Abstract
Data on hemoglobin (Hb) variants in southern Thailand are lacking. This study aimed to reassess the frequency of Hb variants and the clinical aspects of compound heterozygous Hb variant with other hemoglobinopathies. We enrolled 13,391 participants from ten provinces in southern Thailand during 2015–2022. Hb analysis was performed using capillary electrophoresis, and mutations in the HBA and HBB genes were identified using PCR or DNA sequencing. Hb variants were identified in 337 (2.5%) unrelated subjects. Nine β-chain variants, namely Hb Malay (76.9%), Hb C (10.1%), Hb D-Punjab (2.9%), Hb G-Makassar (2.3%), Hb Dhonburi (2.3%), Hb Tak (1.4%), Hb J-Bangkok (1.4%), Hb New York (0.3%), and Hb Hope (0.3%), and four α-chain variants—Hb G-Georgia (HBA1) (0.9%), Hb G-Georgia (HBA2) (0.3%), Hb Q-Thailand (0.6%), and Hb St. Luke’s-Thailand (0.3%)—were identified. The southern population exhibited a distinct spectrum of Hb variants compared to that observed in the populations from other areas. Several compound heterozygous genotypes were also identified. Combining Hb Malay with Hb E or high Hb F determinants did not require a blood transfusion. This study provides essential information for genetic counseling in thalassemia prevention and control programs in this region.
Similar content being viewed by others
Introduction
Hemoglobin (Hb) variants, or abnormal Hb, are hemoglobinopathies resulting from an abnormal structure of the globin chain in the hemoglobin molecule. Several Hb variants, such as Hb E (HBB:c.79G>A) and Hb Malay (HBB:c.59A>G), termed as “thalassemic Hb variants” can lead to reduced Hb variant levels. In Thailand, over 30 types of Hb variants have been reported, with a prevalence rate of 2.4%. However, Hb variants exhibit variations across populations and countries1. The interaction between Hb variants and thalassemia typically manifests as either no or mild clinical phenotypes2,3. Nevertheless, this co-inheritance may result in the misinterpretation of Hb analysis within thalassemia prevention and control programs. For example, certain Hb variants co-migrate within the Hb F zone of capillary electrophoresis (CE), leading to potential misdiagnosis as β-thalassemia disease or high Hb F determinants until molecular diagnosis confirms the specific mutation type4. In addition, the interaction of thalassemic Hb variants with thalassemia can contribute to moderate to severe thalassemia phenotypes, as seen in Hb H with Hb Constant Spring (CS, HBA2:c.427T>C) disease and Hb E/β-thalassemia disease, which are commonly observed in the southeast Asian population5,6. Eight Hb variants in 58 southern populations were previously identified using high-performance liquid chromatography (HPLC) and DNA sequencing7. However, the report did not include Hb Malay, a common Hb variant in southern Thailand and lacked hematological profiles of the combination of Hb Malay with other Hb variants. Up-to-date CE technique is an Hb analysis routinely performed in most laboratories in Thailand. Our center started using this method in 2015. Moreover, there is limited information on the spectrum, prevalence, and clinical phenotypes of Hb variants when co-inherited with other abnormalities in southern Thailand using the CE method. Therefore, this study aimed to reassess the molecular epidemiology of Hb variants and the clinical phenotypes of patients with co-inherited Hb variants and other thalassemia or hemoglobinopathies in the southern population.
Results
Genotypic and phenotypic spectra of Hb variants in the southern Thai population
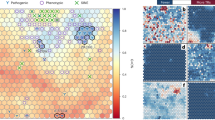
Our reference center received 13,391 samples for thalassemia and hemoglobinopathy diagnoses from 10 provinces in southern Thailand, namely Songkhla, Surat Thani, Nakhon Si Thammarat, Phatthalung, Trang, Phuket, Phangnga, Satun, Yala, and Narathiwat, spanning the period from 2015 to 2022. Routine molecular diagnosis has revealed Hb Malay and Hb Dhonburi mutations and DNA sequencing has revealed 11 distinct Hb variant mutations in 337 (2.5%) unrelated participants, corresponding to 346 chromosomes. The β-chain variant, with nine different mutations, was identified in 338 alleles, and the two most common mutations were Hb Malay (n = 266, 76.9%) and Hb C (HBB:c.19G>A) (n = 35, 10.1%). The remaining seven mutations were Hb D-Punjab (HBB:c.364G>C) (n = 10, 2.9%), Hb G-Makassar (HBB:c.20A>C) (n = 8, 2.3%), Hb Dhonburi (HBB:c.380T>G) (n = 8, 2.3%), Hb Tak (HBB:c.441_442insAC) (n = 5, 1.4%), Hb J-Bangkok (HBB:c.170G>A) (n = 5, 1.4%), Hb New York (HBB:c.341T>A) (n = 1, 0.3%), and Hb Hope (HBB:c.410G>A) (n = 1, 0.3%). In addition, four α-chain variants, namely Hb G-Georgia (HBA1) (HBA1:c.287C>T) (n = 3, 0.9%), Hb G-Georgia (HBA2) (HBA2:c.287C>T) (n = 1, 0.3%), Hb Q-Thailand (HBA1:c.223G>C) (n = 2, 0.6%), and Hb St. Luke’s-Thailand (HBA2:c.287C>G) (n = 1, 0.3%), were identified in seven alleles (Table 1). The distribution of Hb variants among the southern population from ten provinces is shown in Fig. 1. The hematological parameters of 263 (78.0%) subjects with heterozygous Hb variant genotypes are shown in Table 2. Hb analysis showed that Hb Malay and Hb Dhonburi migrated along Hb A (zone 9). Furthermore, three Hb variants—HbTak, Hb Q-Thailand, and Hb G-Georgia—migrated to the Hb F zone (zone 7). Herein, we report Hb G-Georgia (HBA1) for the first time in Thailand (Fig. 2). Hb G-Georgia (HBA1) exhibited lower levels than Hb G-Georgia (HBA2), at 10.3–10.6% vs. 17.0%. Two Hb variants, Hb D-Punjab and Hb St. Luke ’s-Thailand, were migrated in zone 6. Notably, the levels of Hb D-Punjab in a case with compound heterozygous α0-thalassemia/α+-thalassemia (−−/−α) were lower than those in Hb D-Punjab carriers with the normal HBA gene (αα/αα) or heterozygous α+-thalassemia (−α/αα), at 23.6% vs. 32.6–39.3%. Moreover, Hb G-Makassar migrated to the Hb S (HBB:c.20A>T) zone (zone 5). Hb C, Hb Hope, Hb New York, and Hb J-Bangkok were detected in zones 2, 10, 11, and 12, respectively (Fig. 3).
Hematological profiles of patients with homozygous or compound heterozygous Hb variants
The hematological parameters of 61 (18.1%) patients with homozygous or compound heterozygous Hb variant genotypes without blood transfusion are shown in Table 3. The results showed that eight patients with homozygous Hb Malay and 27 patients with compound heterozygous Hb Malay with Hb E displayed mild-to-moderate anemia without a history of blood transfusion. Two patients with Hb Malay and β+-thalassemia (NT-28 (A>G), HBB:c.− 78A>G) exhibited nontransfusion-dependent thalassemia and moderate anemia. In addition, five cases of compound heterozygous Hb Malay with high Hb F determinant mutations, such as HPFH6 (NG_000007.3:g.45595_124872del), δβ0-thalassemia (12.5 kb deletion) (NG_000007.3:g.64383_76994del), Indian del-inv Aγδβ0-thalassemia (NG_000007.3:g.48400_49245del;49246_64567inv;64568_72051del), and Thai del-inv-ins Aγδβ0-thalassemia (NG_000007.3:g.47449_165744del;168412_168590invins;insAAGAAGA), along with one patient with compound heterozygous Hb Malay with β0-thalassemia (3.5 kb deletion, NC_000011.10:g.5224302-5227791del3490bp), exhibited a non-transfusion-dependent thalassemia phenotype. Among nine patients with compound heterozygous Hb C with other hemoglobinopathies, all were asymptomatic or presented mild anemia. This group included six patients with Hb C/Hb E, one patient with Hb C/Hb Malay, and two patients with Hb C/β0-thalassemia (3.5 kb deletion and codon 41 (− C), (HBB:c.126delC)). Interestingly, a patient with compound heterozygous Hb C and β0-thalassemia (3.5 kb deletion) displayed significantly elevated Hb A2 (7.6%) and Hb F (11.6%) levels. In contrast, a patient with compound heterozygous Hb C and β0-thalassemia (codon 41 (− C)) exhibited Hb A2 (3.7%) without detectable Hb F levels. This study reports several Hb variants co-inherited with Hb E in the southern population, including two cases of Hb D-Punjab/Hb E, two cases of Hb G-Makassar/Hb E, one case of Hb J-Bangkok/Hb E, and one case of Hb Hope/Hb E, all of which showed no clinical symptoms. Additionally, two patients with compound heterozygous Hb J-Bangkok with β+-thalassemia [IVS1-5 (G>C), (HBB:c.92 + 5G>C)] exhibiting mild anemia were reported for the first time. Hb analysis of the samples of these patients revealed Hb J-Bangkok in zone 12 (89.5% and 93.0%) and Hb A2 (> 3.5%). Finally, one patient with compound heterozygous Hb Tak and β+-thalassemia (IVS1-5 (G>C)) displayed 5.6% Hb A2 and 91.6% Hb Tak. However, this patient presented with mild anemia without secondary erythrocytosis.
Furthermore, 13 patients with compound heterozygous Hb Malay, harboring various β-thalassemia point mutations [e.g., NT-28 (A>G), codon 17 (A>T) (HBB:c.52A>T), codon 41 (− C), codons 41/42 (− TTCT) (HBB:c.126_129delCTTT), IVS1-1 (G>T) (HBB:c.92 + 1G>T), IVS1-5 (G>C), and IVS2-654 (C>T) (HBB:c.316-197C>T)], were identified as transfusion-dependent thalassemia cases who need regular blood transfusion to manage their clinical complications and survival. The frequency of blood transfusion, clinical history, and hematological profiles in these patients is shown in Table 4.
Table 5 displays a comparative analysis of the spectrum of Hb variants in the population of southern Thailand and the populations from other areas. The findings indicate distinct prevalence patterns of Hb variants across various parts of the country. Hb Malay and Hb C were the predominant variants in the southern population, whereas Hb Hope, Hb Q-Thailand, and Hb J-Bangkok were frequently found in populations from the northern and central regions. The northeastern population presented the prevalence of four common mutations—Hb Q-Thailand, Hb J-Bangkok, Hb Pyrgos (HBB:c.251G>A), and Hb Hope. Moreover, northeastern Thailand populations displayed notable variability in the distribution of Hb variants.
Discussion
This study revisited the molecular spectrum of Hb variants in the southern population across ten provinces through a large-scale survey of specimens received by our center over eight years. Among 13,391 individuals, 337 (2.5%) carried Hb variants, with 263 (78%) identified as Hb variant carriers. The predominant Hb variant was Hb Malay, followed by Hb C. Herein, we report rare Hb variants identified in zone 7, similar to Hb F, including Hb Q-Thailand, Hb Tak, and Hb G-Georgia. To our knowledge, this study is the first to report Hb G-Georgia on the HBA1 gene in Thailand. Hb G-Georgia (HBA1) heterozygote showed lower levels (10.3–10.6%) than Hb G-Georgia (HBA2), 17.0% in a double heterozygote Hb G-Georgia and Hb E in this study and 23.4% in Hb G-Georgia heterozygote reported in a previous study8, which may be explained by approximately 2–3 times lower expression of HBA1 gene than the HBA2 gene9. Interestingly, Hb G-Georgia did not present any Hb A2 variant peak in hemoglobin analysis using the CE technique, observed in both heterozygote Hb G-Georgia and double heterozygote Hb G-Georgia and Hb E. Thus, this could be misconstrued as β+/β+ or β+/β0-thalassemia disease or β+-thalassemia with Hb E disease based on the Hb pattern, reflecting A2FA or EFA. However, the three patients with heterozygous Hb G-Georgia in our study exhibited no clinical symptoms and normal RDW levels. Furthermore, an alkaline denaturation test yielded negative results. Therefore, we performed DNA sequencing to identify this Hb variant. A patient harboring compound heterozygous Hb G-Georgia with α0-thalassemia did not develop Hb H disease4, indicating that Hb G-Georgia is not classified as an α-thalassemia mutation. However, rapid molecular diagnosis is required for proper genetic counseling. Thus, we developed an allele-specific PCR (AS-PCR) for detecting Hb G-Georgia in both HBA1 and HBA2 genes for the first time (Fig. 4). Unlike PCR–RFLP, this technique is simple, rapid, inexpensive, and does not require restriction enzymes4.
Schematic illustrating the primer orientation for the newly developed allele-specific PCR (a). Agarose gel electrophoresis results for the detection of hemoglobin (Hb) G-Georgia (HBA2) (b) and Hb G-Georgia (HBA1) (c). M; 100 bp DNA marker, 1; negative for Hb G-Georgia (HBA2 and HBA1), 2 and 3; positive for Hb G-Georgia (HBA1), 4; positive for Hb G-Georgia (HBA2).
The clinical phenotype of Hb Tak often includes erythrocytosis in patients with compound heterozygous Hb Tak with β-thalassemia, homozygous Hb Tak, and Hb Tak with δβ0-thalassemia10,11,12. However, a patient with Hb Tak and β+-thalassemia (IVS1-5 (G>C)) showed no symptomatic erythrocytosis (Hb 11 g/dL, Hct 37.5%), which might be explained by the underlying disease with an atrial septal defect and failure to thrive.
Thalassemia mutations are common and heterogeneous in southern populations13. We reported the interaction of Hb Malay with other abnormalities resulted in diverse genotypes in 57 (16.9%) patients. The most common genotype was compound heterozygous Hb Malay with Hb E patients (n = 27), manifesting a thalassemia intermedia phenotype without blood transfusion, similar to those reported previously14,15. Accordingly, prenatal diagnosis is deemed unnecessary for couples at risk of developing Hb Malay with Hb E disease to reduce the risk of miscarriage. Nevertheless, postnatal diagnosis and appropriate genetic counseling are imperative. This study showed that Hb Malay with β0-thalassemia, including codon 17 (A>T), codon 41 (− C), codons 41/42 (− TTCT), IVS1-1 (G>T), or β+-thalassemia, including IVS1-5 (G>C) and IVS2-654 (C>T), led to severe anemia, wherein patients required regular blood transfusion. Prenatal diagnosis is thus essential for families with this combination. Conversely, a patient with Hb Malay and β0-thalassemia (3.5 kb deletion) presented with moderate anemia (Hb 9.1 g/dL) without the need for blood transfusion. This milder clinical manifestation could be due to co-inheritance with heterozygous α0-thalassemia, ameliorating clinical severity by balancing the levels of α- and β-globin chains16,17,18,19. Accordingly, this study supports a previous recommendation proposing the inclusion of α0-thalassemia analysis in prenatal diagnosis for fetuses affected with thalassemia disease to make appropriate decisions20. Previous studies reported that β0-thalassemia (3.5 kb deletion) carriers usually exhibit higher Hb A2 and Hb F levels than other β-thalassemia carriers due to point mutations21,22. The positive result in reverse dot blot (RDB) hybridization indicating a homozygous Hb Malay genotype in the Hb Malay with β0-thalassemia (3.5 kb deletion) case, alongside hematological profiles resembling thalassemia intermedia, raises the possibility of misdiagnosis as homozygous Hb Malay. However, the elevated Hb A2 levels (8.7%) compared to those of homozygous Hb Malay (4.5–5.4%) underscored the need for further laboratory investigation into β0-thalassemia (3.5 kb deletion). Subsequently, the true genotype of this patient was found to be Hb Malay with β0-thalassemia (3.5 kb deletion).
Interestingly, a previous study reported that Hb Malay with β+-thalassemia typically manifests as a thalassemia intermedia phenotype without the need for regular blood transfusion15. However, this study reported three cases of Hb Malay with β+-thalassemia (NT-28 (A>G)) exhibiting distinct phenotypes. One patient presented transfusion-dependent thalassemia and splenomegaly; this was potentially influenced by additional abnormalities. Subsequently, gap-PCR was conducted to identify α-globin gene triplication (ααα/αα)23, which, if present, could exacerbate globin chain imbalance and escalate clinical severity24. Despite obtaining a negative result for this patient (data not shown), we propose the application of whole-exome sequencing to comprehensively determine the clinical severity.
In Thailand, the frequency of high Hb F determinants is 1.06%25. The co-occurrence of this abnormality with β-thalassemia can yield diverse clinical phenotypes, ranging from mild to severe anemia, depending on the β-thalassemia genotype25,26,27. However, scant information exists on the clinical phenotypes associated with high Hb F determinants in patients with Hb Malay. We present, for the first time, a case of Hb Malay with Thai del-inv-ins Aγδβ0-thalassemia exhibiting no clinical symptoms. Moreover, combinations of Hb Malay with δβ0-thalassemia (12.5 kb deletion), Indian del-inv Aγδβ0-thalassemia, or HPFH6 also presented only thalassemia intermedia phenotype without the need for blood transfusion. These results suggest that prenatal diagnosis might be unnecessary for couples carrying Hb Malay with high Hb F determinants. However, a postnatal diagnosis should be performed for proper genetic counseling.
Hb J-Bangkok is a β-chain variant occasionally reported in Thailand. A carrier usually presents with normal hematological parameters, with Hb J-Bangkok levels of 44.5 ± 4.7%28. However, we report a case of Hb J-Bangkok carrier with moderate anemia (Hb 7.7 g/dL), potentially affected by an underlying disease but unconfirmed patient-specific condition. Interestingly, we report two cases of Hb J-Bangkok with β+-thalassemia (IVS1-5 (G>C) for the first time. Elevated Hb J-Bangkok levels of 89.5% and 93% in patients with mild anemia (Hb 10.1 and 10.6 g/dL) might suggest the presence of homozygous Hb J-Bangkok. However, this rare variant is infrequently reported in southern populations. Thus, these patients are preferably linked to co-inheritance with β-thalassemia mutation, and molecular diagnosis of β-thalassemia is subsequently performed in these cases.
The levels of Hb E or Hb C in pure Hb E or pure Hb C heterozygotes were higher than those genotypes co-inherited with α0-thalassemia because the α-globin chain prefers to form dimerization with β-globin chain than βE or βC-globin chain. Thus, reduced α-globin chain production in α0-thalassemia contributes to lower Hb E or Hb C levels. However, Hb E levels in compound heterozygous Hb C/Hb E are higher than in Hb E heterozygote. The previous studies reported that the Hb E levels in compound heterozygous Hb C/Hb E could be presented in a wide range from 32.0 to 39.7%14,29. This study reported four cases with compound heterozygous Hb C/Hb E without co-inherited α-thalassemia with Hb E levels ranging from 30.6 to 34.0%, while a compound heterozygous Hb C/Hb E co-inherited α0-thalassemia presented with Hb E levels of 35.6%. Hence, lower levels of Hb E were not observed in a compound heterozygous Hb C/Hb E co-inherited with α0-thalassemia. It might be due to both Hb E and Hb C are positively charged Hb variants, which might have a similar ability to interact with the α-globin chain. Moreover, the decrease of αβC dimer formation leading to an indirect increase in the αβE dimer formation29 as the same as that presented in compound heterozygous Hb S/Hb E disease30.
For two cases of compound heterozygous Hb D-Punjab/Hb E co-inherited with α+-thalassemia, the Hb D-Punjab value (66.6% and 65.6%) is elevated while the Hb E value (24.5% and 29.0%) is the same as the Hb E heterozygote. Hb D-Punjab mutation results in structural protein changes but does not affect the value of Hb D-Punjab production. However, Hb E mutation creates abnormal mRNA splicing, resulting in low output of Hb E. Thus, lower Hb E levels than Hb D-Punjab levels could be observed in the compound heterozygous Hb D-Punjab/Hb E cases. Moreover, co-inherited α+-thalassemia in compound heterozygous Hb D-Punjab/Hb E cases might not much affect the lower production of Hb E levels when compared to Hb E levels of compound heterozygous Hb D-Punjab/Hb E with normal α-globin chain cases in a previous report (24.5% and 29.0% vs 28.4% and 29.3%)31.
The Hb variant spectra in Thailand were compared. Three common Hb variants—Hb Hope, Hb Q-Thailand, and Hb Tak—have been observed in many populations from northern, central, and northeastern Thailand1. However, the southern population showed different common Hb variants, especially compared to the northern population. It could be explained by differing ethnic backgrounds of populations between the north and south. In Thailand, most people belong to the Thai ethnicity. However, each part of the country has different minority ethnic groups. Minor ethnic groups were observed in the northern population, including Lawa, Mon, Shan, Yuan, Khuen, Lue, and Yong32. By contrast, the minority ethnic groups in the southern population are Thai Muslims, Maniq, Moken, Moklen, and Urak Lawoi33. Furthermore, the mitochondrial phylogenetic analysis revealed that the population from the northern area has distinct haplotype groups compared to those of the southern population34. This divergence may be explained by the proximity of southern Thailand to the sea, leading to populations of diverse nationalities due to human migration from neighboring countries such as Malaysia and India, where Hb Malay and Hb D-Punjab are prevalent, respectively35,36. In addition, Hb C is commonly found in West African populations37 and is occasionally reported in Southeast Asian populations of different origins29. Carriers of Hb C are immune to malarial infections38. Accordingly, Hb C is predominantly observed in southern Thailand, where malaria is endemic. Moreover, Hb G-Makassar is frequently observed in the southern population, similar to that in the Malaysian population39. Hb G-Makassar comigrated at the same retention time as that for Hb S, as determined using the CE technique. Thus, molecular testing is essential for differential diagnosis. Furthermore, we reported two cases of Hb G-Makassar with Hb E, presenting mild clinical phenotypes similar to that in a previous report39.
In conclusion, this study demonstrates a distinct spectrum of Hb variants in Thailand. In addition, we describe the clinical aspects of Hb variants in combination with thalassemia or hemoglobinopathies. This information is essential for determining the need to perform prenatal diagnosis in the prevention and control program for thalassemia in this region.
Materials and methods
All laboratory methods were performed following the national guidelines of Thailand for laboratory diagnosis of thalassemia and hemoglobinopathy40. The study protocol was approved by the Human Research Ethics Unit (HREU) of the Faculty of Medicine, Prince of Songkla University (REC 63-458-5-2). Consent was obtained from all 337 participants with Hb variants. Participants who visited or had their blood samples collected were referred to Songklanagarind Hospital from 2015 to 2020. Informed consent was obtained via telephone, followed by sending the documents via the post office. For patients who visited Songklanagarind Hospital from 2021 to 2022, informed consent was obtained when they visited for follow-up.
Each hospital provided hematological profiles and recorded the history of blood transfusion data. The hematological profiles and history of blood transfusion data of the patients from ten provinces were collected from the laboratory requesting program of Songklanagarind Hospital. We collected hematological data, history of blood transfusion, routine molecular diagnosis results, and DNA samples of patients referred to Songklanagarind Hospital from January 2015 to December 2022 for diagnosing thalassemia.
Samples
A total of 337 DNA specimens were obtained from molecular diagnosis at the thalassemia unit at the Department of Pathology, Faculty of Medicine, Prince of Songkla University, southern Thailand.
Hematological analysis
The hematological profiles were obtained from each hospital in the ten provinces. In our center, hematological parameters were obtained from an automated blood cell counter (Sysmex XN 3000; Sysmex, Japan). Hemoglobin analysis of all referred samples was performed using CE technique (Capillarys 2; Sebia, Lisses, France) at our laboratory, and a thorough review of blood transfusion history was conducted.
Molecular analysis
Routine molecular diagnosis was performed to identify β-thalassemia, α-thalassemia, Hb Hb CS, Hb Paksé (PS, HBA2:c.429A>T), and high Hb F determinants using PCR-based techniques. Analysis of point mutations in β-thalassemia involved the examination of Hb Malay, Hb Dhonburi, and β-thalassemia 19 common mutations in southern Thailand were performed using RDB hybridization13. Analysis of β-thalassemia deletion (3.5 kb and 45 kb deletion (NG_000007.3:g.66258_184734del118477)) was carried out through melt-curve analysis41. High Hb F determinants, including δβ0-thalassemia (12.5 kb deletion), Indian del-inv Aγδβ0-thalassemia, HPFH6, and Thai del-inv-ins Aγδβ0-thalassemia (or Siriraj deletion), were identified using multiplex gap-PCR42. Nine α-thalassemia deletion mutations, such as −−SEA (NC_000016.10:g.165397_184700), −−THAI (NC_000016.10:g.149863_183312), −−SA (NG_000006.1:g.19464_43064del23601), −−CR (NC_000016.10:g.144,215_188,841), −−FIL (NG_000006.1:g.11684_43534del31851), −−MED (NG_000006.1:g.24664_41064del16401), -(α)20.5, -α3.7 (NG_000006.1:g.34164_37967del3804) and -α4.2 ((NC_000016.10:g.149863_183312), were identified using multiplex gap-PCR43. Allele-specific PCR was performed to identify Hb CS and Hb PS44. Additional investigation for unidentified Hb variants in the HBA and HBB genes among cases with Hb variant peak by CE method was confirmed by Sanger DNA sequencing using an ABI PRISMTM 3130xl analyzer (Applied Biosystems, Foster City, CA, USA) or by performing barcode-tagged sequencing based on next-generation sequencing on the Illumina MiSeq (Illumina, Inc., San Diego, CA).
Development of allele-specific PCR for identification of Hb G-Georgia in the HBA1 and HBA2 genes
Two AS-PCR conditions were developed for the differential diagnosis of Hb G-Georgia. To identify Hb G-Georgia in HBA1, a 1093 bp fragment generated from primers A17 (5′-GCTCCGCGCCAGCCAATGAG-3′) and A18 (5′-CTGGACTTCGCGGTGGCTC-3′) was used as an internal control. A 588 bp fragment specific to Hb G-Georgia in HBA1 was amplified using primer GG (5′-ACAAGCTTCGGGTGGACCT-3′) and primer A18. For the identification of Hb G-Georgia in HBA2, a 1,117 bp fragment generated from primers A17 and A19 (5′-GCAGGCCTGGCACCTCTCAG-3′) was used as an internal control. A 542 bp fragment specific to Hb G-Georgia in HBA2 was amplified using primers GG and A19 (Fig. 4). Each PCR reaction (25 μL) comprised 50–200 ng genomic DNA, 0.32 pmol of primers A17 and GG, 0.48 pmol of primers A18 or A19, 200 μM dNTPs, 1 M Betaine, 1.75 mM MgCl2, 1.25% DMSO, and 0.5 units of Taq DNA polymerase (Vivantis Technologies, Selangor Darul Ehsan, Malaysia) in 16 mM (NH4)2SO4 and 50 mM Tris–HCl (pH 9.2) buffer, and 0.1% Triton™ X-100. The cycling conditions were as follows: initial denaturation at 95 °C for 5 min, followed by 30 cycles at 94 °C for 1 min, 65 °C for 45 s, and 72 °C for 1 min 20 s, with a final extension at 72 °C for 5 min on a SimpliAmp™ thermal cycler (Thermo Fisher Scientific, Waltham, MA, USA). PCR products were separated via 1.5% agarose gel electrophoresis for 30 min. The amplified fragments were detected under UV light after staining with ethidium bromide (Supplmentary Figure S1).
Data availability
The datasets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Srivorakun, H., Singha, K., Fucharoen, G., Sanchaisuriya, K. & Fucharoen, S. A large cohort of hemoglobin variants in Thailand: Molecular epidemiological study and diagnostic consideration. PLoS ONE 9, e108365. https://doi.org/10.1371/journal.pone.0108365 (2014).
Panyasai, S., Fucharoen, G. & Fucharoen, S. Hemoglobin variants in northern Thailand: Prevalence, heterogeneity and molecular characteristics. Genet. Test Mol. Biomark. 20, 37–43. https://doi.org/10.1089/gtmb.2015.0182 (2016).
Viprakasit, V., Wiriyasateinkul, A., Sattayasevana, B., Miles, K. L. & Laosombat, V. Hb G-Makassar [beta6(A3)Glu–>Ala; codon 6 (GAG–>GCG)]: Molecular characterization, clinical, and hematological effects. Hemoglobin 26, 245–253. https://doi.org/10.1081/hem-120015028 (2002).
Srivorakun, H., Singha, K., Fucharoen, G. & Fucharoen, S. Novel interactions of two alpha-Hb variants with SEA deletion alpha(0)-thalassemia: Hematological and molecular analyses. Hematology 23, 187–191. https://doi.org/10.1080/10245332.2017.1380930 (2018).
Jomoui, W., Tepakhan, W., Satthakarn, S. & Panyasai, S. Molecular spectrum of Hb H disease and characterization of rare deletional alpha-thalassemia found in Thailand. Scand. J. Clin. Lab. Invest. 80, 528–535. https://doi.org/10.1080/00365513.2020.1795921 (2020).
Fucharoen, S. & Winichagoon, P. Haemoglobinopathies in southeast Asia. Indian J. Med. Res. 134, 498–506 (2011).
Saechan, V., Nopparatana, C., Nopparatana, C. & Fucharoen, S. Molecular basis and hematological features of hemoglobin variants in Southern Thailand. Int. J. Hematol. 92, 445–450. https://doi.org/10.1007/s12185-010-0682-x (2010).
Huisman, T. H. et al. Hemoglobin G Georgia or alpha 2–95 Leu (G-2) beta-2. Biochim. Biophys. Acta 200, 578–580. https://doi.org/10.1016/0005-2795(70)90117-0 (1970).
Bowden, D. K. et al. Different hematologic phenotypes are associated with the leftward (-alpha 4.2) and rightward (-alpha 3.7) alpha+-thalassemia deletions. J. Clin. Invest. 79, 39–43. https://doi.org/10.1172/JCI112804 (1987).
Panyasai, S., Sakkhachornphop, S. & Pornprasert, S. Diagnosis of compound heterozygous Hb Tak/beta-thalassemia and HbD-Punjab/beta-thalassemia by HbA(2) levels on capillary electrophoresis. Indian J. Hematol. Blood Transfus 34, 110–114. https://doi.org/10.1007/s12288-017-0810-3 (2018).
Tanphaichitr, V. S., Viprakasit, V., Veerakul, G., Sanpakit, K. & Tientadakul, P. Homozygous hemoglobin Tak causes symptomatic secondary polycythemia in a Thai boy. J. Pediatr. Hematol. Oncol. 25, 261–265. https://doi.org/10.1097/00043426-200303000-00016 (2003).
Prakobkaew, N., Singsanan, S., Fucharoen, G., Surapot, S. & Fucharoen, S. Secondary erythrocytosis caused by hemoglobin Tak/(deltabeta)0-thalassemia syndrome. Acta Haematol. 124, 115–119. https://doi.org/10.1159/000318015 (2010).
Nopparatana, C., Nopparatana, C., Saechan, V., Karnchanaopas, S. & Srewaradachpisal, K. Prenatal diagnosis of alpha- and beta-thalassemias in southern Thailand. Int. J. Hematol. 111, 284–292. https://doi.org/10.1007/s12185-019-02761-4 (2020).
Fucharoen, S., Fucharoen, G., Sanchaisuriya, K. & Surapot, S. Compound heterozygote states for Hb C/Hb Malay and Hb C/Hb E in pregnancy: A molecular and hematological analysis. Blood Cells Mol. Dis. 35, 196–200. https://doi.org/10.1016/j.bcmd.2005.05.004 (2005).
Laosombat, V., Wongchanchailert, M., Sattayesevana, B. & Nopparatana, C. Clinical, hematological and molecular features in Thais with beta-Malay/beta-thalassemia and beta-Malay/HbE. Southeast Asian J. Trop. Med. Public Health 28(Suppl 3), 106–109 (1997).
Sripichai, O. et al. Coinheritance of the different copy numbers of alpha-globin gene modifies severity of beta-thalassemia/Hb E disease. Ann. Hematol. 87, 375–379. https://doi.org/10.1007/s00277-007-0407-2 (2008).
Nuntakarn, L. et al. Molecular, hematological and clinical aspects of thalassemia major and thalassemia intermedia associated with Hb E-beta-thalassemia in Northeast Thailand. Blood Cells Mol. Dis. 42, 32–35. https://doi.org/10.1016/j.bcmd.2008.09.002 (2009).
Winichagoon, P., Fucharoen, S., Chen, P. & Wasi, P. Genetic factors affecting clinical severity in beta-thalassemia syndromes. J. Pediatr. Hematol. Oncol. 22, 573–580. https://doi.org/10.1097/00043426-200011000-00026 (2000).
Charoenkwan, P., Teerachaimahit, P. & Sanguansermsri, T. The correlation of alpha-globin gene mutations and the XmnI polymorphism with clinical severity of Hb E/beta-thalassemia. Hemoglobin 38, 335–338. https://doi.org/10.3109/03630269.2014.952744 (2014).
Yamsri, S. et al. alpha(0)-thalassemia in affected fetuses with hemoglobin E-beta(0)-thalassemia disease in a high-risk population in Thailand. Am. J. Transl. Res. 14, 1315–1323 (2022).
Soontornpanawet, C. et al. Molecular basis of a high Hb A(2)/Hb Fbeta-thalassemia trait: A retrospective analysis, genotype-phenotype interaction, diagnostic implication, and identification of a novel interaction with alpha-globin gene triplication. PeerJ 11, e15308. https://doi.org/10.7717/peerj.15308 (2023).
Tepakhan, W., Kanjanaopas, S. & Srewaradachpisal, K. Association between genetic polymorphisms and Hb F levels in heterozygous beta-thalassemia 3.5 kb deletions. Hemoglobin 44, 338–343. https://doi.org/10.1080/03630269.2020.1811117 (2020).
Liu, Y. T. et al. Rapid detection of alpha-thalassaemia deletions and alpha-globin gene triplication by multiplex polymerase chain reactions. Br. J. Haematol. 108, 295–299. https://doi.org/10.1046/j.1365-2141.2000.01870.x (2000).
Sharma, V. & Saxena, R. Effect of alpha-gene numbers on phenotype of HbE/beta thalassemia patients. Ann. Hematol. 88, 1035–1036. https://doi.org/10.1007/s00277-009-0723-9 (2009).
Singha, K. et al. A large cohort of deletional high hemoglobin F determinants in Thailand: A molecular revisited and identification of a novel mutation. Clin. Chim. Acta 551, 117615. https://doi.org/10.1016/j.cca.2023.117615 (2023).
Fucharoen, S., Pengjam, Y., Surapot, S., Fucharoen, G. & Sanchaisuriya, K. Molecular characterization of (deltabeta)(0)/beta(0)-thalassemia and (deltabeta)(0)-thalassemia/hemoglobin E in Thai patients. Eur. J. Haematol. 67, 258–262. https://doi.org/10.1034/j.1600-0609.2001.00524.x (2001).
Fucharoen, S., Fucharoen, G., Sanchaisuriya, K. & Surapot, S. Molecular characterization of thalassemia intermedia associated with HPFH-6/beta-thalassemia and HPFH-6/Hb E in Thai patients. Acta Haematol. 108, 157–161. https://doi.org/10.1159/000064707 (2002).
Fucharoen, S., Singsanan, S., Sanchaisuriya, K. & Fucharoen, G. Molecular and haematological characterization of compound Hb E/Hb Pyrgos and Hb E/Hb J-Bangkok in Thai patients. Clin. Lab. Haematol. 27, 184–189. https://doi.org/10.1111/j.1365-2257.2005.00665.x (2005).
Sanchaisuriya, K. et al. Molecular characterization of hemoglobin C in Thailand. Am. J. Hematol. 67, 189–193. https://doi.org/10.1002/ajh.1105 (2001).
Huisman, T. H. Combinations of beta chain abnormal hemoglobins with each other or with beta-thalassemia determinants with known mutations: Influence on phenotype. Clin. Chem. 43, 1850–1856 (1997).
Edison, E. S., Shaji, R. V., Chandy, M. & Srivastava, A. Interaction of hemoglobin E with other abnormal hemoglobins. Acta Haematol. 126, 246–248. https://doi.org/10.1159/000329904 (2011).
Kutanan, W. et al. Genetic affinity and admixture of northern Thai people along their migration route in northern Thailand: Evidence from autosomal STR loci. J. Hum. Genet. 56, 130–137. https://doi.org/10.1038/jhg.2010.135 (2011).
Srikummool, M. et al. Forensic and genetic characterizations of diverse southern Thai populations based on 15 autosomal STRs. Sci. Rep. 12, 655. https://doi.org/10.1038/s41598-021-04646-1 (2022).
Jaisamut, K. et al. Unraveling the mitochondrial phylogenetic landscape of Thailand reveals complex admixture and demographic dynamics. Sci. Rep. 13, 20396. https://doi.org/10.1038/s41598-023-47762-w (2023).
Alwi, Z. B. & Syed-Hassan, S. R. Thalassemia in Malaysia. Hemoglobin 46, 45–52. https://doi.org/10.1080/03630269.2022.2057326 (2022).
Srinivas, U., Pati, H. P. & Saxena, R. Hemoglobin D-Punjab syndromes in India: A single center experience on cation-exchange high performance liquid chromatography. Hematology 15, 178–181. https://doi.org/10.1179/102453309X12583347113735 (2010).
Piel, F. B. et al. The distribution of haemoglobin C and its prevalence in newborns in Africa. Sci. Rep. 3, 1671. https://doi.org/10.1038/srep01671 (2013).
Modiano, D. et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature 414, 305–308. https://doi.org/10.1038/35104556 (2001).
Esa, E. et al. Clinical and haematological characteristics of 38 individuals with Hb G-Makassar in Malaysia. EJHaem 4, 940–948. https://doi.org/10.1002/jha2.750 (2023).
Guidelines Review Committee. Laboratory Diagnosis of Thalassemia and Hemoglobinopathy (National Institute of Health of Thailand, 2015).
Tepakhan, W. & Jomoui, W. Rapid molecular detection for differentiation of homozygous HbE and beta0-thalassemia/HbE in samples related with HbE >80% and variable HbF levels. Lab. Med. 52, 232–239. https://doi.org/10.1093/labmed/lmaa065 (2021).
Singha, K., Fucharoen, G., Hama, A. & Fucharoen, S. A novel (A)gammadeltabeta(0)-thalassemia caused by DNA deletion-inversion-insertion of the beta-globin gene cluster and five olfactory receptor genes: Genetic interactions, hematological phenotypes and molecular characterization. Clin. Biochem. 48, 703–708. https://doi.org/10.1016/j.clinbiochem.2015.03.023 (2015).
Jomoui, W., Panyasai, S., Sripornsawan, P. & Tepakhan, W. Revisiting and updating molecular epidemiology of alpha-thalassemia mutations in Thailand using MLPA and new multiplex gap-PCR for nine alpha-thalassemia deletion. Sci. Rep. 13, 9850. https://doi.org/10.1038/s41598-023-36840-8 (2023).
Fucharoen, S. et al. Interaction of hemoglobin E and several forms of alpha-thalassemia in Cambodian families. Haematologica 88, 1092–1098 (2003).
Acknowledgements
This research was financially supported by the Faculty of Medicine, Prince of Songkla University (Grant No. 64-077-2).
Author information
Authors and Affiliations
Contributions
W.T. acquired grants, wrote the main manuscript, and contributed to all aspects of the investigation, including conceptualization, data collection, laboratory investigation, data analysis, and revision of the final manuscript. S.K. and K.S. were involved in sample preparation and molecular analysis. C.W., C.N., C.K., and S.B. were involved in sample collection and hemoglobin analysis. T.P. and P.S. were involved in conceptualization, data collection, and data analysis. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tepakhan, W., Kanjanaopas, S., Sreworadechpisal, K. et al. Molecular epidemiology and hematological profiles of hemoglobin variants in southern Thailand. Sci Rep 14, 9255 (2024). https://doi.org/10.1038/s41598-024-59987-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59987-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.