Abstract
Major depressive disorder (MDD) is accompanied by activated neuro-immune pathways, increased physiosomatic and chronic fatigue-fibromyalgia (FF) symptoms. The most severe MDD phenotype, namely major dysmood disorder (MDMD), is associated with adverse childhood experiences (ACEs) and negative life events (NLEs) which induce cytokines/chemokines/growth factors. To delineate the impact of ACE + NLEs on physiosomatic and FF symptoms in first episode (FE)-MDMD, and examine whether these effects are mediated by immune profiles. ACEs, NLEs, physiosomatic and FF symptoms, and 48 cytokines/chemokines/growth factors were measured in 64 FE-MDMD patients and 32 normal controls. Physiosomatic, FF and gastro-intestinal symptoms belong to the same factor as depression, anxiety, melancholia, and insomnia. The first factor extracted from these seven domains is labeled the physio-affective phenome of depression. A part (59.0%) of the variance in physiosomatic symptoms is explained by the independent effects of interleukin (IL)-16 and IL-8 (positively), CCL3 and IL-1 receptor antagonist (inversely correlated). A part (46.5%) of the variance in physiosomatic (59.0%) symptoms is explained by the independent effects of interleukin (IL)-16, TNF-related apoptosis-inducing ligand (TRAIL) (positively) and combined activities of negative immunoregulatory cytokines (inversely associated). Partial least squares analysis shows that ACE + NLEs exert a substantial influence on the physio-affective phenome which are partly mediated by an immune network composed of interleukin-16, CCL27, TRAIL, macrophage-colony stimulating factor, and stem cell growth factor. The physiosomatic and FF symptoms of FE-MDMD are partly caused by immune-associated neurotoxicity due to T helper (Th)-1 polarization and M1 macrophage activation and relative lowered compensatory immunoregulatory protection.
Similar content being viewed by others
Introduction
Using a novel precision psychiatry methodology that incorporates machine learning techniques, we recently demonstrated that there are two qualitatively distinct phenotypes of major depressive disorder (MDD), namely Major Dysmood Disorder (MDMD) and Simple Dysmood Disorder (SDMD)1,2,3. MDMD is distinguished from SDMD by a higher recurrence of illness (ROI), which is derived from the number of depressive episodes and the number of lifetime suicidal attempts and ideation4. In addition, patients with MDMD score higher on lifetime and current suicidal behaviors and attempts, cognitive deficits, and disabilities, than those with SDMD1,3. In contrast to SDMD, MDMD is associated with elevated levels of pure depressive symptoms (such as depressed mood, feelings of guilt, suicidal ideation, and decreased interest)5, pure anxiety symptoms (anxious mood, tension, fears, and anxious behavior observed during the interview)6, insomnia (early, middle, and late), symptoms of melancholia (including psychomotor retardation, psychomotor agitation, diurnal variation, and early awakening), and total sums on both the Hamilton Depression (HAMD) and Anxiety (HAMA) Rating Scales1,3,5,6.
However, rating scales such as the HAMD and HAMA score high on psychosomatic symptoms, including anxiety somatic, somatic gastro-intestinal, general somatic, genital symptoms, and hypochondriasis of the HAMD5, and somatic anxiety, sensory, cardiovascular, gastrointestinal (GIS), genitourinary, and respiratory symptoms of the HAMA6. Multiple studies have consistently demonstrated that psychosomatic symptoms play a significant role in both “endogenous” depression, and depression resulting from medical conditions7,8,9,10. Nevertheless, due to the discovery that the psychosomatic symptoms of depression have a biological basis (as evidenced below), we have chosen to redefine this collection of symptoms as "physiosomatic" instead of “psychosomatic”8,11. Therefore, "psycho" was substituted for "physio" to emphasize that somatic symptoms are of a biological nature, rather than suggesting that they may have a psychological origin11.
A comparative analysis revealed a notable disparity in the intensity of physiosomatic symptoms between individuals diagnosed with MDMD and the control group1,2,12 Additional physiosomatic symptoms that resemble those found in chronic fatigue syndrome (CFS) and fibromyalgia can be evaluated through the utilization of the Fibro-Fatigue (FF) scale13. These symptoms include muscle pain, muscle tension, fatigue, autonomic and GIS symptoms, headache, and a flu-like malaise14,15. Nevertheless, there is a lack of available data regarding the potential increase in FF symptoms in individuals with MDMD compared to those with SDMD.
Significantly, individuals diagnosed with MDMD and those experiencing their first episode of MDMD (FE-MDMD) exhibit notable elevations in neuro-immune, autoimmune, and gut-brain pathways. These pathways encompass specific modifications in the cytokine network, gut microbiome, gut dysbiosis, heightened translocation of Gram-negative bacteria, increased atherogenicity, and diminished antioxidant, and neurotrophic defenses16,17,18. There are now many publications and meta-analyses indicating that MDD is accompanied by increased levels of pro-inflammatory cytokines and chemokines and by an immune-inflammatory response19,20,21,22,23.
Nevertheless, the neuro-immune disorders observed in MDD can be attributed solely to the neuro-immune abnormalities present in MDMD, while there is no association between activated immune-inflammatory pathways and SDMD2,24. The immune characteristics that are of significance in MDMD encompass the activation of classical M1 macrophages, alternative M2 macrophages, T helper (Th)-1 cells, and Th-17 cells. These immune profiles lead to the activation of the immune-inflammatory response system (IRS) and an increase in the levels of neurotoxic (NT) immune products2,24. According to the study conducted by24, the activation of Th-1 cells and the increased presence of interleukin (IL)-16, tumor necrosis factor (TNF)-α, and TNF-related apoptosis-inducing ligand (TRAIL) are responsible for inducing IRS activation in MDMD. The augmented immune-associated neurotoxicity (Immu-NT) observed in individuals with MDMD is predominantly attributed to heightened concentrations of IL-16, TNF-α, IL-6, CCL2, CCL11, CXCL1, and CXCL1024. On the other hand, individuals diagnosed with MDMD demonstrate heightened concentrations of immune substances that possess negative immunoregulatory or anti-inflammatory properties, including IL-10, IL-4, soluble IL-1 receptor antagonist (sIL-1RA), and sIL-2R24. The relationship between depression, as a construct derived from measures such as HAMD, HAMA, and other depression scores, and activated immune profiles has been found to be highly predictive. However, it remains unclear whether the neuro-immune pathways responsible for MDMD are associated with physiosomatic and FF symptoms in MDMD.
It is widely recognized that experiencing multiple adverse childhood experiences (ACEs) and negative life events (NLEs) is associated with an elevated risk of developing MDD, anxiety disorders, and suicidal behaviors in adulthood4,25,26,27,28,29,30,31. Emerging research suggests that there is a significant association between ACEs, the combination of ACEs and NLEs within the past year, and the development of MDD, with a particular emphasis on FE-MDMD32. These factors have been found to strongly predict various aspects of MDD, such as the severity of depression and anxiety symptoms, lifetime history and current presence of suicidal behaviors, as well as physiosomatic symptoms2,24. The association between the occurrence of FE-MDMD and current suicidal behaviors is primarily linked to the cumulative impact of ACEs and NLEs3.
Furthermore, the occurrence of ACEs is strongly correlated with the activation of immune-inflammatory cytokines, chemokines and growth factor responses, oxidative stress reactions, alterations in the gut microbiome, and diminished antioxidant and neurotrophic responses in later stages of life1,4,16,33. In addition, ACEs have been found to be linked with the activation of M1 and T cytokines, polarization towards a Th-1 response, heightened expression of T cell activation markers such as CD4, CD40L, and CD71, as well as increased IL-16 signaling. Moreover, ACEs have been associated with elevated levels of chemokines including CCL2, CCL5, CCL27, CXCL9, and CXCL10, as well as growth factors such as stem cell factor (SCF), stem cell growth factor (SCGF), hepatic growth factor (HGF), and platelet-derived growth factor (PDGF)16,33. Furthermore, the effects of ACE + NLEs on the depression phenotype are mediated by activated neuroimmune pathways and Th-1 cell activation16.
There is a substantial body of literature indicating that the physiosomatic and FF symptoms associated with MDD may be explained by the activation of neuro-immune pathways in both the peripheral and central nervous systems. The pathways involved in this context encompass M1 and Th-1 pathways, along with the participation of distinct immune molecules, namely IL-6, IL-1β, and TNF-α34,35,36,37,38,39.
The specific mechanisms through which ACEs or ACE + NLEs could impact the physiosomatic and FF symptoms of MDD/MDMD remain uncertain. It is unclear whether these effects are mediated by the activation of the IRS, specific immune phenotypes like Th-1 or M1 phenotypes, or specific cytokines, chemokines, and growth factors such as IL-6, IL-1β, and TNF-α. Therefore, the precise hypotheses of this study are as follows: the impact of ACEs and NLEs on physiosomatic and FF symptoms is influenced by increased activity of the IRS and Immu-NT, specifically through the release of certain M1 cytokines such as IL-6, IL-1β, and TNF-α.
Materials and methods
Participants
In accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)40, a senior psychiatrist at Alhakiem Hospital in Najaf, Iraq, identified and recruited 64 individuals with FE-MDD and 32 healthy controls. Moreover, all patients were diagnosed with MDD, single episode, indicating they experience their first MDD episode, in the current study labeled as FE-MDMD. All patients diagnosed with MDD were observed to be in the acute phase of the disease, with no indications of complete or partial remission. It should be noted that all FE-MDD patients met the diagnostic criteria for MDMD as detailed by Maes, Vasupanrajit, et al. (2023)16. The diagnosis of MDMD versus SDMD is made using machine learning techniques (support vector machine) and as such there are no easy case definitions that can be applied. Nevertheless, MDMD is the more severe subtype in terms of higher ROI and/or severity of depression and anxiety. For example, when both the HAMD and the HAMA exceed twenty-two points, MDMD may be diagnosed even in patients with a first episode.
In addition, the senior psychiatrist recruited healthy controls from the same geographical area to serve as the control group. This group included hospital staff and patients' acquaintances. All participants were enlisted between October 2021 and March 2022. Exclusions were made for those with chronic liver or kidney disease, women who were pregnant or nursing, and those with a history of multiple sclerosis, Parkinson's disease, stroke, or Alzheimer's disease. Similarly, psoriasis, rheumatoid arthritis, inflammatory bowel disease, cancer, type 1 diabetes, and scleroderma exhibited the same pattern. In addition, we excluded participants who had experienced an acute COVID-19 infection, severe or critical COVID-19 disease, Long COVID, or a COVID-19 infection within the previous six months. Our study did not include individuals taking immunosuppressive or immunomodulatory medications, nor did it include those taking therapeutic doses of antioxidants or omega-3 supplements (last three months).
The process of meticulously selecting the control group was accorded significant consideration. Excluded from the control group were participants with a documented lifetime history of clinical depression or dysthymia, a family history of depression, mania, psychosis, or substance use disorder, or a history of suicide. Dysthymia (excluding cases of double depression), schizophrenia, schizoaffective disorder, bipolar disorder, autism spectrum disorders, substance use disorders (excluding nicotine dependence), post-traumatic stress disorder, psycho-organic disorders, generalized anxiety disorder, and obsessive–compulsive disorder were excluded from the study.
Before participating in the study, all participants, or, if applicable, their parents or legal custodians, provided informed consent in writing. Document No. 18/2021 indicates that the Ethics Committee of the College of Medical Technology at the Islamic University of Najaf in Iraq has approved this investigation. The research was conducted in accordance with both Iraqi and international ethical and privacy regulations. In prominent documents such as the Declaration of Helsinki by the World Medical Association, the Belmont Report, the CIOMS Guideline, and the International Conference on Harmonization of Good Clinical Practice, a variety of non-exhaustive principles are outlined. Our organization's institutional review board (IRB) is committed to upholding the highest standards of quality, ensuring precise compliance with the International Guideline for the Conduct of Safe Human Research (ICH-GCP).
Clinical assessments
The physical, mental, and behavioral health of the participants was evaluated by a senior psychiatrist who used a systematic interview and standardized procedures. The senior psychiatrist collected sociodemographic, clinical, and psychological information through semi-structured interviews. The same professional utilized the HAMD and the HAMA to evaluate the severity of depression and anxiety, respectively. In the current study, all physiosomatic symptoms of the HAMD and HAMA were excluded to compute pure depression and pure anxiety scores, respectively. The former concept was conceptualized as a collection of symptoms including depressed mood, feelings of guilt, suicidal ideation, and decreased interest. The score for pure anxiety was determined by adding the scores for anxious mood, tension, fears, and anxious behavior observed during the interview. The physiosomatic symptom score was calculated as a z unit-based composite score based on the sum of the z scores of HAMD and HAMA physiosomatic symptoms, namely anxiety somatic, somatic GIS, and genitourinary symptoms, hypochondriasis, somatic sensory, cardiovascular, GIS, genitourinary, autonomic, and respiratory symptoms. The pure FF symptoms were calculated as the sum of the FF items after exclusion of non-physiosomatic symptoms: muscle pain, muscle tension, fatigue, autonomic, gastro-intestinal symptoms, headache, and a flu-like malaise (all FF scale items after exclusion of all non-physiosomatic symptoms, including cognitive deficits, and sadness). GIS symptoms were conceptualized as a z unit-based composite score based on symptoms of the HAMA and FF scores, namely somatic, and GIS symptoms. Insomnia was conceptualized as a z value-based composite, namely sum of insomnia (HAMA item), insomnia early + insomnia middle + insomnia late (HAMD items) + sleep disorders (FF). Melancholia was conceptualized as a composite based on the z scores of insomnia late, psychomotor retardation, psychomotor agitation, loss of weight, and diurnal variation. This study utilized two items of the Columbia Suicide Severity Rating Scale (C-SSRS) to evaluate suicidal behaviors: the quantification of suicide attempts within the previous year and the assessment of the frequency of suicidal ideation within the previous three months41. A composite score of suicidal behaviors (SB) was calculated by adding the weighted z scores for the HAMD suicide item, the number of suicide attempts, and the frequency of suicidal ideation.
The ACEs Questionnaire42 was used to measure the extent of adverse childhood experiences. There are a total of twenty-eight items on the scale, which comprise the scoring of ten distinct domains. These include (1) mental trauma, (2) physical trauma, (3) sexual abuse, (4) mental neglect, (5) physical neglect, (6) witnessing domestic violence involving the mother, (7) presence of a family member with drug abuse issues, (8) presence of a family member with depression or mental illness, (9) experiencing the loss of a parent due to separation, death, or divorce, and (10) having an incarcerated family member. Various ACE scores were calculated, including the total sum of all ACEs (termed total ACE), the sum of physical trauma, mental neglect, and family member with substance abuse (termed ACE247), and the sum of ACE247 and a family member in prison (termed ACE24710). In addition, the NLEs scale was used to assess the incidence of NLEs in the preceding year43. For the purposes of this investigation, the following factors were considered: a serious accident, the death of a family member or close friend, divorce or separation, unemployment, job loss, alcohol-related issues, drug-related issues, witnessing physical altercations or assaults, experiences of abuse or violent crime, encounters with law enforcement difficulties, problem gambling, familial incarceration, overcrowding in the household, and instances of discrimination. Consequently, we performed calculations to ascertain the combined effects of ACE and the occurrence of one or more NLEs, which we referred to as ACE + NLEs. Body mass index (BMI) was calculated by dividing the participants' weight in kilograms by the square of their height in meters. Using the diagnostic criteria specified in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), tobacco use disorder was evaluated.
Biochemical assays
In this investigation, a disposable syringe and serum tubes were used to obtain a 5 mL venous blood sample from each participant while they were fasting. Blood samples were collected between 8:00 and 11:00 in the morning. The serum and blood cells were effectively separated following centrifugation at 35,000 revolutions per minute (rpm). The serum was then transferred into small Eppendorf containers and stored at − 80 °C until its use in the biomarker assays was deemed necessary. Bio-Plex Multiplex Immunoassay kits (Bio-Rad Laboratories Inc., Hercules, USA) were used to measure the levels of forty-eight cytokines/chemokines/growth factors in the serum of all participants. For measuring the concentrations of these proteins in blood serum, we employed a fluorescence-based method. The researchers measured the fluorescence intensity (FI) of each protein and used the values obtained after subtracting the blank value24,44. The Electronic Supplementary File (ESF) comprises Table 1, which provides an exhaustive listing of the analytes identified by our research. In addition, each analyte's respective gene ID and alternate names are listed in this table. The coefficients of variation (CV) for all analytes in the assay were less than 11.0%. To determine the concentrations of the analytes, we employed the manufacturer-supplied standards. Subsequently, the out-of-range (OOR) concentration rate was calculated, i.e., the proportion of concentrations that fell below the minimum detectable level. Cytokine/chemokine/growth factor levels were excluded from the statistical analysis when more than 80% of the concentrations were below the lowest OOR value. Consequently, IFN-α2, IL-3, IL-7, and IL-12p40 were excluded from our study. Prevalences (measurable or not) were assigned to analytes whose levels exceeded the OOR in 20% to 40% of the cases. These variables were incorporated into the analysis as dummy variables (0 = not measurable, 1 = measurable). These prevalence rates were taken into account in the calculation of our composite immune profile scores. As a result, diverse immune profiles were computed, as described in Table 2 of the ESF. These profiles covered all analytes with the exception of those with detectable concentrations below 20%24,44,45. Table 2 of the ESF lists the variables used to generate M1, M2, M1/M2 (z M1–z M2), Th1, Th2, Th1/Th2 (z Th-1–z Th-2), Th-17, IRS, CIRS, IRS/CIRS (z IRS–z CIRS), and Immu-NT profiles. In addition, the individual cytokines, chemokines, and growth factors were analyzed to ascertain whether there were significant differences in immune profiles between the study groups.
Statistics
This study's statistical analyses were performed with IBM SPSS 29, Windows version. To compare continuous variables between study groups, statistical tests such as analysis of variance (ANOVA) and the Kruskal–Wallis test were utilized. Multiple post-hoc comparisons (Fisher’s exact probability test) were performed to examine differences between subgroups, i.e. between controls and two MDMD subgroups. In contrast, nominal variable comparisons were performed using contingency table analysis, specifically the Chi-square test. In addition, Pearson's and point-biserial correlation coefficients were used to analyze the relationships between scale variables and binary variables. False discovery rate (FDR) p value correction was applied to adjust for multiplicity. The researchers used manual multiple regression analysis to investigate the impact of ACEs, NLEs, and additional demographic factors on the immune profiles. In a similar manner, these analyses examined the influence of different predictor variables, namely immune profiles, ACEs, and NLEs, on the manifestations of depression. In addition, automatic forward stepwise regressions were employed. A significance level of p = 0.05 was used to determine the inclusion of variables, and a significance level of p = 0.06 was used to determine their exclusion. This method made it easier to determine which variables should be included in the final regression model and which ones should be excluded. For each variable included in the final regression models, the standardized coefficients, t-statistics, and exact p values were calculated. In addition, we determined the F statistics, their respective p values, and the effect magnitude using the partial Eta squared. Using appropriate statistical measures, the presence of multicollinearity and collinearity in the data was extensively examined. A tolerance limit of 0.25 and a threshold for the variance inflation factor of four were used. In addition, for the purpose of this analysis, we utilized the condition index and variance proportions derived from the collinearity diagnostics table. Using the White test and a modified variation of the Breusch-Pagan test, heteroskedasticity was identified. All the preceding analyses employed two-tailed tests. A significance level of 0.05 or lower was regarded as statistically significant. We show significance levels as exact p values, unless the p value is lower than 0.001. The latter cases are shown as p < 0.001 (or 0.000 in the partial least squares figures). As required, we utilized transformations such as logarithmic, square-root, rank-based inversed normal (RINT), and a Winsorization technique to obtain a normal distribution for our data indicators.
Partial least squares (PLS) analysis was used to investigate the causal relationships between ACEs and NLEs, immunological profiles, and the physiosomatic symptoms of depression. The output variable was a latent vector derived from the diverse symptom domains, and ACE + NLEs and immune profiles as explanatory variables. In addition, the immune profiles were allowed to mediate the effects of ACE + NLEs on the clinical assessment scores. Only when both the external and internal models met the predetermined quality criteria was a comprehensive partial least squares (PLS) analysis conducted. These quality criteria are: (a) Confirmatory tetrad analysis (CTA) verifies that the latent vectors derived from the indicators have not been incorrectly specified as reflective models. (b) The blindfolding procedure reveals that the cross-validated redundancy of the construct is adequate. (c) The latent vectors exhibit strong construct and convergence validity, as indicated by composite reliability values greater than 0.80, Cronbach's alpha values greater than 0.70, and average variance extracted (AVE) values greater than 0.5. (d) At a significance level of p < 0.001, all loadings on the extracted latent vectors exceed 0.65. (e) The model fit is regarded satisfactory if the standardized root squared residual (SRMR) is less than 0.08. Consequently, a thorough pathway analysis is conducted using PLS-Structural Equation Modeling (PLS-SEM). In the analysis, SmartPLS software and 5,000 bootstrap samples were utilized. Path coefficients and their respective p values were calculated. In addition, specific indirect effects, total indirect effects (mediated effects), and total effects were calculated if the model quality data met the specified conditions. The estimated minimum sample size is 103 based on a power analysis (conducted with G*Power 3.1.9.4) using a linear multiple regression analysis with an estimated effect size of 0.111 (corresponding to approximately 10% of the variance explained), a significance level (alpha) of 0.05, and statistical power of 0.8, with three covariates.
Ethical approval and consent to participate
The College of Medical Technology at The Islamic University of Najaf, Iraq (18/2021) approved the research project. Our IRB follows the International Guideline for Human Research Safety, as well as the World Medical Association Declaration of Helsinki, The Belmont Report, the CIOMS Guideline, and the International Conference on Harmonization of Good Clinical Practice, and our study was conducted in accordance with all applicable Iraqi and international ethics and privacy laws. (ICH-GCP).
Informed consent statement
All participants and their parents or legal guardians signed a written consent form.
Results
Socio-demographic and clinical data
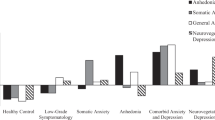
Table 1 shows the sociodemographic and clinical data of the participants (controls and MDMD patients) divided into three groups using a PC extracted from 6 symptom domains (pure_depression, pure_anxiety, physiosomatic, insomnia, melancholia, and gastro-intestinal symptoms), and the major immune profiles (M1, Th-1, IRS, CIRS) and cytokines/chemokines (IL-16, TRAIL, CLC27, SCGF, M-CSF) (labeled: PC_immune + phenome) that differentiate controls from patients. We were able to extract one validated PC from these 15 variables (KMO = 0.839, Bartlett’s test of sphericity = 1155.581, df = 36, p < 0.001, EV = 61.5, all loading of the 15 variables are > 0.694). Consequently, using a visual binning method the PC score was divided in three groups using − 0.606 and + 0.608. As such, the MDMD group was divided into two subgroups namely severe versus less severe MDMD and these were compared with healthy controls. Table 1 shows the mean values of the PC_immune + phenome scores. There was a strong association between these groups and the clinical diagnosis (controls versus MDMD patients): the PC-derived class with a PC score < − 0.606 were the 32 controls, while the patients were divided into those with severe symptoms and immune aberrations (labeled “severe MDMD”) versus milder symptoms and immune disorders (“milder MDMD”).
Table 1 shows that there were no significant differences in age, sex, education, marital status, BMI, and previous COVID-19 infection between the three study groups. Pure_depression, pure_anxiety, pure_FF, and insomnia scores were higher in patients than controls, while physiosomatic, melancholia, and GIS scores were different between the three groups and increased from controls to milder MDMD to severe MDMD. Autonomic symptoms were significantly increased in the latter group as compared to controls. There were no significant differences in the use of fluoxetine (20–40 mg per day), amitriptyline (150–200 mg per day), escitalopram (10–20 mg per day), olanzapine (5–15 mg per day), and mirtazapine (15–30 mg per day just before sleep) between the two depression study groups. There were twenty-six drug-naïve patients (never taken any antidepressant) and the medicated/unmedicated ratio was not significantly different between both depression subgroups. Moreover, there were no significant differences in the associations between physiosomatic symptoms and immune profiles between drug-naïve patients and those using antidepressants. As a consequence, we have combined these two subgroups.
Immune profiles and cytokine/chemokine/growth factor measurements
Table 2 shows the measurements of the immune profiles in controls versus both depression subgroups. Classical M1, Th-1, Th-2, IRS, CIRS and the Immu-NT profiles differed significantly between the three subgroups and increased from controls to the milder MDMD group to severe MDMD. There were no differences in the zM1–zM2 and zIRS–zCIRS scores between the three groups, whereas the zTh-1–zTh-2 score was higher in the milder MDMD group than in controls. The alternative M2 profile was significantly higher in MDMD patients than in controls, while Th-17 was significantly increased in the severe MDMD group as compared with controls and the milder MDMD group.
ESF, Fig. 1 shows the mean (SE) z values of all cytokine levels (z values with the mean value of controls set at 0) in both the less severe and severe MDMD groups. sIL-1RA (p < 0.001), sIL-2R (p < 0.001), IL-6 (p = 0.002), IL-9 (p < 0.001), IL-16 (p < 0.001), IL-18 (p < 0.001), TNF-α (p = 0.001), TNF-β (p < 0.001), and TRAIL (p < 0.001) were significantly higher in MDMD than in controls. There was a trend towards lowered CCL3 in MDMD patients as compared with controls (p = 0.306). ESF, Fig. 2 shows the mean (SE) z values of all chemokines/growth factor levels (z values with the mean value of controls set at 0) in the milder and severe MDMD groups. The following chemokines/growth factors were significantly higher in patients than controls: CCL2 (p < 0.001), CCL4 (p < 0.001), CCL5 (p < 0.001), CCL11 (p < 0.001), CCL27 (p < 0.001), CXCL1 (p < 0.001), CXCL9 (p < 0.001), CXCL10 (p < 0.001), M-CSF (p < 0.001), GM-CSF (p = 0.012), SCF (p < 0.001), SCGF (p < 0.001), SDF1 (p < 0.001), NGF (p = 0.011), PDGF (p < 0.001), and HGF (p < 0.001). All significant p values remained significant using a FDR of 0.05.
ESF, Fig. 1 shows that IL-1β (p = 0.043), sIL-2R (p < 0.001), IL-4 (p < 0.001), IL-6 (p = 0.023), IL-16 (p = 0.001), IL-17 (p = 0.005), IL-18 (p = 0.029), MIF (p = 0.004), and TRAIL (p = 0.018) were significantly higher in severe MDMD than in less severe MDMD. We found that CCL2 (p = 0.013), CCL7 (p = 0.036), CCL11 (p = 0.012), CCL27 (p < 0.001), CXCL9 (p = 0.007), M-CSF (p = 0.006), SCF (p = 0.001), SCGF (p = 0.006), and FGF (p = 0.039) were significantly higher in severe MDMD than in milder MDMD. However, after p-correction using a FDR of p = 0.05, IL-1β, IL-6, IL-18, CCL7 and FGF were no longer significant.
Intercorrelation matrix
Table 3 shows the correlation between the major symptom domains and the immune profiles. There were no significant associations between any of the immune profiles and autonomic symptoms. The zM1–zM2 and zIRS–zCIRS scores were not correlated with all symptom domains. The M1 classical, Th-1, IRS, CIRS, and Immu-NT profiles were significantly correlated with all symptom domains (except autonomic symptoms). The alternative M2 profile was associated with all symptom domains, except pure FF, GIS, and autonomic symptoms. Th-2 was significantly correlated with pure_depression, pure_anxiety, melancholia, and insomnia. Th-17 was associated with all symptom domains except pure_FF and autonomic symptoms. The zTh-1–zTh-2 profile was significantly correlated with all domains, except melancholia, insomnia, and autonomic symptoms.
Results of multiple regression analysis
Table 4 shows the results of different multiple regression analyses with the symptom domains as dependent variables and the cytokines/chemokines/growth factors as dependent variables, while allowing for the effects of age, sex, smoking, education, and BMI. Since we found that multiple CIRS cytokines were significant in the final regression analysis we also entered the CIRS score as an additional explanatory variable. Regression #1 shows that 48.9% of the variance in the pure_depression score was explained by IL-16 and SCGF (both positively) and CCL3 (inversely). Regression #2 shows that 45.4% of the variance in the pure_anxiety score was explained by IL-16 and IL-17 (both positively) and CCL3 (inversely). Regression #3 shows that 59.0% of the variance in physiosomatic symptoms was explained by IL-16, IL-8, and male sex (all positively associated) and CCL3 and sIL-1RA (both inversely). Figures 1 and 2 show the partial regression of the physiosomatic symptom domain on IL-16 and CCL3, respectively. We found that 46.5% of the variance in the pure_FF score was explained by IL-16 and TRAIL (positively) and inversely by the CIRS score (regression #4). We found that 53.2% of the variance in melancholia was explained by the regression on IL-16 and SCGF (both positively) and CCL3 (inversely). A large part of the variance in GIS was predicted by IL-16, CCL1, TRAIL, and IL-8 (all positively) and CCL3 (inversely). Up to 54.1% of the variance in the insomnia score was predicted by IL-16 and SCGF (both positively) and sIL-1RA (inversely). Only a small part of the variance in autonomic symptoms was explained by TRAIL.
Effects of ACEs and NLEs on the phenome are mediated by immune aberrations.
Since there are associations between ACE + NLEs and the immune profiles as well as symptom domains33, we also examined whether the regression of the symptoms domains on the immune products was influenced by ACE + NLEs. Table 5 shows the results of multiple regression analyses which included the significant effects of ACE + NLEs on the phenome data. Thus, the ACE + NLEs score had a significant effect on all symptom domains, except autonomic symptoms. The results of this Table also show that cytokines/chemokines/growth factors had significant effects on the symptom domains beyond the effects of ACE + NLEs. In all cases, the explained variances were significantly enhanced by entering the ACE + NLEs values with around 3% (variance explained), except for GIS (no significant improvement in explained variance).
Results of PLS analysis
The first PLS model conceptualized the phenome of depression as a latent vector extracted from physiosomatic and FF symptoms, as well as pure_depression, pure_anxiety, GIS symptoms, melancholia, and insomnia. The ACEs + NLEs index was composed as a formative model. Based on the results of the multiple regression and correlation analyses, we entered the most significant cytokines/chemokines/growth factors predicting the phenome, and in addition included the effects of ACE + NLEs on the immune variables (together with age and sex). The composite reliability (0.956), Cronbach’s alpha (0.945) and AVE (0.759) of the phenome latent vector were more than adequate, as was the model quality fit with an SRMR of 0.047. All Q2 predicted values for the manifest and latent variables were positive using PLSpredict, indicating that the model outperforms the naivest benchmark as shown in Fig. 3. We observed that the phenome of depression was best predicted by ACE + NLEs, IL-16, TRAIL (all positively), and sIL-1RA and CCL3 (both inversely). The latter five predictors explained 69.6% of the variance in the phenome of depression. In addition, part of the variance in IL-16 (26.8%), TRAIL (19.4%) and sIL-1RA (6.4%) was explained by ACE + NLEs. Age was associated with CCL3 and sex with TRAIL. As such, there were significant specific indirect effects of ACE + NLEs on the phenome which were partly mediated by IL-16 (t = 4.40, p < 0.001) and TRAIL (t = 2.16, p = 0.040). The direct and total indirect (t = 5.99, p < 0.001) effects of ACE + NLEs on the phenome explain the strong total effects of ACE + NLEs on the phenome (t = 13.21, p < 0.001).
Results of Partial Least Squares analysis. The phenome of depression was conceptualized as a latent vector extracted from physiosomatic (Ph..som) and Fibro-fatigue (FF) symptoms, as well as pure depression (P-dep), pure anxiety (P-anx), gastro-intestinal (GIS) symptoms, melancholia (Melanch), and insomnia symptoms. Adverse childhood experiences + negative life events (ACEs + NLEs), interleukin (IL)-16, TNF-related apoptosis-inducing ligand (TRAIL), soluble IL-1 receptor antagonist (sIL-1RA) and CCL3 predict 69.6% of the variance in the physio-affective phenome of depression. Part of the variance in IL-16 (26.8%), TRAIL (19.4%) and sIL-1RA (6.4%) is explained by ACE + NLEs. Shown are path coefficients (with exact pvalues), loadings (with p values) or weights (with p values). Figures in blue circles: variance explained.
Consequently, we have constructed a second PLS model by integrating different cytokines/chemokine/growth factors into one latent vector, labeled “immune network,” including IL-16, TRAIL, CCL27, M-CSF, and SCGF. This factor showed adequate convergence and construct validity as indicated by an AVE value of 0.736, composite reliability of 0.933, and Cronbach’s alpha of 0.910. Moreover, the overall model fit was adequate with an SRMR value of 0.043. We found that 68.8% of the variance in the phenome of depression was explained by this immune network (positively associated) and CCL3 (inversely associated). ACE + NLEs had significant effects on the phenome, comprising direct effects (see Fig. 4) as well as specific indirect effects (t = 6.55, p < 0.001) which were partially mediated by the immune network. Both effects of ACE + NLEs culminated in strong total effects (t = 13.41, p < 0.001). On the other hand, there was no significant effect of age on the phenome (t = 1.62, p = 0.106).
Results of partial least squares analysis. The phenome of depression was conceptualized as a latent vector extracted from physiosomatic (Ph..som) and Fibro-fatigue (FF) symptoms, as well as pure depression (P-dep), pure anxiety (P-anx), gastro-intestinal (GIS) symptoms, melancholia (Melanch), and insomnia. Adverse childhood experiences + negative life events (ACEs + NLEs), CCL3, and a factor extracted from 5 cytokines/chemokines/growth factors (labeled: immune network) explained 68.6% of the variance in the physio-affective phenome. IL-16: interleukin (IL)-16, TRAIL: TNF-related apoptosis-inducing ligand, M-CSF: macrophage colony stimulating factor, SCGF: stem cell growth factor. Shown are path coefficients (p values) and loadings (p values) or weights (p values). Figures in blue circles: variance explained.
Discussion
The physio-affective phenome of depression
The first major discovery of this study indicates that within MDMD patients, the physiosomatic, FF, and GIS symptom domains are associated with the same factor as pure depression, pure anxiety, melancholia symptoms, and insomnia. This suggests that the various symptom domains are closely interconnected expressions of the same latent construct, specifically the phenome of depression. Previous studies have demonstrated that physiosomatic symptoms are associated with the same phenome factor as pure depressive and anxiety symptoms7,46. To emphasize the influence of interconnected affective and physiosomatic symptoms on the manifestation of MDD, this construct has been renamed as the physio-affective phenome7,47. Furthermore, various medical and neuro-psychiatric disorders exhibit a latent construct encompassing symptoms of depression, anxiety, and physiosomatic manifestations. This phenomenon can be observed in conditions such as acute COVID-19 infection48, Long COVID49, relapsing–remitting multiple sclerosis (RRMS)50, schizophrenia51, and epilepsy52. Furthermore, FF symptoms belong to the same latent construct which loads highly on affective and physiosomatic symptoms. The aforementioned interconnected relationships among FF symptoms and the phenome of depression were previously identified in the context of MDD7, acute COVID-19 infection53, RRMS50, Long COVID54, schizophrenia55, and end-stage renal disease56.
The interrelationship between affective, physiosomatic, and FF symptoms is well-established, indicating that these symptoms are interconnected and should not be considered distinct phenomena. This implies that major depressive disorder (MDD), bipolar disorder, and various psychiatric disorders exhibit a physio-affective phenome. These findings also suggest that the physiosomatic, FF and GIS symptom domains, as well as pure depression and anxiety, melancholia, and insomnia, are associated with similar underlying mechanisms that involve both central and peripheral aberrations. This observation suggests that MDMD is a condition that affects the entire body and that affective symptoms are merely one component of the comprehensive clinical presentation.
The physio-affective phenome is predicted by immune activation
The results of the present study show that physiosomatic and FF symptoms of MDMD are best predicted by a) IRS, Th-1 activation, increased Immu-NT, Th-1 polarization, and M1 activation; b) a combination of increased IRS products (especially IL-16, IL-8 or TRAIL), lowered CIRS components (sIL-1RA), and lowered levels of a chemokine (CCL3), and c) an immune network comprising IL-16, TRAIL, CCL27, M-CSF and SCGF (all positively) and CCL3 (inversely). These results indicate that T cell activation (IL-16, Th-1 polarization) is a significant factor in the development of physiosomatic, FF, and GIS symptoms.
IL-16 is elevated in major depressive disorder57 and is a crucial cytokine in FE-MDMD24. IL-16 and other proinflammatory cytokines are associated with elevated depression and neuroticism scores in hepatitis C patients receiving cytokine-based immunotherapy58.59,60. IL-16 induces T cell signaling via the CD4 molecule on Th cells, resulting in the upregulation of activation markers such as HLA-DR + and CD25 + cells. Prenatal depression is associated with elevated IL-16 levels in the prefrontal cortex and hippocampus in animal models61 and contributes to neuroinflammation in animal models of autoimmune encephalomyelitis by activating T cells62. CCL27 or CTACK (cutaneous T cell-attracting chemokine) is a pro-inflammatory chemokine that attracts memory T cells (T lymphocyte-associated antigen) and this chemokine is essential for T cell-mediated cutaneous inflammation63,64. Such interactions may explain the co-occurrence of depression and "psychocutaneous disorders" like psoriasis, which is accompanied by elevated CCL27 levels65. Moreover, CCL27 is a key regulator of immune homeostasis in mucosal tissues and the epidermis66, and CCL27 KO-mice exhibit CR10 + T cell infiltration in the reproductive tract and lungs66.
Another cytokine that is strongly associated with physiosomatic and FF symptoms is TRAIL. TRAIL signals apoptosis by binding to death receptors and activating the caspase pathway67. This cytokine has strong neurotoxic effects, even leading to neurodegeneration and neuronal death, mediated by the TRAIL-R2/DR5 receptor and caspase activation68. Recent evidence suggests, however, that TRAIL may modulate the IRS and T cell activation and shows anti-inflammatory effects67. TRAIL inhibits the activation of T cells, indicating that this cytokine functions as an activator of an anti-inflammatory pathway independent of apoptosis.
Many patients with MDD exhibit T cell activation, as measured by flow cytometry and T cell activation markers including HLA-DR+ and CD7+ CD25+ T cells69,70. We have argued, based on these and other findings, that cell-mediated activation, and specifically T cell activation, is a key factor in MDD37. CD3+ CD71+, CD3+ HLADR+, and CD4+ CD71+ T cell phenotypes have been demonstrated to differentiate MDMD from SDMD71. In addition, the numbers of CD3+ CD40L, CD4+ CD40L, CD4+ HLADR+, and CD8+ CD40L+ cells are substantially higher in MDMD than in healthy controls, whereas SDMD patients occupy an intermediate position71.
Importantly, physiosomatic and FF symptoms are strongly associated with Th-1 cytokine profiles, but not with Th-2 profiles. Th-2 cytokines (such as IL-4) have negative immune-regulatory and anti-inflammatory effects and are essential for CIRS activity. As a result, a relative decrease in Th-2 functions may result in attenuated immunoregulatory Th-2 effects, thereby enhancing the deleterious effects of Th-1 and IRS activation. Similarly, our results demonstrate that decreased CIRS activity plays a crucial role in FF symptoms. Even more significant is the fact that relative decreases in sIL-1RA levels (another component of CIRS) contribute to physiosomatic symptoms and the physio-affective phenome of depression. There is evidence that both MDD and MDMD are associated with elevated sIL-1RA serum/plasma concentrations24,72. Maes and Carvalho (2018) report that soluble sIL-RA may inhibit both IL-1α and IL-1β signaling, thereby exerting potent anti-inflammatory effects. Recent findings indicate that the phenome of MDMD is significantly predicted by activated T and B cells, whereas T regulatory cells (such as CD25 + FoxP3 + GARP +) are inversely associated with the phenome71. Based on the IRS/CIRS theory of depression73, we may conclude that increased Th-1 (IL-16), TRAIL and M1 activities, and relative deficiencies in CIRS (including Th-2 cytokines and sIL-1RA) are major contributors to physiosomatic and FF symptoms.
The analyses that we conducted in the current study suggest that a decrease in CCL3 may contribute to the severity of physiosomatic and FF symptoms, which is another potentially significant finding. CCL3 (or macrophage inflammatory protein) is a pro-inflammatory chemokine that recruits activated immune cells to inflammatory sites and increases the expression of pro-inflammatory M1 cytokines including IL-1β, TNF-α, and IL-674. Previously, we found that whole blood-stimulated CCL3 production in the culture supernatant of patients with MDMD was significantly lower than that of healthy controls75. In addition, CCL3 was one of the few cytokines/chemokines whose serum concentrations were lower in the serum of MDMD patients compared with controls24. Consequently, we can hypothesize that relative decreases in CCL3 secretion may play a role in the depression phenome. CCL3 mediates fever independent of prostaglandin (PGE2) and elevates PGE2 in cerebrospinal fluid76,77,78. Endogenous PGE2 possesses context-dependent anti-inflammatory properties and contributes to the resolution of inflammation79,80. In addition, CCL3 has neuroprotective properties, such as protecting dopaminergic neurons, and influencing traumatic brain injuries81.
Effects of ACE and NLEs on the phenome are mediated by the immune network
The third major finding is that the combined independent effects of immune aberrations and ACE + NLEs significantly predict the physiosomatic and FF symptoms of MDMD, and the effects of ACE + NLEs on the phenome are partially mediated by increased IL-16 and TRAIL, whereas ACE-activation of sIL-1RA levels may have protective effects. Our second PLS analysis revealed that ACE + NLEs exert a substantial influence on an immune network composed of IL-16, CCL27, TRAIL, MCSF, and SCGF, which partially mediates the effects of ACE + NLEs on the phenome, including physiosomatic and FF symptoms. SCGF (CLECC11a) activates different hematopoietic progenitor cells and activates cell signaling networks including glycogen synthase kinase 3, β-catenin and Wnt pathways, which play a major role in MDD, physiosomatic and FF symptoms2,82. M-CSF is a macrophage differentiation factor that is essential for microglial activation and neuroinflammation83,84.
Together, ACEs and NLEs appear to induce a pro-inflammatory network composed of cytokines, chemokines, and growth factors. These findings extend those of a previous paper31, which demonstrated that ACEs elicit a network of cytokines/chemokines/growth factors measured in the culture supernatant of stimulated whole blood from MDMD patients. In fact, both the present investigation (conducted on Iraqi patients) and our previous report (conducted on Thai patients)2 demonstrate that ACEs activate M1, Th-1, Th-17, IRS, and CIRS profiles and significantly increase Immu-NT. In addition, ACEs were associated with elevated levels of sIL-1RA, IL-9, IL-12p70, PDGF, and TNF-α in both studies.
Maes et al.2 demonstrated that ACEs induce stimulated but not unstimulated levels of cytokines/chemokines/growth factors, suggesting that the latter factors are sensitized in MDMD patients. Future stressors could then reactivate these sensitized networks, according to the hypothesis. Since NLEs increase the serum levels of IL-16, CXCL12, M-CSF, SCGF, TRAIL, IL-18, PDGF, sIL-2R, sIL-1RA, SCF, and IL-9, and ACE + NLEs have a greater effect on CXCL12 and M-CSF than ACEs, we can conclude that NLEs may indeed reactivate the sensitized ACE-induced immune responses. However, ACE + NLEs also had significant direct effects on the MDMD phenome, indicating the involvement of other pathways, such as gut-brain pathways, gut-dysbiosis, oxidative stress pathways, and decreased antioxidants and neurotrophic protection4,16,29,71.
Limitations
This paper might have been more intriguing had we assessed additional significant biomarkers of MDD, such as oxidative and nitrosative stress, which are linked to IRS activation1. An additional concern pertains to the utilization of a machine-learning-derived classification for MDMD, a more severe subtype of the disorder. Consequently, the generalizability of the results from this investigation to moderate subtype of depression (SDMD) or the overarching group MDD1,12,18 may be limited. Moreover, it is imperative that our findings be replicated in diverse nations and cultures.
Conclusions
The findings of this study indicate that physiosomatic, FF and GIS symptoms are strongly associated with depression, anxiety, melancholia, and insomnia domains of MDMD. The first factor derived from these six different domains is designated as the physio-affective phenome of depression. A larger part of the variances in the physio-affective phenome, and the physiosomatic FF and GIS symptom domains is explained by immune variables. Specifically, IL-16, TRAIL and IL-8 have a positive correlation with these symptoms, while CCL3 and sIL-1RA exhibit an inverse correlation. The application of PLS analysis reveals that the combination of ACEs and NLEs has a significant impact on the physio-affective phenome. This influence is partially mediated by an immune network consisting of interleukin-16, CCL27, TRAIL, M-CSF, and SCGF. Overall, the physiosomatic and FF symptoms of FE-MDMD are attributed, in part, to Immu-NT linked to Th-1 polarization and M1 macrophage activation, as well as a decrease in CIRS protection. IL-16, Th-1 polarization, M1 activation and Immu-NT are new drug targets to treat the physiosomatic and FF symptoms of MDMD, and to desensitize the upregulated cytokine network and deprogram the detrimental effects of ACEs and NLEs.
Data availability
The corresponding author (MM) will make the SPSS file used in the current study available upon receipt of an appropriate request and once the author has fully exploited the data.
References
Maes, M. et al. Towards a new model and classification of mood disorders based on risk resilience, neuro-affective toxicity, staging, and phenome features using the nomothetic network psychiatry approach. Metab. Brain Dis. 36, 509–521. https://doi.org/10.1007/s11011-020-00656-6 (2021).
Maes, M. et al. The immune profile of major dysmood disorder: Proof of concept and mechanism using the precision nomothetic psychiatry approach. Cells https://doi.org/10.3390/cells11071183 (2022).
Maes, M. & Almulla, A. F. Research and diagnostic algorithmic rules (RADAR) and RADAR plots for the first episode of major depressive disorder: Effects of childhood and recent adverse experiences on suicidal behaviors, neurocognition and phenome features. Brain Sci. 13, 714 (2023).
Maes, M. et al. Development of a novel staging model for affective disorders using partial least squares bootstrapping: Effects of lipid-associated antioxidant defenses and neuro-oxidative stress. Mol. Neurobiol. 56, 6626–6644. https://doi.org/10.1007/s12035-019-1552-z (2019).
Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56 (1960).
Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 50(1), 48–63 (1959).
Al-Hakeim, H. K., Al-Naqeeb, T. H., Almulla, A. F. & Maes, M. The physio-affective phenome of major depression is strongly associated with biomarkers of astroglial and neuronal projection toxicity which in turn are associated with peripheral inflammation, insulin resistance and lowered calcium. J. Affect. Disord. 331, 300–312. https://doi.org/10.1016/j.jad.2023.03.072 (2023).
Anderson, G., Maes, M. & Berk, M. Biological underpinnings of the commonalities in depression, somatization, and chronic fatigue syndrome. Med. Hypotheses 78, 752–756. https://doi.org/10.1016/j.mehy.2012.02.023 (2012).
Al-Hakeim, H. K., Hadi, H. H., Jawad, G. A. & Maes, M. Intersections between copper, β-arrestin-1, calcium, FBXW7, CD17, insulin resistance and atherogenicity mediate depression and anxiety due to type 2 diabetes mellitus: A nomothetic network approach. J Pers Med. https://doi.org/10.3390/jpm12010023 (2022).
Maes, M. et al. In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatr. Scand. 127, 344–354. https://doi.org/10.1111/j.1600-0447.2012.01908.x (2013).
Maes, M. et al. IgM-mediated autoimmune responses directed against anchorage epitopes are greater in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) than in major depression. Metab. Brain Dis. 27, 415–423. https://doi.org/10.1007/s11011-012-9316-8 (2012).
Maes, M., Moraes, J. B., Congio, A., Vargas, H. & Nunes, S. Research and diagnostic algorithmic rules (RADAR) for mood disorders, recurrence of illness, suicidal behaviours, and the patient’s lifetime trajectory. Acta Neuropsychiatr. 35, 104–117. https://doi.org/10.1017/neu.2022.31 (2023).
Zachrisson, O., Regland, B., Jahreskog, M., Kron, M. & Gottfries, C. G. A rating scale for fibromyalgia and chronic fatigue syndrome (the FibroFatigue scale). J. Psychosom. Res. 52, 501–509. https://doi.org/10.1016/s0022-3999(01)00315-4 (2002).
Morris, G. et al. Myalgic encephalomyelitis/chronic fatigue syndrome: From pathophysiological insights to novel therapeutic opportunities. Pharmacol Res 148, 104450. https://doi.org/10.1016/j.phrs.2019.104450 (2019).
Maes, M. et al. In myalgic encephalomyelitis/chronic fatigue syndrome, increased autoimmune activity against 5-HT is associated with immuno-inflammatory pathways and bacterial translocation. J. Affect. Disord. 150, 223–230. https://doi.org/10.1016/j.jad.2013.03.029 (2013).
Maes, M. et al. Adverse childhood experiences and reoccurrence of illness impact the gut microbiome, which affects suicidal behaviors and the phenome of major depression: Towards enterotypic-phenotypes. Acta Neuropsychiatr. https://doi.org/10.1017/neu.2023.21 (2023).
Simeonova, D., Stoyanov, D., Leunis, J. C., Murdjeva, M. & Maes, M. Construction of a nitro-oxidative stress-driven, mechanistic model of mood disorders: A nomothetic network approach. Nitric Oxide 106, 45–54. https://doi.org/10.1016/j.niox.2020.11.001 (2021).
Maes, M. Precision nomothetic medicine in depression research: A new depression model, and new endophenotype classes and pathway phenotypes, and a digital self. J. Pers. Med. https://doi.org/10.3390/jpm12030403 (2022).
Köhler, C. A. et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 135, 373–387. https://doi.org/10.1111/acps.12698 (2017).
Jayakumar, S. et al. A systematic review and meta-analysis of the evidence on inflammation in depressive illness and symptoms in chronic and end-stage kidney disease. Psychol. Med. 53, 5839–5851. https://doi.org/10.1017/s0033291722003099 (2023).
Colasanto, M., Madigan, S. & Korczak, D. J. Depression and inflammation among children and adolescents: A meta-analysis. J. Affect. Disord. 277, 940–948. https://doi.org/10.1016/j.jad.2020.09.025 (2020).
Smith, K. J., Au, B., Ollis, L. & Schmitz, N. The association between C-reactive protein, Interleukin-6 and depression among older adults in the community: A systematic review and meta-analysis. Exp. Gerontol. 102, 109–132. https://doi.org/10.1016/j.exger.2017.12.005 (2018).
Leighton, S. P. et al. Chemokines in depression in health and in inflammatory illness: A systematic review and meta-analysis. Mol. Psychiatry 23, 48–58. https://doi.org/10.1038/mp.2017.205 (2018).
Almulla, A. F., Abbas Abo Algon, A., Tunvirachaisakul, C., Al-Hakeim, H. K. & Maes, M. T helper-1 activation via interleukin-16 is a key phenomenon in the acute phase of severe, first-episode major depressive disorder and suicidal behaviors. J. Adv. Res. https://doi.org/10.1016/j.jare.2023.11.012 (2013).
Agnew-Blais, J. & Danese, A. Childhood maltreatment and unfavourable clinical outcomes in bipolar disorder: A systematic review and meta-analysis. Lancet Psychiatry 3, 342–349. https://doi.org/10.1016/S2215-0366(15)00544-1 (2016).
Paykel, E. S., Emms, E. M., Fletcher, J. & Rassaby, E. S. Life events and social support in puerperal depression. Br. J. Psychiatry 136, 339–346. https://doi.org/10.1192/bjp.136.4.339 (1980).
Kendler, K. S., Karkowski, L. M. & Prescott, C. A. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 156, 837–841 (1999).
Kraaij, V., Arensman, E. & Spinhoven, P. Negative life events and depression in elderly persons: A meta-analysis. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 57, P87–P94 (2002).
Maes, M. et al. Early life trauma predicts affective phenomenology and the effects are partly mediated by staging coupled with lowered lipid-associated antioxidant defences. Biomol. Concepts 9, 115–130 (2018).
Abe, Y., Sirichokchatchawan, W., Sangkomkamhang, U., Satthapisit, S. & Maes, M. Adverse childhood experiences combined with emotional and physical abuse by the partner predict antenatal depression. J. Affect. Disord. 298, 194–201. https://doi.org/10.1016/j.jad.2021.10.099 (2022).
Maes, M. et al. Adverse childhood experiences predict the phenome of affective disorders and these effects are mediated by staging, neuroimmunotoxic and growth factor profiles. Cells 11, 1564 (2022).
Maes, M. & Almulla, A. F. Research and Diagnostic Algorithmic Rules (RADAR) and RADAR plots for the first episode of major depressive disorder: Effects of childhood and recent adverse experiences on suicidal behaviors, neurocognition and phenome features. medRxiv. https://doi.org/10.1101/2022.12.18.22283606 (2022).
Almulla, A. F., Ali Abbas Abo, A. & Michael, M. Adverse childhood experiences and recent negative events activate immune and growth factor pathways, which are associated with first episode major depression and suicidal behaviours. medRxiv. https://doi.org/10.1101/2023.06.19.23291597 (2023b).
Maes, M., Anderson, G., Kubera, M. & Berk, M. Targeting classical IL-6 signalling or IL-6 trans-signalling in depression?. Expert Opinion Therap. Targets 18, 495–512. https://doi.org/10.1517/14728222.2014.888417 (2014).
Anderson, G., Berk, M., Dean, O., Moylan, S. & Maes, M. Role of immune-inflammatory and oxidative and nitrosative stress pathways in the etiology of depression: Therapeutic implications. CNS Drugs 28, 1–10. https://doi.org/10.1007/s40263-013-0119-1 (2014).
Maes, M., Mihaylova, I., Kubera, M. & Ringel, K. Activation of cell-mediated immunity in depression: Association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Progress Neuro Psychopharmacol. Biol. Psychiatry 36, 169–175 (2012).
Maes, M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Progress Neuro Psychopharmacol. Biol. Psychiatry 35, 664–675 (2011).
Morris, G. & Maes, M. Increased nuclear factor-κB and loss of p53 are key mechanisms in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Med Hypotheses 79, 607–613. https://doi.org/10.1016/j.mehy.2012.07.034 (2012).
Morris, G., Berk, M., Galecki, P. & Maes, M. The emerging role of autoimmunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Mol. Neurobiol. 49, 741–756. https://doi.org/10.1007/s12035-013-8553-0 (2014).
American Psychiatric Association, A,. Diagnostic And statistical Manual of Mental Disorders: DSM-5 (American Psychiatric Association, Arlington, 2013).
Posner, K. et al. The Columbia–Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry 168, 1266–1277. https://doi.org/10.1176/appi.ajp.2011.10111704 (2011).
Felitti, V. J. et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 14, 245–258. https://doi.org/10.1016/s0749-3797(98)00017-8 (1998).
Buri, J. Negative life events scale for students (NLESS). Coll. Stud. J. 52, 361–367 (2018).
Rachayon, M. et al. In vitro effects of cannabidiol on activated immune-inflammatory pathways in major depressive patients and healthy controls. Pharmaceuticals (Basel) https://doi.org/10.3390/ph15040405 (2022).
Thisayakorn, P., Thipakorn, Y., Tantavisut, S., Sirivichayakul, S. & Maes, M. Delirium due to hip fracture is associated with activated immune-inflammatory pathways and a reduction in negative immunoregulatory mechanisms. BMC Psychiatry 22, 369. https://doi.org/10.1186/s12888-022-04021-y (2022).
Anderson, G., Berk, M. & Maes, M. Biological phenotypes underpin the physio-somatic symptoms of somatization, depression, and chronic fatigue syndrome. Acta Psychiatr. Scand. 129, 83–97. https://doi.org/10.1111/acps.12182 (2014).
Maes, M. et al. Lowered quality of life in long COVID is predicted by affective symptoms, chronic fatigue syndrome, inflammation and neuroimmunotoxic pathways. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph191610362 (2022).
Al-Jassas, H. K., Al-Hakeim, H. K. & Maes, M. Intersections between pneumonia, lowered oxygen saturation percentage and immune activation mediate depression, anxiety, and chronic fatigue syndrome-like symptoms due to COVID-19: A nomothetic network approach. J. Affect. Disord. 297, 233–245. https://doi.org/10.1016/j.jad.2021.10.039 (2022).
Maes, M. et al. Lowered quality of life in Long COVID is strongly predicted by affective symptoms and chronic fatigue syndrome which are associated with inflammatory processes during the acute infectious phase and consequent neuroimmunotoxic pathways. medRxiv,. https://doi.org/10.1101/2022.07.25.22277980 (2022).
Almulla, A. F. et al. Mood symptoms and chronic fatigue syndrome due to relapsing-remitting multiple sclerosis are associated with immune activation and aberrations in the erythron. Brain Sci. 13, 1073 (2023).
Kanchanatawan, B. et al. In Schizophrenia, depression, anxiety, and physiosomatic symptoms are strongly related to psychotic symptoms and excitation, impairments in episodic memory, and increased production of neurotoxic tryptophan catabolites: A multivariate and machine learning study. Neurotox. Res. 33, 641–655. https://doi.org/10.1007/s12640-018-9868-4 (2018).
Maes, M. et al. Increased oxidative stress toxicity and lowered antioxidant defenses in temporal lobe epilepsy and mesial temporal sclerosis: Associations with psychiatric comorbidities. Mol. Neurobiol. 57, 3334–3348. https://doi.org/10.1007/s12035-020-01949-8 (2020).
Almulla, A. F., Al-Hakeim, H. K. & Maes, M. Chronic fatigue and affective symptoms in acute and long COVID are attributable to immune-inflammatory pathways. Psychiatry Clin. Neurosci. 77, 125–126. https://doi.org/10.1111/pcn.13514 (2023).
Al-Hakeim, H. K., Al-Rubaye, H. T., Al-Hadrawi, D. S., Almulla, A. F. & Maes, M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol. Psychiatry https://doi.org/10.1038/s41380-022-01836-9 (2022).
Maes, M. et al. In schizophrenia, chronic fatigue syndrome- and fibromyalgia-like symptoms are driven by breakdown of the paracellular pathway with increased zonulin and immune activation-associated neurotoxicity. CNS Neurol. Disord. Drug Targets 22, 215–225. https://doi.org/10.2174/1871527321666220806100600 (2023).
Asad, H. N., Al-Hakeim, H. K., Moustafa, S. R. & Maes, M. A causal-pathway phenotype of chronic fatigue syndrome due to hemodialysis in patients with end-stage renal disease. CNS Neurol. Disord. Drug Targets 22, 191–206. https://doi.org/10.2174/1871527321666220401140747 (2023).
Timothy, R. P. et al. Assessing 42 inflammatory markers in 321 control subjects and 887 major depressive disorder cases: BMI and other confounders and overall predictive ability for current depression. bioRxiv, 327239. https://doi.org/10.1101/327239 (2018).
Pawlowski, T. et al. Depression and neuroticism in patients with chronic hepatitis C: Correlation with peripheral blood mononuclear cells activation. J. Clin. Virol. 60, 105–111. https://doi.org/10.1016/j.jcv.2014.03.004 (2014).
Mathy, N. L. et al. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology 100, 63–69. https://doi.org/10.1046/j.1365-2567.2000.00997.x (2000).
Hall, G. et al. Structure of a potential therapeutic antibody bound to interleukin-16 (IL-16): Mechanistic insights and new therapeutic opportunities. J. Biol. Chem. 291, 16840–16848. https://doi.org/10.1074/jbc.M115.709303 (2016).
Posillico, C. K. & Schwarz, J. M. An investigation into the effects of antenatal stressors on the postpartum neuroimmune profile and depressive-like behaviors. Behav. Brain Res. 298, 218–228. https://doi.org/10.1016/j.bbr.2015.11.011 (2016).
Hridi, S. U. et al. Increased Levels of IL-16 in the central nervous system during neuroinflammation are associated with infiltrating immune cells and resident glial cells. Biology 10(6), 472. https://doi.org/10.3390/biology10060472 (2021).
Morales, J. et al. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc. Natl. Acad. Sci. U. S. A. 96, 14470–14475. https://doi.org/10.1073/pnas.96.25.14470 (1999).
Homey, B. et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat. Med. 8, 157–165. https://doi.org/10.1038/nm0202-157 (2002).
Ye, G., Ren, X.-Z., Qi, L., Wang, L. & Zhang, Y. CGRP modulates the pathogenetic process of psoriasis via promoting CCL27 secretion in a MAPK-and NF-κB signaling pathway-dependent manner. Biomed. Res. 28, 6319–6325 (2017).
Davila, M. L. et al. CCL27 is a crucial regulator of immune homeostasis of the skin and mucosal tissues. iScience 25, 104426. https://doi.org/10.1016/j.isci.2022.104426 (2022).
Chyuan, I. T. & Hsu, P. N. TRAIL regulates T cell activation and suppresses inflammation in autoimmune diseases. Cell. Mol. Immunol. 17, 1281–1283. https://doi.org/10.1038/s41423-020-0410-2 (2020).
Di Benedetto, G. et al. TRAIL-R deficient mice are protected from neurotoxic effects of amyloid-β. Int. J. Mol. Sci. https://doi.org/10.3390/ijms231911625 (2022).
Maes, M. et al. Evidence for a systemic immune activation during depression: Results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychol. Med. 22, 45–53. https://doi.org/10.1017/S0033291700032712 (1992).
Maes, M. et al. A significantly increased expression of T cell activation markers in depression: Additional evidence for an inflammatory process during that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 17, 241–255 (1993).
Maes, M., Rachayon, M., Jirakran, K., Sughondhabirom, A. & Sodsai, P. Effects of recurrence of illness and adverse childhood experiences on effector, cytotoxic, and regulatory T cells, and cannabinoid receptor-bearing B cells in major depression, an autoimmune disorder. medRxiv. https://doi.org/10.1101/2023.06.11.23291243 (2023).
Maes, M., Smith, R. & Scharpe, S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology 20(2), 111–116 (Elsevier, Netherlands, 1995).
Maes, M. & Carvalho, A. F. The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol. Neurobiol. 55, 8885–8903. https://doi.org/10.1007/s12035-018-1016-x (2018).
Zhang, C. et al. Regulatory T cells were recruited by CCL3 to promote cryo-injured muscle repair. Immunol. Lett. 204, 29–37. https://doi.org/10.1016/j.imlet.2018.10.004 (2018).
Rachayon, M., Jirakran, K., Sodsai, P., Sughondhabirom, A. & Maes, M. T cell activation via the CD40 ligand and transferrin receptor and deficits in T regulatory cells are associated with major depressive disorder and severity of depression. medRxiv. https://doi.org/10.1101/2023.05.03.23289312 (2023).
Soares, D. M. et al. CCL3/MIP-1α is not involved in the LPS-induced fever and its pyrogenic activity depends on CRF. Brain Res. 1269, 54–60. https://doi.org/10.1016/j.brainres.2009.03.003 (2009).
Soares, D. M., Hiratsuka Veiga Souza, F., Fabrício, A. S., Javier Miñano, F. & Petto-Souza, G. E. CCL3/macrophage inflammatory protein-1alpha induces fever and increases prostaglandin E2 in cerebrospinal fluid of rats: effect of antipyretic drugs. Brain Res. 1109, 83–92. https://doi.org/10.1016/j.brainres.2006.06.026 (2006).
Miñano, F. J. et al. Macrophage inflammatory protein-1: Unique action on the hypothalamus to evoke fever. Brain Res. Bull. 24, 849–852. https://doi.org/10.1016/0361-9230(90)90150-X (1990).
Scher, J. U. & Pillinger, M. H. The anti-inflammatory effects of prostaglandins. J. Investig. Med. 57, 703–708. https://doi.org/10.2310/JIM.0b013e31819aaa76 (2009).
Frolov, A., Yang, L., Dong, H., Hammock, B. D. & Crofford, L. J. Anti-inflammatory properties of prostaglandin E2: Deletion of microsomal prostaglandin E synthase-1 exacerbates non-immune inflammatory arthritis in mice. Prostaglandins Leukot. Essent. Fatty Acids 89, 351–358. https://doi.org/10.1016/j.plefa.2013.08.003 (2013).
Leggio, L. et al. Small extracellular vesicles secreted by nigrostriatal astrocytes rescue cell death and preserve mitochondrial function in Parkinson’s disease. Adv. Healthc. Mater. 11, 2201203. https://doi.org/10.1002/adhm.202201203 (2022).
Maes, M. et al. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates—Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology 20, 127–150. https://doi.org/10.1007/s10787-011-0111-7 (2012).
Smith, A. M. et al. M-CSF increases proliferation and phagocytosis while modulating receptor and transcription factor expression in adult human microglia. J. Neuroinflamm. 10, 85. https://doi.org/10.1186/1742-2094-10-85 (2013).
Ghia, J.-E. et al. Role of M-CSF-dependent macrophages in colitis is driven by the nature of the inflammatory stimulus. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G770–G777 (2008).
Acknowledgements
The authors acknowledge the Al-Hakeem General Hospital workers for their efforts in data collection.
Funding
The C2F program at Chulalongkorn University in Thailand, grant number 64.310/436/2565 to AFA, the Thailand Science Research, and Innovation Fund at Chulalongkorn University (HEA663000016), and a Sompoch Endowment Fund (Faculty of Medicine) MDCU (RA66/016) to MM all provided funding for the project.
Author information
Authors and Affiliations
Contributions
A.F.A. and M.M. designed the study. A.F.A. and P.S. quantified the biomarkers in the blood serum. M.M. conducted the study's statistical analysis. The work is written and edited by M.M., A.A., B.Z., A.A.A. and P.S. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maes, M., Almulla, A.F., Zhou, B. et al. In major dysmood disorder, physiosomatic, chronic fatigue and fibromyalgia symptoms are driven by immune activation and increased immune-associated neurotoxicity. Sci Rep 14, 7344 (2024). https://doi.org/10.1038/s41598-024-57350-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57350-1
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.