Abstract
In this work, the electrochemical generation of phenothiazin-5-ium (PTZox) from the direct oxidation of phenothiazine (PTZ) in a water/acetonitrile mixture using a commercial carbon anode and conventional stainless steel cathode is reported. PTZox is a reactive intermediate with high potential synthetic applications, which is used in this paper for the synthesis of new phenothiazine derivatives. In this work a novel and simple electrochemical methodology for the synthesis of some bis(phenylsulfonyl)-10H-phenothiazine derivatives was established. In this paper, a mechanism for PTZ oxidation in the presence of arylsulfinic acids has been proposed based on the results obtained from voltammetric and coulometric experiments as well as spectroscopic data of the products. These syntheses are performed in a simple cell by applying constant current under mild conditions and at room temperature with high atom economy.
Similar content being viewed by others
Introduction
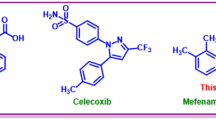
Electrochemistry is a powerful tool to identify the mechanism of reactions as well as the synthesis of organic and inorganic compounds1,2,3. In this research, we deal with three categories of compounds with valuable medicinal properties. The first category involves phenothiazines. Phenothiazine has various biological activities, such as neuroleptic, antiemetic and anti-histaminic activities, which have made it interesting to scientists4. Phenothiazine derivatives are also used as anti-cancer, antipyretic, anticonvulsant, analgesic, anti-fungal, anti-bacterial, anti-malarial, antiinflammatory, and immunosuppressive agents4,5,6. Prominent examples of widely used phenothiazine derivatives include trifluorophenazine, chlorpromazine, thioridazine, fluphenazine and perphenazine (Fig. 1, top row). From the electrochemical point of view, phenothiazine is a useful compound in photovoltaic7 and electrochemical applications8,9,10,11. Also due to their interesting chemical and physical properties, phenothiazines are an important and widely used building block for a wide range of applications in different fields of chemistry12. For example, these compounds are widely used in the optoelectronic industry due to the presence of electron-rich nitrogen and sulfur atoms in their molecules13. Also, since phenothiazines have a reversible redox process with low oxidation potential, they are widely used in perovskite solar cells14.
The second category of compounds are sulfones. Sulfone drugs are useful to treat many diseases, like anti-inflammatory15, antimicrobial16, anti-cancer17, and anti-malaria18. Diarylsulfones are useful in biological fields and pharmaceutical aims as antifungal, antimicrobial, anticancer, antibacterial and HIV treatment agents and also can be used to prepare other drugs19,20. Prominent examples of widely used sulfone and diarylsulfone drugs are shown in Fig. 1, middle row. The third category of compounds are sulfonamides. Sulfonamides are the antibiotics that are applied in to treat bacterial infections21. In addition, they are used as anti-cancer, anti-inflammatory and antiviral drugs22,23,24,25,26. Additionally, type II diabetes treatment activity is reported from some of these organic drugs27. Moreover various type of diseases such as coronary artery disease, asthma28, Alzheimer's29 and respiratory diseases30 have been treated by sulfonamide drugs which are called sulfa drugs. Examples of the most commonly used sulfonamides are shown in Fig. 1, bottom row.
The medicinal properties of these three groups of compounds prompted us to prepare new molecules containing three moieties of phenothiazine, sulfone, and sulfonamide. The presence of these three moieties in one molecule can have high potential in variable medicinal and biological properties, and maybe the synergistic effect of these groups will intensify the medicinal properties and/or reduce the side effects of the synthesized molecule. To achieve this idea, the electrochemical oxidation of phenothiazine in the presence of some arylsulfinic acids has been carried out in an undivided cell using a carbon electrode. This work presents a sustainable and efficient one-step strategy with high atom economy under ambient conditions without using any catalyst for the synthesis of novel compounds with medicinal potential.
Experimental data
Apparatus and reagents
Voltammetric and controlled potential coulometric experiments were carried out using an Autolab model PGSTAT20 potentiostat/galvanostat (Metrohm-Autolab, Netherland) equipped with a working electrode (glassy carbon disc, 1.8 mm diameter), a counter electrode (platinum wire) and a reference electrode (Ag/AgCl) (3M KCl). All electrodes are prepared from Azar Electrode Company (Urmia, Iran). The alumina slurry from Iran Alumina Co. (0.1–3.0 μm) was used to polish the glassy carbon electrode. The working electrode (anode) used in macroscale electrolysis consists of a set of four soft carbon rods (diameter 8 mm and length 6 cm) placed at a distance of 3 cm from each other on the edges of a square. The counter electrode (cathode) consists of a stainless steel porous cylinder (area 25 cm2) placed in the center of the square (Fig. 2). The electrolysis was carried out using a DC power supply model Dazheng ps-303D in an undivided cell equipped with a magnetic stirrer at room temperature. Controlled potential coulometry was performed in the same cell with an Ag/AgCl (3M KCl) reference electrode using a BEHPAJOOH-C2056 potentiostat.
Perkin-Elmer 1760 X device, German Bruker spectrometer (model: Avance 300 MHz), Agilent-5973C mass spectrometer and Barnstead Electrothermal 9100 instrument were used to record FTIR, NMR, MS spectra, and melting point, respectively. The NMR chemical shifts were related to the residual solvent signal. Phenotiazine (PTZ) and aryl sulfinic acid sodium salt, benzenesulfinic acid (BSA), 4-toluenesulfinic acid (TSA) and 4-chlorobenzenesulfinic acid (CSA) sodium salts) were obtained from Sigma-Aldrich and used without further purification. The buffer solution with pH value of 2.0 was prepared by 0.2 M phosphoric acid (pro-analysis grade from E. Merck). The pH values were adjusted by sodium hydroxide.
General procedure for synthesis of bis(phenylsulfonyl)-10H-phenothiazine derivatives (2a–2e)
The electroorganic synthesis of bis(phenylsulfonyl)-10H-phenothiazine derivatives (2a–2e) has been carried out under controlled potential as well as constant current conditions at room temperature. In controlled potential electrolysis PTZ (0.25 mmol) and arylsulfinic acid (0.5 mmol) were electrolyzed at 0.55 V versus Ag/AgCl (3M KCl) in the mixture of water (phosphate buffer, pH 2.0, c = 0.2 M)/acetonitrile (50/50, v/v) (80 ml). The progress of the electrolysis was monitored by periodically recording the decrease of the oxidation peak current in cyclic voltammetry and also by using TLC on silica gel (ethyl acetate/n-hexane: 40/60). The samples on the TLC were visualized with a UV lamp (254 nm). To activate the electrode surface, the electrolysis is stopped for a while and the carbon anodes are washed with acetone. Electrolysis was terminated when the oxidation peak current reached 5% of the initial value. In this condition, the amount of electricity consumed is equal to 100 C. At the end of the electrolysis, the contents were placed at room temperature to reduce the volume of the solution (evaporation) to half and lead to the precipitation of the products. The crude precipitate was filtered and washed several times with water. After drying the product was purified by thin layer chromatography on silica gel GF250-60 with ethyl acetate/n-hexane (40/60 V/V). Product yield is calculated by weighing the pure product.
Electrolysis under constant current conditions has also been performed by applying a current density of 1.25 mA/cm2 (30 mA) for 78 min (consumption of 140 C electricity) for oxidation of PTZ in the presence of BSA as well as other nucleophiles (TSA and CSA) under similar conditions as reported for electrolysis in controlled potential method.
Characteristics of the products

3,10-bis(phenylsulfonyl)-10H-phenothiazine (C24H19NO4S3) (2a)
Yellow powder (47.5 mg), isolated yield: 39%. Mp: 153–156 °C: 1H NMR, δ ppm (300 MHz, DMSO-d6), 6.2 (s, 1H, aromatic), 6.55 (d, J = 3.9 Hz, 2H, aromatic), 6.67–7.06 (m, 4H, aromatic), 7.49 (m, 7H, aromatic), 7.90 (d, J = 4.8 Hz, 3H, aromatic). 13C NMR, δ ppm (75 MHz, DMSO-d6): 114.7, 115.9, 118.0, 123.6, 127.4, 127.6, 128.8, 129.7, 130.0, 130.2, 132.8, 133.7, 134.0, 134.4, 140.3, 142.4, 147.0. IR (KBr) (cm−1): 2958, 2921, 2851, 1731, 1603, 1563, 1470, 1446, 1384, 1320, 1153, 1111, 1075, 722. MS (m/z) (EI, 70 EV) (relative intensity): 77 (30), 154 (40), 198 (90), 339 (100), 396 (20), 479 (M, 30).

3,7-bis(phenylsulfonyl)-10H-phenothiazine (C24H19NO4S3) (2b)
Cream powder (60.0 mg), isolated yield: 50%. Mp: 120–123 °C. 1H NMR, δ ppm (300 MHz, DMSO-d6), 6.76 (d, J = 8.4 Hz, 2H, aromatic), 7.46 (d, J = 5.3 Hz, 2H, aromatic), 7.53 (d, J = 3.5 Hz, 1H, aromatic), 7.55 (d, J = 2.1 Hz, 1H, aromatic), 7.61 (d, J = 9.2 Hz, 3H, aromatic),7.66 (d, J = 6.7 Hz, 2H, aromatic), 9.72 (s, 1H, NH). 13C NMR, δ ppm (75 MHz, DMSO-d6): 115.5, 117.7, 125.8, 127.5, 128.7, 130.1, 133.9, 135.0, 142.1, 145.0. UVmax (EtOH): 274 nm31. IR (KBr) (cm−1): 3583, 3494, 3095, 2921, 1680, 1654, 1606, 1563, 1509, 1419, 1317, 1229, 1142, 745, 687.31 MS (m/z) (EI, 70 EV) (relative intensity): 77 (80), 107 (60), 291 (30), 338 (50), 396 (20), 479 (M, 100).

3,10-ditosyl-10H-phenothiazine (C26H21NO4S3) (2c)
Yellow powder (42.5 mg), isolated yield: 34%. Mp: 160–162 °C. 1H NMR, δ ppm (300 MHz, DMSO-d6), 2.37 (s, 6H, CH3), 6.68–7.04 (m, 4H, aromatic), 7.39 (d, J = 9.0 Hz, 9H, aromatic), 7.50 (dd, J = 12.2 Hz, 2H, aromatic), 7.80 (d, J = 8.1 Hz, 3H, aromatic), 9.2 (s, 1H, aromatic). 13C NMR, δ ppm (75 MHz, DMSO-d6): 21.5, 114.6, 115.5, 115.9, 117.6, 117.9, 123.6, 125.6, 127.4, 128.4, 130.6, 133.8, 135.3, 139.2, 140.3, 144.5, 144.9, 146.8. IR (KBr) (cm−1): 3053, 2958, 2921, 2851, 1703, 1630, 1600, 1534, 1499, 1438, 1347, 1297,1260, 1113, 1019, 886, 770, 717. MS (m/z) (EI, 70 EV) (relative intensity): 55 (25), 91 (30), 154(30), 198 (60), 353 (100), 507 (M, 15).

3,7-ditosyl-10H-phenothiazine(C26H21NO4S3) (2d)
Cream powder (57.5 mg), isolated yield: 47%. Mp: 130–132 °C. 1H NMR, δ ppm (300 MHz, DMSO-d6), 2.37 (s, 6H, CH3), 6.67 (d, J = 6.9 Hz, 1H, aromatic), 6.72 (d, J = 8.4 Hz, 1H, aromatic), 6.82 (dd, J = 7.5 Hz, 1H, aromatic), 6.92 (d, J = 7.8 Hz, 1H, aromatic), 6.99 (d, J = 6.3 Hz, 1H, aromatic), 7.04 (d, J = 7.8 Hz, 1H, aromatic), 7.39 (m, J = 4.2 Hz, 4H, aromatic), 7.79 (dd, J = 10.8 Hz, 1H, aromatic), 7.78 (d, J = 8.1 Hz, 3H, aromatic), 9.23 (s, 1H, NH). 13C NMR, δ ppm (75 MHz, DMSO): 21.5, 114.6, 115.5, 115.8, 117.9, 123.6, 125.5, 126.8, 127.5, 128.2, 128.5, 130.5, 133.8, 139.6, 140.3, 144.3, 146.8. IR (KBr) (cm−1): 3403, 3334, 3093, 2922, 2852, 1569, 1474, 1434, 1326, 1157, 1114, 1086, 828, 745, 616, 575. MS (m/z) (EI, 70 EV) (relative intensity): 65 (15), 91 (30), 154 (40), 198 (80), 353 (100), 507 (M, 10).

3,7-bis((4-chlorophenyl) sulfonyl)-10H-phenothiazine (C24H15Cl2NO4S3) (2e)
Cream powder (67.5 mg), isolated yield: 50%. Mp: 145–148 °C. 1H NMR, (300 MHz, DMSO-d6), 6.48 (d, J = 7.8 Hz, 1H, aromatic), 6.54 (t, J = 8.4 Hz, 2H, aromatic), 6.68 (d, J = 6.3 Hz, 1H, aromatic), 6.77 (t, J = 7.8 Hz, 1H, aromatic), 7.18 (d, J = 2.1 Hz, 1H, aromatic), 7.29 (dd, J = 10.2 Hz, 2H, aromatic), 7. 43(d, J = 8.4 Hz, 3H, aromatic), 7.70 (d, J = 8.7 Hz, 2H, aromatic), 9.36 (s, 1H, NH). 13C NMR, δ ppm (75 MHz, DMSO): 114.7, 115.7, 115.8, 118.0, 123.6, 125.8, 126.7, 129.3, 130.2, 132.7, 138.7, 140.3, 141.4. IR (KBr) (cm−1): 3438, 3028, 2918, 2850, 2709, 1578, 1566, 1457, 1223, 1155, 1121, 1009, 815, 682. MS (m/z) (EI, 70 EV) (relative intensity): 51 (35), 77 (45), 158 (40), 266 (50), 407 (100), 547 (M, 10).
Results and discussion
Electrochemical study of PTZ in the presence of arylsulfinic acids
The cyclic voltammogram of PTZ (1.0 mM) in a solution of phosphate buffer 0.2 M, pH 2.0)/acetonitrile (50/50 v/v) in scan rate of 100 mV/s is shown in Fig. 3, part I, curve a. It reveals a quasi-reversible two-electron process involving oxidation of PTZ to phenothiazine-5-ime (PTZox) (peak A1 at 0.55 V vs. Ag/AgCl) and reduction of PTZox to PTZ (peak C1 at 0.45 V vs. Ag/AgCl)10. The peak current ratio (IPC1/IPA1) close to one which illustrates no side reaction is accrued in the time scale of voltammetry10. Figure 3, part I, curve b is the cyclic voltammogram of PTZ in the presence of 1.0 mM benzenesulfinic acid (BSA). Compared to cyclic voltammogram a, the following changes have occurred in the cyclic voltammogram of b. The first change is the removal of the cathode peak C1, which confirms the reaction between PTZox and BSA. The second change is the appearance of an ill-defined irreversible peak A2 and a new reversible redox peak (A3 and C3) at more positive potentials. The peak A2 is related to the oxidation of the adduct formed from the reaction of PTZox with BSA (PTZ-BSA) (INT1) to INT1ox (Fig. 3, part I, curve b). The positive shift of peak A2 compared to peak A1 can be justified based on the electron withdrawing property of BSA bound to PTZ. After the electrochemical generation of INT1ox, another rapid chemical reaction occurs and a BSA binds to INT1ox. This chemical reaction causes the cathodic peak corresponding to the reduction of INT1ox (C2) to be eliminated. Finally, the anodic and cathodic peaks A3 and C3 are related to the oxidation and reduction of final products (2b, 2d, 2e), respectively. The occurrence of oxidation of the final products at more positive potentials than the potential of peak A2 confirms the presence of two BSA groups in the structure of the final products. The third change is the shift of EpA1 towards less positive potentials. This is another confirmation of the reaction between electrochemically generated PTZox and BSA32.
Part I: (a) Cyclic voltammogram of PTZ (1.0 mM). (b) Cyclic voltammogram of PTZ (1.0 mM) in the presence of 1.0 mM BSA. (c) Cyclic voltammogram of BSA (1.0 mM). Potential scan rate: 100 mV/s. inset: Cyclic voltammogram of PTZ (1.0 mM) in the presence of 0.5 mM BSA. Potential scan rate: 25 mV/s. Parts II and III: Cyclic voltammograms of PTZ (1.0 mM) in the presence of 0.5 mM BSA at different scan rates. Scan rates from a to f are: 10, 25, 50, 100, 300 and 500 mV/s. Solvent: phosphate buffer (pH 2.0, c = 0.2 M)/acetonitrile mixture (50/50 v/v). All experiments were performed at room temperature using a GC electrode.
In Fig. 3, part I, curve c is related to BSA in the absence of the PTZ. The peak observed in this cyclic voltammogram is attributed to the one electron oxidation of BSA to the corresponding radical33. An important point regarding the peak current ratio (IpC1/IpA1) is the dependence on the nucleophile (BSA) concentration and on the potential scan rate. As shown in the Fig. 3, part I, inset, the peak current ratio (IpC1/IpA1) increases with decreasing BSA concentration. Comparison of this voltammogram with voltammogram b shows that decreasing the concentration of BSA slows down the reaction rate of BSA with PTZox and leads to more PTZox remaining on the electrode surface, which leads to an increase in the cathodic peak current (IpC1). The effect of scan rate on the cyclic voltammograms of PTZ/BSA mixture is shown in Fig. 3, parts II and III. As can be seen, at low scan rates such as 10 mV/s (part II, curve a), peaks A1, A2, and A3 are clearly observed in the anodic cycle, and peaks C1 and C3 are also observed in the cathodic scan. Increasing the potential scan rate causes remarkable two changes in the current of the peaks. The first change is related to the gradual decrease of the anodic peaks A2 and A3 with the increase of the scan rate and the second change is related to the increase of IpC1 with increasing scan rate. When the scan rate increases, there is not enough time for the BSA to react with PTZox. In such a situation, most of the PTZox molecules participate in the cathodic reaction, which results in an increase in IpC1. On the one hand, the current of anodic peaks A2 and A3 decrease, because these peaks are related to the oxidation of mono-substituted and di-substituted PTZs, respectively, which are not formed under these conditions.
Based on the obtained electrochemical data, the synthesis of bis(phenylsulfonyl)-10H-phenothiazine derivatives (2a–e) was carried out. The products after separation and purification were identified by various spectroscopic methods such as IR, NMR and MS. Based on spectroscopic data as well as voltammetric results, the following mechanism is proposed for the oxidation of PTZ in the presence of BSA (Fig. 4). This mechanism can be extended to other nucleophiles (4-toluenesulfinic acid, TSA and 4-chlorobenzenesulfinic acid, CSA). According to the proposed mechanism, initially PTZ is converted into its oxidized form (phenothiazin-5-ium, PTZOX) by losing two electrons and one proton. In the next step, PTZOX is attacked by anion resulting from deprotonation of arylsulfinic acids (RSO2−) and forms the first intermediate (INT1) [3-(arylsulfonyl)-10H-phenothiazine] after aromatization. It should be noted that the formation of other isomers of INT1 is also possible. However, due to the presence of sulfonium atom in the structure of PTZOX, it is suggested that C3 atom is the most favorable site for nucleophilic attack of arylsulfinic acids.
The oxidation of INT1 in the next step causes the formation of INT1OX. There are a few important points regarding this step. First, unlike PTZ, whose two rings A and B are similar, INT1 rings A and B are not the same, and due to the presence of an electron-withdrawing arylsulfinic group in ring A, oxidation takes place on the B-ring. Like PTZOX, INT1OX is attacked by another arylsulfinic anion, leading to the final product after aromatization (Fig. 4). As seen in Fig. 4, two types of products are formed in the reaction of arylsulfinic anion with INT1OX. One of these products is formed by the attack of arylsulfinic anion on the nitrogen atom of INT1OX (path I). This compound is a sulfone-sulfonamide product. The second product results from an attack similar to the first arylsulfinic attack (path II), which leads to the formation of the sulfone-sulfone product. These products were separated from each other by thin layer chromatography.
To further investigate the oxidation of PTZ in the presence of BSA, the double potential step chronoamperometry method was also used (Fig. 5). To achieve this goal, based on PTZ cyclic voltammogram (Fig. 3, part I, curve a), the electrode potential is changed from an initial value of 0.20 V to a potential of 0.55 V in the forward step (oxidation step) and then to 0.40 V in the reverse step (reduction step). The generated current was recorded for 12 s in both step. Figure 5 curve a shows the chronoamperometry of PTZ (1.0 mM) in phosphate buffer (pH 2.0, c = 0.2 M/acetonitrile mixture solution (50/50 v/v). The forward current corresponds to the oxidation of PTZ to PTZox and the reverse current corresponds to the reduction of PTZox to PTZ. In this method, the theoretical current ratio for a reversible system at 2τ and τ (Ir (2τ)/If (τ)) is 0.29332,34,35,36.
Double potential step chronoamperograms of 1.0 mM PTZ: (a) in the absence, (b) in the presence of 1.0 mM BSA. E1 = 0.20 V vs. Ag/AgCl, E2 (oxidation step) = 0.55 V vs. Ag/AgCl and E3 (reduction step) = 0.40 V vs. Ag/AgCl. Solvent: phosphate buffer (pH 2.0, c = 0.2 M)/acetonitrile mixture (50/50 v/v). All experiments were performed at room temperature using a GC electrode.
The experimental current ratio (Ir (2τ)/If (τ)) obtained for PTZ is equal to 0.25, which is slightly less than the theoretical value but close to it. This result confirms the relative stability of oxidized PTZ (PTZox) in the time scale of the experiment. Repeating this experiment for the oxidation of PTZ in the presence of BSA and measuring the current at 2τ and τ (Ir (2τ)/If (τ)) shows two important facts. First, the forward currents in these two experiments are exactly the same, and second, the reverse current (Ir (2τ)) in the presence of BSA is zero. These results confirm that PTZox is removed from the electrode surface due to its reaction with BSA on the time scale of the experiment.
Due to the fundamental difference in the structure of the synthesized products (3,10-bis(phenylsulfonyl)-10H-phenothiazine, 2a and 3,7-bis(phenylsulfonyl)-10H-phenothiazine, 2b), this section is dedicated to investigating the electrochemical behavior of the synthesized products 2a and 2b. Figure 6 curve a, shows the cyclic voltammogram of 2b. The cyclic voltammogram displays a quasi-reversible redox process ascribed to the oxidation of 2b to 2box and vice versa. Comparing the cyclic voltammogram of 2b with that of PTZ (Fig. 6 curve b) shows that the oxidation potential of 2b is about 140 mV more anodic than the PTZ oxidation potential. The peak shift can be expected due to the difference in the structure of 2b (the presence of two electron withdrawing sulfonyl groups) with PTZ. In addition, the presence of two sulfonyl groups in the structure of 2b has caused its oxidized form (2box) to become more unstable than the oxidized form of PTZ (PTZox) and thus participate in subsequent chemical reactions. As a result, the current of its cathodic peak (C3) becomes lower than the current of cathodic peak of PTZ (C1). Figure 6 curve d, shows the cyclic voltammogram of 2a. In similar conditions, unlike the cyclic voltammogram of 2b, the cyclic voltammogram of 2a shows the presence of an irreversible process. The main reason for this difference is the attachment of a sulfonyl group to the nitrogen atom. This binding causes the two-electron oxidation of the 2a to form a highly unstable 2aox compound (Fig. 4).
(a) Cyclic voltammogram of 1.0 mM 2b. (b) Cyclic voltammogram of 1.0 mM PTZ. (c) Cyclic voltammogram of 1.0 mM PTZ in the presence of 1.0 mM BSA. (d) Cyclic voltammogram of 1.0 mM 2a. Potential scan rate: 50 mV/s. Solvent: phosphate buffer (pH 2.0, c = 0.2 M)/acetonitrile mixture (50/50 v/v). Working electrode: glassy carbon electrode. All experiments were performed at room temperature.
According to the proposed mechanism in Fig. 4, the structure of the product formed via pathway II (for example 2b) is similar to that of PTZ and therefore, its general electrochemical behavior should also be similar to PTZ. In Fig. 6, we studied and compared the cyclic voltammogram of product 2b with the cyclic voltammogram of PTZ. Based on this, the redox reactions producing the anodic and cathodic peaks A3 and C3 is shown in Fig. 7. The electrochemical oxidation of 2a was also studied (Fig. 6, curve d). Replacing the hydrogen atom in the PTZ molecule with a sulfinic group has a great effect on the stability of the oxidized product (PTZOX). The removal of the hydrogen atom attached to the nitrogen atom as a proton and giving its electron to the mother molecule causes the relative stability of the oxidized forms of molecules PTZ and 2b (PTZOX and 2box). In contrast to these molecules, the replacement of the hydrogen atom with a sulfinic group in molecule 2a has caused the oxidized molecule (2aox) to be in the dication form. This compound is very unstable and quickly participates in subsequent chemical processes such as ring cleavage37,38, dimerization reactions39,40, hydroxylation reactions41,42, hydrolysis43,44 and/or sulphoxide formation45. The occurrence of these reactions makes the cyclic voltammogram of 2a shows the behavior of an irreversible system.
In this part, it is necessary to consider the possibility of formation of different isomers in the oxidation of PTZ in the presence of arylsulfinic acids. Figure 8 shows the structures that may be formed in the oxidation of PTZ in the presence of arylsulfinic acids. The steric energy calculations show that due to the steric hindrance caused by the presence of two arylsulfinic groups in the ortho position to each other, it is not possible to form molecules such as I, X, XIV and XV.
As discussed earlier, it seems that due to the presence of sulfonium atom in PTZOX structure, beside nitrogen atom, C1 and C3 atoms are the most favorable sites for nucleophilic attack. Accordingly, the probability of formation of molecules IX, XII and XVII is very low and therefore they are excluded from the set of possible structures. It seems that the first nucleophilic attack leads to the formation of one of the following intermediates (INTA, INTB and INTC) shown in Fig. 9.
As discussed, the second oxidation step of the INTA leads to the corresponding dication, which is a very unstable compound and does not appear to be capable of conversion to our desired product (molecule III, 2a). Therefore, we do not consider this pathway possible in the oxidation of PTZ in the presence of arylsulfinyl acids. However, the second oxidation step of the intermediates INTB and INTC leads to the compounds I, III, VI, VII, XI and XVI. By removing compound I for the reasons mentioned earlier, the only remaining compound with the presence of a sulfinic group on the nitrogen atom is compound III (2a). By excluding, compounds I and III, the 1H NMR spectrum of the product was compared with the simulated 1H NMR spectra of compounds VI, VII, XI and XVI. This comparison shows that the experimental spectrum is the most consistent with the simulated spectrum for compound VI (Fig. 10).
Controlled potential coulometry
Controlled potential coulometry was carried out in a solution containing 0.25 mmol of PTZ and 0.5 mmol BSA in phosphate buffer (pH 2.0, c = 0.2 M/acetonitrile solution mixture (50/50, v/v) at 0.55 V versus Ag/AgCl. In order to better understand what happens during coulometry, cyclic voltammograms of the electrolyzed solution were recorded during coulometry (Fig. 11). These voltammograms at different time intervals show that the peak A1 current decreases in the progress of electrolysis. The second change observed in cyclic voltammograms is the appearance of peak A2 and its relative increase with the progress of coulometry. Plotting the values of peak A1 current versus the amount of electricity consumed in order to determine the number of electrons consumed in this process shows that peak A1 disappears with the consumption of about 104 coulombs of electricity (Fig. 11, inset).
Cyclic voltammograms of 0.25 mmol PTZ in the presence of 0.5 mmol BSA during controlled-potential coulometry at 0.55 V vs. Ag/AgCl, in phosphate buffer (pH = 2.0, c = 0.2 M)/acetonitrile mixture (50/50 v/v). Cyclic voltammograms from a to f are: 20, 40, 60, 80, 95 and 104 C. Scan rate: 100 mV/s. Inset: Variation of A1 peak current (IpA1) vs. charge consumed. Working electrode: glassy carbon electrode. All experiments were performed at room temperature.
According to the electricity consumption and performing the necessary calculations, 4.1 electrons are assigned to each molecule of PTZ. This number is slightly higher than 4 due to electrolysis in an undivided cell. The number of electrons obtained in this experiment (n = 4) is consistent with that reported in Fig. 4. According to Fig. 4, two electrons are used to oxidize PTZ to PTZox (peak A1) and the other two electrons are used to oxidize intermediate INT1 to INT1ox (peak A2). It should be noted that, the lack of increase in IpA3 and IpC3 during electrolysis is due to the insolubility of final products in electrolysis solution (see experimental section).
Constant current synthesis and optimization of effective parameters
In order to facilitate the synthesis of these compounds in a way that can be used by all researchers, in this section, the synthesis of these compounds in constant current mode has been examined and the effective parameters in improving the efficiency and purity of the products, such as applied current density, amount of electricity, solution pH, electrode material and solvent mixture are optimized by one factor at a time method. The current density is one of the most important parameters in electrosynthesis of organic and inorganic compounds, which affects the yield and purity. In this section, the electrochemical synthesis of products (2a and 2b) was investigated at different current densities from 0.41 to 2.08 mA/cm2 (Fig. 12, part I) while other parameters are kept constant (see the caption of Fig. 12). The results showed that the highest product yield (89%) was obtained at a current density of 1.25 mA/cm2. At current densities less than 1.25 mA/cm2, the amount of overvoltage is not sufficient to oxidize PTZ and/or intermediates. On the other hand, at current densities higher than the optimal current density (1.25 mA/cm2), oxidation of the solvent, supporting electrolyte and/or over-oxidation of the product will reduce the production yield (Fig. 12, part I). To optimize the amount of electricity, the electrochemical synthesis of 2a and 2b was investigated at different amount of electricity and at current density of 1.25 mA/cm2 while other parameters are kept constant. The results showed that the highest product yield was obtained at Q = 140 C. The higher amount of electricity consumed in the constant current method compared to the controlled potential method is due to the inherent difference of the two methods in the selective consumption of electricity.
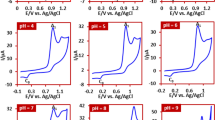
Part I: The effect of current density on the product yield (both 2a and 2b). The amounts of PTZ and BSA 0.25 and 0.5 mmol, respectively. Q = 140 C. Solvent: phosphate buffer (pH = 2.0, c = 0.2 M)/acetonitrile mixture (50/50 v/v). Anode and cathode material: carbon and stainless steel, respectively. Part II: The effect of solution pH on the yield of product. Applied current density: 1.25 mA/cm2. Other conditions are the same as part I. All experiments were performed at room temperature.
The effect of solution pH on the yield and purity of products was also investigated (Fig. 12, part II). For this purpose water with different pH values of 1.0, 2.0, 6.0, 8.0 and 10.0/acetonitrile (50/50 v/v) solution mixtures are prepared. Phosphate buffer (0.2 M) was used to prepare solutions with pH values 2.0, 6.0 and 8.0. For pH = 1.0, perchloric acid solution (0.1 M) and for pH = 10.0, carbonate buffer (0.2 M) was used. Other conditions such as current density (1.25 mA/cm2) and electricity consumption (140 C) were constant in all experiments. Figure 12, part II shows that the optimum pH is 2.0 for highest product yield. The instability of PTZox in alkaline solutions and participation in side reactions such as dimerization10,39,40 and/or hydroxylation10,41,42 is the main reason for the low yield of the product in alkaline solutions. On the other hand, the one-electron oxidation of PTZ in highly acidic solutions10 and the instability of cation radicals along with nucleophile protonation, reduce the product yield in highly acidic solutions.
In this section, the effect of electrode materials on product efficiency is investigated and the results are given in Table 1. As can be seen, the highest product yield was achieved with carbon anode and stainless steel cathode in aqueous phosphate buffer (pH, 2.0, c = 0.2M)/acetonitrile mixture (50/50 v/v). The last optimization in this work was performed on the solvent system. The use of organic co-solvent is due to the low solubility of PTZ in water. Therefore, solvents such as ethanol and acetonitrile have been used as co-solvents. It should be noted that PTZ is easily soluble in acetone. But due to the unexpected reactivity of acetone as a solvent, this compound was not examined as a co-solvent.
The effect of solvent system on product yield is shown in Table 2. As can be seen, the highest product yield was achieved in aqueous phosphate buffer (pH, 2.0, c = 0.2 M)/acetonitrile mixture (50/50 v/v). It should be noted that increasing the percentage of acetonitrile in the mixture does not have a significant effect on the yield of the product. In addition, our research has shown that replacing ethanol with acetonitrile in the solvent mixture decreases yield. The decrease may be due to the low solubility of PTZ in ethanol. A large increase in the percentage of ethanol in the water/ethanol mixture increases the solubility of PTZ (slightly), but on the other hand, it causes a marked decrease in the solubility of the supporting electrolyte. Regarding the solution pH, it should be noted that increasing the pH from 2.0 decreases the yield of 2a and 2b. Increasing pH causes deprotonation of PTZH+ and coupling reaction of PTZ with PTZox (dimerization reaction)10. This reaction competes with the reaction between arylsulfinic acids and PTZox. In other words, decreasing the pH causes the protonation of PTZ (formation of PTZH+) and suppresses the dimerization reaction. It should be noted that due to the low basicity of arylsulfinate anions (pKa < 2.0)47, decreasing the pH does not have a significant effect on their nucleophilicity.
Conclusion
In this study, the electrochemical synthesis of five new phenothiazine derivatives (bis(phenylsulfonyl)-10H-phenothiazine derivatives) in water/acetonitrile mixture was carried out in a one-pot process through the electrochemical generation of phenothiazin-5-ium (PTZox) in the presence of arylsulfinic acids. Our results show that two different types of products (bis(phenylsulfonyl)-10H-phenothiazine derivatives) are formed during the electrolysis: (1) Phenothiazine-sulfonamide-sulfone derivatives. In these products, one arylsulfinic group is attached to the nitrogen atom and the other group is attached to the carbon atom. (2) Phenothiazine-disulfone derivatives. In these products, both arylsulfinic groups are attached to carbon atoms. In addition to the controlled potential method, the synthesis of these compounds by performing electrolysis in constant current mode has also been successful. In this research, a mechanism for the oxidation of PTZ in the presence of arylsulfinic acids was also proposed based on the data obtained from cyclic voltammetric, chronoamperometric and controlled-potential coulometric studies along with the structure of the synthesized products. This mechanism is depicted in Fig. 4. According to Fig. 4, PTZ is converted to the final product (bis(phenylsulfonyl)-10H-phenothiazine derivatives) through the ECEC mechanism. The insolubility of the final product in the electrolysis solution is one of the factors that prevent the re-oxidation of the final product. Finally, we hope that the synergistic effect of the groups added to the phenothiazine molecule will intensify the medicinal properties and/or reduce the side effects of the synthesized molecules.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Heard, D. M. & Lennox, A. Electrode materials in modern organic electrochemistry. Angew. Chem. Int. Ed. 59, 18866–18884 (2020).
Leech, M. C. & Lam, K. A. practical guide to electrosynthesis. Nat. Rev. Chem. 6, 275–286 (2022).
Zivari-Moshfegh, F., Khoram, M. M. & Nematollahi, D. Green electrochemical synthesis of silver sulfadiazine microcrystals. RSC Adv. 9, 24105–24109 (2019).
Lodarski, K. et al. Discovery of butyrylcholinesterase inhibitors among derivatives of azaphenothiazines. J. Enzyme Inhib. Med. Chem. 30, 98–106 (2015).
Pluta, K., Morak-Młodawska, B. & Jeleń, M. Recent progress in biological activities of synthesized phenothiazines. Eur. J. Med. Chem. 46, 3179–3189 (2011).
Jaszczyszyn, A. et al. Chemical structure of phenothiazines and their biological activity. Pharmacol. Rep. 64, 16–23 (2012).
Mentré, F. et al. Dose regimen of favipiravir for Ebola virus disease. Lancet Infect. Dis. 15, 150–151 (2015).
Aktaş, A., Tüzün, B., Aslan, R., Sayin, K. & Ataseven, H. New anti-viral drugs for the treatment of COVID-19 instead of favipiravir. J. Biomol. Struct. Dyn. 39, 7263–7273 (2021).
Jiang, Y., Xu, K. & Zeng, C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 118, 4485–4540 (2017).
Mohamadighader, N., Nematollahi, D. & Saraei, M. A. comprehensive study on electrochemical oxidation of phenothiazine in water-acetonitrile mixture: Electrosynthesis of phenothiazine dimers. Electrochim. Acta 425, 140706 (2022).
Scott, K. A. & Njardarson, J. T. Analysis of US FDA-approved drugs containing sulfur atoms. In Sulfur Chemistry 1–34 (Springer, 2019).
Zhou, J. et al. Extended phenothiazines: synthesis, photophysical and redox properties, and efficient photocatalytic oxidative coupling of amines. Chem. Sci. 13, 5252–5260 (2022).
Padhy, H. J. et al. Synthesis and applications of low-bandgap conjugated polymers containing phenothiazine donor and various benzodiazole acceptors for polymer solar cells. J. Polym. Sci. Part A Polym. Chem. 48, 4823–4834 (2010).
Ullah, A. et al. Novel phenothiazine-based self-assembled monolayer as a hole selective contact for highly efficient and stable p-i-n perovskite solar cells. Adv. Energy Mater. 12, 2103175 (2022).
Tozkoparan, B., Küpeli, E., Yeşilada, E. & Ertan, M. Preparation of 5-aryl-3-alkylthio-l,2,4-triazoles and corresponding sulfones with antiinflammatory–analgesic activity. Bioorganic Med. Chem. Lett. 15, 1808–1814 (2007).
Hwang, S. H. et al. Synthesis and structure–activity relationship studies of urea-containing pyrazoles as dual inhibitors of cyclooxygenase-2 and soluble epoxide hydrolase. J. Med. Chem. 54, 3037–3050 (2011).
Kudryavtsev, K. V., Bentley, M. L. & McCafferty, D. G. Probing of the cis-5-phenyl proline scaffold as a platform for the synthesis of mechanism-based inhibitors of the Staphylococcus aureus sortase SrtA isoform. Bioorganic Med. Chem. Lett. 17, 2886–2893 (2009).
Guruswamy, B., Arul, R. K., Chaitan, M. V. S. R. K. & Darsi, S. S. P. K. Synthesis and biological evaluation of novel β-hydroxy benzimidazolyl sulfone fluoroquinolones by selective oxidation using ammonium molybdate catalysed H2O2. Eur. J. Chem. 4, 329–335 (2013).
Al-Said, M. S., Ghorab, M. M. & Nissan, Y. M. Dapson in heterocyclic chemistry, part VIII: Synthesis, molecular docking and anticancer activity of some novel sulfonylbiscompounds carrying biologically active 1,3-dihydropyridine, chromene and chromenopyridine moieties. Chem. Cent. J. 6, 1–14 (2012).
Kamble, R. B., Chavan, S. S. & Suryavanshi, G. An efficient heterogeneous copper fluorapatite (CuFAP)-catalysed oxidative synthesis of diaryl sulfone under mild ligand-and base-free conditions. N. J. Chem. 43, 1632–1636 (2019).
Anderson, R., Groundwater, P. W., Todd, A. & Worsley, A. Antibacterial Agents: Chemistry, Mode of Action, Mechanisms of Resistance and Clinical Applications (John Wiley & Sons, 2012).
Shoaib Ahmad Shah, S., Rivera, G. & Ashfaq, M. Recent advances in medicinal chemistry of sulfonamides. Rational design as anti-tumoral, anti-bacterial and anti-inflammatory agents. Mini Rev. Med. Chem. 13, 70–86 (2013).
Scozzafava, A., Owa, T., Mastrolorenzo, A. & Supuran, C. T. Anticancer and antiviral sulfonamides. Curr. Med. Chem. 10, 925–953 (2003).
Supuran, C. T., Casini, A. & Scozzafava, A. Protease inhibitors of the sulfonamide type: Anticancer, antiinflammatory, and antiviral agents. Med. Res. Rev. 23, 535–558 (2003).
Rakesh, K. P. et al. Recent development of sulfonyl or sulfonamide hybrids as potential anticancer agents. Med. Chem. 18, 488–505 (2018).
Pant, S. M. et al. Design, synthesis, and testing of potent, selective hepsin inhibitors via application of an automated closed-loop optimization platform. J. Med. Chem. 61, 4335–4347 (2018).
Nishioka, H., Tooi, N., Isobe, T., Nakatsuji, N. & Aiba, K. BMS-708163 and Nilotinib restore synaptic dysfunction in human embryonic stem cell-derived Alzheimer’s disease models. Sci. Rep. 6, 33427 (2016).
Pennington, L. D. et al. Discovery and structure-guided optimization of diarylmethanesulfonamide disrupters of glucokinase–glucokinase regulatory protein (GK–GKRP) binding: Strategic use of a N→ S (nN→ σ* S-X) interaction for conformational constraint. J. Med. Chem. 58, 9663–9679 (2015).
Gillman, K. W. et al. Discovery and evaluation of BMS-708163, a potent, selective and orally bioavailable γ-secretase inhibitor. ACS Med. Chem. Lett. 1, 120–124 (2010).
Sugimoto, H. et al. An orally bioavailable small molecule antagonist of CRTH2, ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro. J. Pharmacol. Exp. Ther. 305, 347–352 (2003).
Lafferty, J. J., Garvey, E., Nodiff, E. A., Thompson, W. E. & Zirkle, C. L. The synthesis of phenothiazines. VII. Methyl- and arylsulfonylation of phenothiazine and its 10-substituted derivatives. J. Org. Chem. 27, 1346–1351 (1962).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications (Wiley, 2001).
Nematollahi, D., Joudaki, M., Khazalpour, S. & Pouladi, F. Electrochemical oxidation of sulfinic acids: Efficient oxidative synthesis of diaryl disulfones. J. Electrochem. Soc. 164, G65–G70 (2017).
Zivari-Moshfegh, F., Javanmardi, F. & Nematollahi, D. A comprehensive electrochemical study on anti-tuberculosis drug rifampicin. Investigating reactions of rifampicin-quinone with other anti-tuberculosis drugs, isoniazid, pyrazinamide and ethambutol. Electrochim. Acta 457, 142487 (2023).
Zivari-Moshfegh, F. & Nematollahi, D. An eco-friendly electrochemical process for the formation of a new desloratadine derivative and its antibacterial susceptibility. Report of a new type of ortho-quinhydrone complex. Electrochim. Acta 421, 140518 (2022).
Zivari-Moshfegh, F. & Nematollahi, D. New insights into co-administration of anti-tuberculosis drug rifampicin with acetaminophen and vitamin C: Strong electrochemical evidence for the detoxification. J. Electrochem. Soc. 170, 095501 (2023).
Varmaghani, F., Nematollahi, D., Mallakpour, S. & Esmaili, R. Electrochemical oxidation of 4-substituted urazoles in the presence of arylsulfinic acids: An efficient method for the synthesis of new sulfonamide derivatives. Green Chem. 14, 963–967 (2012).
Varmaghani, F. & Nematollahi, D. Electrochemical study of 1,2-dihydropyridazine-3,6-dione in protic and aprotic solvents: Oxidative ring cleavage and reduction. Electrochim. Acta 56, 6089–6096 (2011).
Salehzadeh, H., Nematollahi, D. & Rafiee, M. Electrochemical dimerization of 4-methylesculetin: Synthesis and kinetic study of a highly-oxygenated dimer. J. Electroanal. Chem. 650, 226–232 (2011).
Shahparast, S., Nematollahi, D., Sharafi-Kolkeshvandi, M. & Goljani, H. Direct electrochemical dimerization of N, N′-diphenylbenzidine. J. Electrochem. Soc. 166, G47 (2019).
Nematollahi, D., Shayani-Jam, H., Alimoradi, M. & Niroomand, S. Electrochemical oxidation of acetaminophen in aqueous solutions: Kinetic evaluation of hydrolysis, hydroxylation and dimerization processes. Electrochim. Acta 54, 7407–7415 (2009).
Souri, Z., Masoudi Khoram, M., Nematollahi, D., Mazloum-Ardakani, M. & Alizadeh, H. A. green protocol for the electrochemical synthesis of a fluorescent dye with antibacterial activity from imipramine oxidation. Sci. Rep. 12, 4921 (2022).
Beiginejad, H. & Nematollahi, D. Electrochemical oxidation of 2, 5-diethoxy-4-morpholinoaniline in aqueous solutions. Electrochim. Acta 114, 242–250 (2013).
Beiginejad, H., Nematollahi, D., Varmaghani, F. & Bayat, M. Efficient factors on the hydrolysis reaction rate of some para-aminophenol derivatives in acidic pHs. J. Electrochem. Soc. 160, H469 (2013).
Blankert, B. et al. Electrochemical, chemical and enzymatic oxidations of phenothiazines. Electroanalysis 17, 1501–1510 (2005).
Zhang, C., Liu, J. & Chen, B. Effect of CeO2 and graphite powder on the electrochemical performance of Ti/PbO2 anode for zinc electrowinning. Ceram. Int. 44, 19735–19742 (2018).
Jamshidi, M., Nematollahi, D., Bayat, M. & Salahifar, E. Unsymmetrical diaryl sulfones through electrochemical oxidation of fast violet B in the presence of aryl sulfinic acids. J. Electrochem. Soc. 163, G211–G218 (2016).
Acknowledgements
The authors acknowledge the Bu-Ali Sina University Research Council and Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS) for their support of this work.
Author information
Authors and Affiliations
Contributions
N.M. investigation, conceptualization, methodology, writing—original draft. F.Z.M. investigation, conceptualization, methodology, writing—original draft. D.N. writing—review & editing, supervision, project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamadighader, N., Zivari-Moshfegh, F. & Nematollahi, D. Electrochemical generation of phenothiazin-5-ium. A sustainable strategy for the synthesis of new bis(phenylsulfonyl)-10H-phenothiazine derivatives. Sci Rep 14, 4276 (2024). https://doi.org/10.1038/s41598-024-53620-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53620-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.