs at retest resulted significantly shorter than test for both observers (p < 0.001). Finally, Bland–Altman plots of the test–retest measurements (see Supplementary Fig. 3S online) show a proportional bias for observer 1 (Spearman Rho = − 0.29; p = 0.008), indicating that the longer the NIBUT the shorter the retest compared to test. No proportional bias was found for the observer 2 (Spearman Rho = − 0.04; p = 0.74).
Discussion
Two different experiments were carried out to evaluate the NIBUT assessment of Sirius+, a recently developed Placido-based topographer integrated with a Scheimpflug tomographer. Even though its clinical application has been already reported in the literature41,42,43,44, no data about its level of agreement with other devices/procedures, and repeatability is available. To clarify the discussion of the results obtained in the two experiments, the outcomes have been divided into specific paragraphs.
Agreement between NIBUT procedures and fBUT
The first part of the study showed that NIBUT was longer than fBUT, independently from the device employed, and this result is in agreement with the literature12,15,45,46,47. However, elsewhere in literature automatic NIBUT was also found to be shorter than fBUT27,48. It has been proposed that the shorter fBUT might be induced by the instillation of fluorescein which would reduce the stability of the tear film15,19. When the amount of instilled fluorescein is reduced, the difference between NIBUT and fBUT decreases15. However, it has also been found that increasing the delivered volume of fluorescein solution by the glass rod technique (micropipette) lengthened fBUT47,49. In a recent paper, NIBUT measurements were carried out with Sirius+ without and with fluorescein that caused a prolongation in the NIBUT, labelled as “de-naturation” of the tear film50.
In the present study, a caveat of the difference between fBUT and NIBUTs might be the fact that the sequence of the measurements was not fully randomised: due to its invasiveness fBUT was carried out always at the end. Despite washout intervals, this practice may have contributed to decreased tear film stability, increasing the difference between fBUT and NIBUTs.
Furthermore, another source of shorter times with fBUT might be the different area covered by fBUT and NIBUT assessments. In many participants, the shadow of the lashes on the superior area of the Placido rings (Fig. 1) made the measurement impossible in this area for both the manual and the automated assessment of NIBUT procedures. Moreover, the Placido rings were reflected only in a reduced area of the cornea (Fig. 1). This made the area covered by the fBUT procedure larger than the NIBUT procedure, then with the fBUT procedure, it was possible to detect breaks in zones not covered by NIBUT procedures.
Agreement between NIBUT procedures
Looking at the NIBUT procedures, the first thing to highlight is that the subjective assessment of NIBUT of the EasyTear View+ and the Polaris are extremely close to the findings of Bandlitz et al.30 (12.2 ± 6.6 s and 12.0 ± 6.4 s, respectively), who collected data with the same paradigm (two sessions in the same day) on individuals with very similar age (24.2 ± 3.6 years vs 23.1 ± 2.1 years in this work). The present study showed no difference between Polaris and Sirius+ in both eyes and between EasyTear View+ and Sirius+ in the right eye. However, few comparisons displayed a statistical difference (see Table 2). This result is not clear to interpret. Considering that the three NIBUT procedures are non-invasive and based on a “concentric ring grid”, the results might be expected to be similar, as reported for four NIBUT devices (EasyTear View+ , Keratograph 5 M, Polaris, and Tearscope Plus)30. However, other studies evidenced a poor agreement between different NIBUT procedures51,52. Furthermore, NIBUT values in healthy population, measured by grids or Placido discs, have shown extreme variability, ranged between 10 and 50 s19,22,23. Therefore, it should be considered that many factors could induce variability, such as different age and ethnicity of the subjects assessed, the various sizes, brightness and coverage (e.g., due to corneal curvature) of Placido discs21, and the fact that for some instruments it is still requested a manual (subjective) judgment46. Earlier studies comparing automatic and manual NIBUTs consistently found differences28,31, but variations in instrument features rather than the detection method (automatic vs manual) may have contributed to these differences. For example, the comparison between automated software to achieve a NIBUT by a topographer (Keratograph) and a manual NIBUT performed by Keeler Tearscope showed a shorter time with the former28. Also Markulli et al. found that NIBUT of healthy people was significantly greater with the Tearscope-Plus (15.9 ± 10.7 s) than NIBUT achieved with Oculus Keratograph 5 M (8.2 ± 3.5 s)31. These results might be because these releases of the software were extremely sensitive to minimal changes in the projected rings (deformation). As for the difference between the two eyes (no difference in right eye among the 3 procedures, and differences limited to EasyTear View+ and the two other NIBUTs in left eye) the only difference in the procedure that might have caused a bias is the missing randomised order between the two eyes (right always first; see Fig. 2). However, this is a simple association with no clear meaningful cause-effect reason.
NIBUT and fBUT reliability
The first experiment provided information also about the intra-observer repeatability of the measurements. All the procedures showed high values of CP, CR, and CV in both eyes and the test and the retest session (Table 3). fBUT values resulted slightly lower, especially at the retest. The intrasession repeatability of another topography-based NIBUT (VX120 + system) was recently investigated53, revealing a within-subject standard deviation (Sw) of three consecutive measurements of 0.86. This value is notably smaller (from 3.5 to 8 times), than the Sw values derived from the coefficients of Table 3 for the four procedures investigated in the present study. However, it is possible that the low Sw value in Molina-Martìn et al.’s study53 might be biased by the extremely narrow range of NIBUT in the sample, which is anomalous for healthy subjects. The repeatability coefficients achieved in the present study values might indicate poor reliability of the four procedures, but they could also be linked to the intrinsic very high variability of the phenomenon.
This second hypothesis seems to find a confirmation looking at the values of the repeatability in the second experiment, where the NIBUT is performed on the same video (so the variability of the tear film is nonexistent, and only the variability of the measurement by the observer remains). Finally, the test–retest reliability appeared good for Sirius+ and also for the other two NIBUT procedures (Table 4) in agreement to the results of Bandlitz et al.30.
Agreement between manual and automatic NIBUT performed by Sirius+
Despite the strong correlation between automatic and manual NIBUTs (first and overall average), automatic assessment always provided shorter values than manual measures (of about 1.1 and 0.3 s for the first manual assessment and the average of all assessments, respectively). The difference of about 1 s between the two comparisons depends on the shortening of the manual NIBUT with the repeated measures. This reduction is likely due to the observer’s awareness of the point where the break-up occurs, which affects the following measures. In the first manual measurement, the observer assessed the keratoscopic disc’s projection without any information on the break-up location. Nonetheless, in the subsequent measurements, the observer was allowed to scan more specifically the area where the break-up was previously spotted, then potentially shortening the time. This outcome is something to consider because in clinical practice also the manual NIBUT is quickly transforming for the rapid diffusion of systems that can make a video of the projection of a grid (systems that can be mounted on a video slit lamp such as the Polaris or the EasyTear View+, etc.), allowing the observer to subjectively evaluate offline the recording several times.
However, it can be highlighted that the differences of 1.1 and 0.3 s are clinically negligible (the statistical significance was certainly linked to the big sample studied). Unfortunately, to the best of our knowledge, there is no previous research that compared automatic vs manual assessment of NIBUT in the same event (video of the same projection). More often, comparisons between automatic and manual assessment have been carried out on the same subjects, but in different moments and using different instruments28,31. Therefore, it is not possible to compare the results of the present study to other studies.
The second experiment showed also a difference between the two observers. One possible explanation might be the different clinical experience of the two observers. The more experienced observer produced longer measures. This might depend on a more prudent judgment that brings the more experienced observer to get the time only when a break-up happened (therefore a discontinuation and no distortion of rings) or a better sensitivity to the break-up of the less experienced observer.
In terms of reliability, the coefficients (Table 7) are very good for both observers. It is interesting to notice that in this case the coefficients are much better (lower values) than the one achieved for manual assessment in the first experiment (Table 4). As already reported in the discussion, this seems to demonstrate that the poor reliability achieved in the NIBUT procedures performed in the first experiment is likely due to the intrinsic variability of the tear film stability. When the phenomenon is the same (second experiment) the intra-observer variability is almost negligible. Also, the test–retest reliability for both the observers is extremely good confirming that the manual assessment of NIBUT is reliable.
The two experiments of the study present some limitations. In the first experiment, the participants were mainly university students, therefore the age was young with a narrow SD. Moreover, the participants were healthy subjects, not selected for DED, although the NIBUT range as well as the variability of symptoms (OSDI score range varied between 0 and 39.6) resulted quite wide.
Conclusions
The effort to engineer NIBUT devices able to perform an automated measure of tear film stability has made available many different commercialised instruments. However, every “new entry” such as the one investigated in this study, is not interchangeable with other NIBUT devices as well as fluorescein-based procedures. Likely, the difficulty in finding a good level of agreement and repeatability among the several NIBUT devices might be represented by the tear film itself which is a complex system, difficult to model and measure. However, the automated algorithm that measures the NIBUT in the device here studied provided clinically negligible differences from the manual measures achieved on the same video of Placido disc projection.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Willcox, M. D. P. et al. TFOS DEWS II tear film report. Ocul. Surf. 15, 366–403. https://doi.org/10.1016/j.jtos.2017.03.006 (2017).
King-Smith, P. E. et al. The thickness of the human precorneal tear film: Evidence from reflection spectra. Investig. Ophthalmol. Vis. Sci. 41, 3348–3359 (2000).
Wolffsohn, J. S. et al. TFOS DEWS II diagnostic methodology report. Ocul. Surf. 15, 539–574. https://doi.org/10.1016/j.jtos.2017.05.001 (2017).
Sweeney, D. F., Millar, T. J. & Raju, S. R. Tear film stability: A review. Exp. Eye Res. 117, 28–38. https://doi.org/10.1016/j.exer.2013.08.010 (2013).
Kojima, T. et al. A new noninvasive tear stability analysis system for the assessment of dry eyes. Investig. Ophthalmol. Vis. Sci. 45, 1369–1374. https://doi.org/10.1167/iovs.03-0712 (2004).
Norn, M. S. Desiccation, of the precorneal film: I. Corneal wetting-time. Acta Ophthalmol. 47, 865–880. https://doi.org/10.1111/j.1755-3768.1969.tb03711.x (1969).
Downie, L. E., Keller, P. R. & Vingrys, A. J. An evidence-based analysis of Australian optometrists’ dry eye practices. Optom. Vis. Sci. 90, 1385–1395. https://doi.org/10.1097/OPX.0000000000000087 (2013).
Turner, A. W., Layton, C. J. & Bron, A. J. Survey of eye practitioners’ attitudes towards diagnostic tests and therapies for dry eye disease. Clin. Exp. Ophthalmol. 33, 351–355. https://doi.org/10.1111/j.1442-9071.2005.01026.x (2005).
Smith, J., Nichols, K. K., Baldwin, E. K. & Kimelblatt, S. Current patterns in the use of diagnostic tests in dry eye evaluation. Cornea 27, 656–662. https://doi.org/10.1097/QAI.0b013e3181605b95 (2008).
Vanley, G. T., Leopold, I. H. & Gregg, T. H. Interpretation of tear film breakup. Arch. Ophthalmol. 95, 445–448. https://doi.org/10.1001/archopht.1977.04450030087010 (1977).
Lee, J. H. & Kee, C. W. The significance of tear film break-up time in the diagnosis of dry eye syndrome. Korean J. Ophthalmol. 2, 69–71. https://doi.org/10.3341/kjo.1988.2.2.69 (1988).
Mengher, L. S., Bron, A. J., Tonge, S. R. & Gilbert, D. J. Effect of fluorescein instillation on the pre-corneal tear film stability. Curr. Eye Res. 4, 9–12. https://doi.org/10.3109/02713688508999961 (1985).
Korb, D. R., Greiner, J. V. & Herman, J. Comparison of fluorescein break-up time measurement reproducibility using standard fluorescein strips versus the dry eye test (DET) Method. Cornea 20, 811–815. https://doi.org/10.1097/00003226-200111000-00007 (2001).
Pult, H. & Riede-Pult, B. H. A new modified fluorescein strip: Its repeatability and usefulness in tear film break-up time analysis. Contact Lens Anterior Eye 35, 35–38. https://doi.org/10.1016/j.clae.2011.07.005 (2012).
Mooi, J. K., Wang, M. T. M., Lim, J., Müller, A. & Craig, J. P. Minimising instilled volume reduces the impact of fluorescein on clinical measurements of tear film stability. Contact Lens Anterior Eye 40, 170–174. https://doi.org/10.1016/j.clae.2017.01.004 (2017).
Cho, P. Stability of the precorneal tear film: A review. Clin. Exp. Optom. 74, 19–25. https://doi.org/10.1111/j.1444-0938.1991.tb04603.x (1991).
Nichols, K. K., Mitchell, G. L. & Zadnik, K. The repeatability of clinical measurements of dry eye. Cornea 23, 272–285. https://doi.org/10.1097/00003226-200404000-00010 (2004).
Han, S. B., Liu, Y. C., Mohamed-Noriega, K., Tong, L. & Mehta, J. S. Objective imaging diagnostics for dry eye disease. J. Ophthalmol. 2020, 1–10. https://doi.org/10.1155/2020/3509064 (2020).
Mengher, L. S., Bron, A. J., Tonge, S. R. & Gilbert, D. J. A non-invasive instrument for clinical assessment of the pre-corneal tear film stability. Curr. Eye Res. 4, 1–7. https://doi.org/10.3109/02713688508999960 (1985).
Patel, S., Murray, D., McKenzie, A., Shearer, D. S. & McGrath, B. D. Effects of fluorescein on tear breakup time and on tear thinning time. Optom. Vis. Sci. 62, 188–190. https://doi.org/10.1097/00006324-198503000-00006 (1985).
Craig, J. P., Blades, K. & Patel, S. Tear lipid layer structure and stability following expression of the meibomian glands. Ophthalmic Physiol. Opt. 15, 569–574. https://doi.org/10.1016/0275-5408(95)00071-K (1995).
Mohidin, N., Bay, T. C. & Yap, M. Non-invasive tear break-up time in normal Malays. Clin. Exp. Optom. 85, 37–41. https://doi.org/10.1111/j.1444-0938.2002.tb03070.x (2002).
Guillon, M., Styles, E., Guillon, J. P. & Maïssa, M. C. Preocular tear film characteristics of nonwearers and soft contact lens wearers. Optom. Vis. Sci. 74, 273–279. https://doi.org/10.1097/00006324-199705000-00022 (1997).
Goto, T., Zheng, X., Okamoto, S. & Ohashi, Y. Tear film stability analysis system: Introducing a new application for videokeratography. Cornea 23, S56–S70. https://doi.org/10.1097/01.ico.0000136685.88489.70 (2004).
Goto, T. et al. A new method for tear film stability analysis using videokeratography. Am. J. Ophthalmol. 135, 607–612. https://doi.org/10.1016/S0002-9394(02)02221-3 (2003).
Gumus, K. et al. Noninvasive assessment of tear stability with the tear stability analysis system in tear dysfunction patients. Investig. Ophthalmol. Vis. Sci. 52, 456–461. https://doi.org/10.1167/iovs.10-5292 (2011).
Hong, J. et al. Assessment of tear film stability in dry eye with a newly developed keratograph. Cornea 32, 716–721. https://doi.org/10.1097/ICO.0b013e3182714425 (2013).
Best, N., Drury, L. & Wolffsohn, J. S. Clinical evaluation of the Oculus Keratograph. Contact Lens Anterior Eye 35, 171–174. https://doi.org/10.1016/j.clae.2012.04.002 (2012).
García-Marqués, J. V., Martínez-Albert, N., Talens-Estarelles, C., García-Lázaro, S. & Cerviño, A. Repeatability of non-invasive keratograph break-up time measurements obtained using oculus keratograph 5M. Int. Ophthalmol. 41, 2473–2483. https://doi.org/10.1007/s10792-021-01802-4 (2021).
Bandlitz, S. et al. Agreement and repeatability of four different devices to measure non-invasive tear breakup time (NIBUT). Contact Lens Anterior Eye 43, 507–511. https://doi.org/10.1016/j.clae.2020.02.018 (2020).
Markoulli, M., Duong, T. B., Lin, M. & Papas, E. Imaging the tear film: A comparison between the subjective keeler tearscope-plusTM and the objective oculus® keratograph 5M and LipiView® interferometer. Curr. Eye Res. 43, 155–162. https://doi.org/10.1080/02713683.2017.1393092 (2018).
Tian, L., Qu, J. H., Zhang, X. Y. & Sun, X. G. Repeatability and reproducibility of noninvasive keratograph 5M measurements in patients with dry eye disease. J. Ophthalmol. https://doi.org/10.1155/2016/8013621 (2016).
Wilkins, A., Sihra, N. & Smith, I. N. How precise do precision tints have to be and how many are necessary?. Ophthalmic Physiol. Opt. 25, 269–276. https://doi.org/10.1111/j.1475-1313.2005.00279.x (2005).
Brahim, I., Lamard, M., Benyoussef, A. A. & Quellec, G. Automation of dry eye disease quantitative assessment: A review. Clin. Exp. Ophthalmol. 50, 653–666. https://doi.org/10.1111/ceo.14119 (2022).
Wang, M. T. M., Murphy, P. J., Blades, K. J. & Craig, J. P. Comparison of non-invasive tear film stability measurement techniques. Clin. Exp. Optom. 101, 13–17. https://doi.org/10.1111/cxo.12546 (2018).
Fernández, J. et al. Agreement and repeatability of objective systems for assessment of the tear film. Graefe’s Arch. Clin. Exp. Ophthalmol. 256, 1535–1541. https://doi.org/10.1007/s00417-018-3986-9 (2018).
Armstrong, R. A. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol. Opt. 33, 7–14. https://doi.org/10.1111/opo.12009 (2013).
Bland, J. M. Statistica Medica (Apogeo Editore, 2009).
Koo, T. K. & Li, M. Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155–163. https://doi.org/10.1016/j.jcm.2016.02.012 (2016).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 33, 159–174. https://doi.org/10.2307/2529310 (1977).
Fattoh, R., Mounir, A., Anbar, M. & Mohammed, O. A. Changes in noncontact meibography and noninvasive tear break-up time test with contact lenses usage. Clin. Ophthalmol. 16, 1427–1437. https://doi.org/10.2147/OPTH.S362307 (2022).
Ozulken, K., Aksoy Aydemir, G., Tekin, K. & Mumcuoğlu, T. Correlation of non-invasive tear break-up time with tear osmolarity and other invasive tear function tests. Semin. Ophthalmol. https://doi.org/10.1080/08820538.2020.1730916 (2020).
Tavazzi, S. et al. Effects of aqueous-supplementing artificial tears in wearers of biweekly replacement contact lenses vs wearers of daily disposable contact lenses. Clin. Optom. 12, 75–84. https://doi.org/10.2147/OPTO.S249078 (2020).
Penbe, A. et al. The effects of facial mask use on ocular surface parameters and tear film cytokine profile in prolonged use. Int. Ophthalmol. https://doi.org/10.1007/s10792-023-02661-x (2023).
Cho, P. & Douthwaite, W. The relation between invasive and noninvasive tear break-up time. Optom. Vis. Sci. 72, 17–22. https://doi.org/10.1097/00006324-199501000-00004 (1995).
Nichols, J. J., Nichols, K. K., Puent, B., Saracino, M. & Mitchell, G. L. Evaluation of tear film interference patterns and measures of tear break-up time. Optom. Vis. Sci. 79, 363–369. https://doi.org/10.1097/00006324-200206000-00009 (2002).
Lan, W., Lin, L., Yang, X. & Yu, M. Automatic noninvasive tear breakup time (TBUT) and conventional fluorescent TBUT. Optom. Vis. Sci. 91, 1412–1418. https://doi.org/10.1097/OPX.0000000000000418 (2014).
Bhandari, V., Reddy, J. K., Relekar, K., Ingawale, A. & Shah, N. Non-invasive assessment of tear film stability with a novel corneal topographer in Indian subjects. Int. Ophthalmol. 36, 781–790. https://doi.org/10.1007/s10792-016-0186-7 (2016).
Johnson, M. E. & Murphy, P. J. The effect of instilled fluorescein solution volume on the values and repeatability of TBUT measurements. Cornea 24, 811–817. https://doi.org/10.1097/01.ico.0000154378.67495.40 (2005).
Acet, Y. & Dağ, Y. Changes caused by fluorescein in the tear film evaluated with hybrid break-up time test as a new method—Part Two: Its effect on breakup locations and other quantitative values. Photodiagnosis Photodyn. Ther. 43, 103137. https://doi.org/10.1016/j.pdpdt.2023.103651 (2023).
Lee, R., Yeo, S., Aung, H. T. & Tong, L. Agreement of noninvasive tear break-up time measurement between tomey RT-7000 auto refractor-keratometer and oculus keratograph 5M. Clin. Ophthalmol. 10, 1785–1790. https://doi.org/10.2147/OPTH.S110180 (2016).
Martínez-Plaza, E., Molina-Martín, A. & Piñero, D. P. Agreement of tear break-up time and meniscus height between medmont E300 and visionix VX120+. Appl. Sci. 12, 4589. https://doi.org/10.3390/app12094589 (2022).
Molina-Martín, A., de Fez, D. & Piñero, D. P. Repeatability of non-invasive break-up time measures with a new automated dry eye platform in healthy eyes. Int. Ophthalmol. 40, 2855–2864. https://doi.org/10.1007/s10792-020-01470-w (2020).
Acknowledgements
Aurora Spagnolli, Luca Schroder, and Federica Miglio for their help in data collection. CSO for providing the instruments and the technical support for the study and for funding the open-access publication.
Author information
Authors and Affiliations
Contributions
F.Z., and S.T. conceived the study. F.Z., G.C.R. conducted the experiment. F.Z., G.C.R., E.P. and S.T. analysed the results. All authors wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zeri, F., Rizzo, G.C., Ponzini, E. et al. Comparing automated and manual assessments of tear break-up time using different non-invasive devices and a fluorescein procedure. Sci Rep 14, 2516 (2024). https://doi.org/10.1038/s41598-024-52686-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52686-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.