Abstract
Current approaches to diagnosing male infertility inadequately assess the complexity of the male gamete. Beyond the paternal haploid genome, spermatozoa also deliver coding and non-coding RNAs to the oocyte. While sperm-borne RNAs have demonstrated potential involvement in embryo development, the underlying mechanisms remain unclear. In this study, 47 sperm samples from normozoospermic males undergoing fertility treatment using donor oocytes were sequenced and analyzed to evaluate associations between sperm RNA elements (exon-sized sequences) and blastocyst progression. A total of 366 RNA elements (REs) were significantly associated with blastocyst rate (padj < 0.05), some of which were linked to genes related to critical developmental processes, including mitotic spindle formation and both ectoderm and mesoderm specification. Of note, 27 RE-associated RNAs are predicted targets of our previously reported list of developmentally significant miRNAs. Inverse RE-miRNA expression patterns were consistent with miRNA-mediated down-regulation. This study provides a comprehensive set of REs which differ by the patient’s ability to produce blastocysts. This knowledge can be leveraged to improve clinical screening of male infertility and ultimately reduce time to pregnancy.
Similar content being viewed by others
Introduction
Infertility affects an estimated 186 million people globally and has considerable psychological, emotional and economic consequences for individuals and couples struggling to conceive and undergoing fertility treatment1,2. While male factors contribute to half of all instances of subfertility, current strategies for diagnosing and managing the infertile male are insufficient3,4. Spermatozoal quantity, motility and morphology are assessed through laboratory semen analyses5,6. In some cases, the workup may include evaluating general and reproductive health through blood hormone testing, reproductive history and physical examination5,6. However, 30–50% of infertile males are classified as idiopathic, having an uncertain cause of subfertility7.
The complexity of spermatozoa and their potential contributions to conception and offspring development are becoming better understood3,8. Beyond carrying the paternal haploid genome, sperm also contain large and small, coding and non-coding RNAs, as well as other epigenetic signatures and proteins9. Since mature sperm have a limited cytoplasm, a condensed nucleus, and are largely transcriptionally and translationally inert, these various elements were previously assumed to lack functions beyond spermatogenesis10,11. However, accumulating evidence suggests that, upon delivery to the oocyte, they may have important roles in development of the early embryo12. For instance, animal studies interfering with sperm RNA activity through gene knockdown, RNase treatment, or using otherwise RNA-deficient sperm have suggested they are indispensable to normal fertilization, embryo development and live birth outcome13,14,15. It has also been demonstrated that the sperm RNA profile may undergo dramatic changes as sperm migrate through the male reproductive tract, specifically throughout epididymal transit, suggesting that particular RNAs may be selectively delivered to sperm through epididymosomes prior to fertilization and are responsive to the environment16,17,18. Interestingly, sperm RNAs, including miRNAs and lncRNAs, may be altered by paternal states such as chronic stress and anxiety, and they provide a potential mechanism for paternally-mediated inheritance of affective behaviour19,20. However, while selective RNA delivery during epididymal transit is one proposed mechanism of sperm epigenetic modulation, the dynamicity of the sperm RNA profile in response to paternal influences is not fully understood.
Clinically, sperm RNAs have been reported to be associated with male fertility status. Jodar et al. identified 648 sperm RNA elements that are common among fertile couples21. When all of these sequences were present in sperm from idiopathic infertile couples, they were significantly more likely to achieve live birth outcomes by timed intercourse or intrauterine insemination, compared to couples lacking one or more of these species21. Recently, our group compared the small RNA profiles of male patients utilizing donor oocytes, who had variable outcomes through assisted reproductive technology (ART), despite normozoospermic presentations22. We observed several hundred sperm-borne small RNAs, including 93 micro-RNAs (miRNAs), whose dysregulated expression were associated with blastocyst rates. These data suggest that sperm small RNAs delivered to presumably competent donor oocytes could contribute directly to the embryo’s ability to complete preimplantation development22. However, this focused small RNA capture and analysis excludes larger RNA species, which comprise the majority of the RNA elements previously reported by Jodar et al., and further analyzed by Burl et al21,23.
In the present study, next-generation sequencing and RNA-seq analysis tools (REDa [RNA Element Discovery algorithm] and TII [Transcript Integrity Index]) were used to evaluate associations between sperm RNA elements and blastocyst progression among normozoospermic males using donor oocytes. We report 33 and 333 RNA Elements (REs), which had increased abundance in sperm samples from couples with high and low blastocyst rates, respectively, some of which were linked to genes related to important developmental processes, including mitotic spindle formation, as well as ectoderm and mesoderm development. We identified 27 RE-associated RNAs (RE-RNAs) that are targets of our previously reported miRNAs which were associated with successful embryo development to the blastocyst and we observed their corresponding target gene depletion consistent with miRNA-mediated down-regulation.
Methods
Methods related to the selection of study subjects, sample preparation and RNA extraction have been reported in our previous study, in which small RNAs were isolated and profiled from an overlapping patient population at our centre22.
Patient population
Approval for this study was provided by Veritas IRB (Quebec, CA; IRB protocol number 2021–2343- 7435–1). All methods, data collections, and analyses were performed in accordance with the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2; 2022) and the Personal Health Information Protection Act (PHIPA; 2004). All participants gave informed consent for the collection and use of their semen and information for research. A total of 47 male patients undergoing IVF using donor oocytes at CReATe Fertility Centre, Toronto, Canada were included in the study. All included patients had an unremarkable semen analysis (normozoospermic; no oligozoospermia, asthenozoospermia or teratozoospermia) and had no other known cause of subfertility. Donor ova were fertilized using either a single sperm provider or sperm from each member of a same-sex male couple (50/50 IVF). Same-sex couples (16 males) were separated into two groups (high and low), based on their blastulation rates. Blastocyst rates were determined through dividing the number of resulting blastocysts by the number of 2PN zygotes obtained. Only couples with a difference in blastulation rate greater than 35% and a minimum of 4 ova fertilized by each partner were included. Single sperm providers (31 males) were subsequently divided into low, average, and high groups based on their relationship to the mean blastulation rate for all male-male couples in the study; cut-offs of one standard deviation higher and lower than the mean were used for the high and low groups, respectively. A minimum of 3 fertilized ova was used for single sperm providers. Patient characteristics and relevant IVF cycle data are summarized in Table 1. Embryology data for individual study subjects is included in Supplementary Table 1. Note that donor age data for 4 patients was unavailable from the biobank database.
Sample preparation
Fresh human semen samples were collected by ejaculation. Samples were incubated at room temperature for no more than 60 min and were assessed for: (1) motility and morphology by computer-aided semen analysis (CASA) using the HTM-CEROS Sperm Analyzer (Hamilton Thorne); and (2) morphology according to WHO recommendations24. Patient information and samples were collected by members of the CReATe Biobank, a certified CTRNet Biobank Program. Spermatozoa was isolated from seminal plasma 24 h after washing; semen was centrifuged at 420 g for 10 min and the spermatozoal pellet was resuspended in 0.5 mL of sperm wash medium and stored at − 80 °C.
RNA isolation
RNA extraction procedures were adapted from an existing sperm RNA isolation protocol, described previously22,25. Sperm counts were estimated using the CountessTM 3 Automated Cell Counter (Invitrogen) and a starting input of 2 million sperm was used for RNA extraction. Total RNA was isolated using the QIAzol phenol/guanidine-based RNeasy Kit (Qiagen), according to the manufacturer’s instructions. Elution of purified RNA was performed using nuclease-free water. Samples were treated with DNAse I (Thermo Scientific) to remove any single- or double-stranded DNA contamination and starting RNA input was evaluated by Qubit™ RNA HS Assay Kit (Invitrogen). The miRNeasy Kit (Qiagen) was further used to isolate the small RNA species which were analyzed previously22.
Library preparation and sequencing
The SeqPlex RNA Amplification Kit (Sigma Aldrich) was used to reverse-transcribe the total RNA and amplify the resulting double-stranded cDNA through primer-mediated amplification, according to manufacturer instructions. Primers were subsequently eliminated, and DNA was purified using the Zymo DNA Clean & Concentrator-5 (Zymo Research). The NEBNext Ultra II DNA Library Prep Kit (New England BioLabs) was used to prepare and amplify barcoded libraries from purified cDNA. Individual large RNA libraries underwent bead-based size-selection with SPRI beads (Beckman Coulter). Final library traces were assessed using the Bioanalyzer 2100 (Agilent technologies). Resulting large RNA libraries had an average length of roughly 270 bp. Sequencing was performed using the NextSeq 550 Sequencing System (Illumina) for library normalization. Pooled large RNA libraries were denatured and diluted, according to the manufacturer’s protocols, and sequenced at 1 × 150 cycles. Samples were re-pooled using NextSeq reads to calculate normalization, size-selected, re-quantified, and the final sequencing data was collected using the NovaSeq 6000 Sequencing System (Illumina). An S2 NovaSeq flow cell was used, with a read length of 100 bp, paired-end, and a target of roughly 85 million reads per sample.
Bioinformatics and statistical analysis
Sequence processing and analyses were performed as previously described26,27. Briefly, reads were first aligned to the telomere-2-telomere (T2T) genome (T2T-CHM13v2.0) followed by processing with the RNA Element Discovery algorithm (REDa), which identifies exon-sized RNA fragments called RNA Elements (REs), originating from both exonic and intronic regions, as well as those 10 KB from a known exon, and those greater than 10 KB from a known exon (novel orphan)26. Reads were normalized per kilobase of exon model per million mapped reads (RPKM) to compare expression levels among samples28. Welch’s one-way ANOVA was used to test for significant differences in RE and read categories among groups.
Transcript integrity was evaluated using the transcript integrity index (TII) algorithm aligned to the Gencode hg38 version 41 genome, as described previously, to evaluate the quality of RNA present in the sperm samples, despite the high levels of fragmentation that are inherent in sperm27. Differences in human genome alignment versions are due to the TII algorithms requirements of running in R version 3.6.0, which inhibits use of the T2T genome. Transcripts were considered intact if the transcript received a TII score greater than 0.5, which indicates at least half of the transcript was covered by a minimum of 5 reads per million (RPM). Samples not meeting TII thresholds were excluded from the analysis. Transcript integrity was also visually confirmed by the UCSC Genome Browser T2T genome track.
Identified REs were filtered to include only those with greater than 1 RPKM in at least a third of the samples to remove those with low abundance. Mfuzz (version 2.54.0) clustering of group median RPKM was undertaken in R, set for 8 clusters with a membership probability cutoff of 0.4. A Kruskal Wallis test in R was used to apply a p-value for each RE that was present in the cluster patterns. Principal component analysis (PCA) of significant REs was undertaken and a plot was generated using Clustvis (https://biit.cs.ut.ee/clustvis/). A singular value decomposition PCA method was used with imputation of missing values. The Pareto scaling approach was used to scale rows by the square root of the standard deviation.
Gene ontology enrichment analysis was completed using the following programs: EnrichR (https://maayanlab.cloud/Enrichr/); GeneMANIA (cytoscape module version 3.5.2); STINGdb (version 11.5); MsigDB (Human MSigDB v2023.1.Hs); and Metascape (v3.5.20230501). For patterns containing multiple clusters, ontology analysis was undertaken using these major patterns, rather than individual clusters. Significant Mfuzz RE-associated RNAs were compared to miRNA target gene expression. TargetScan and miRtarbase were used to determine and catalog target gene names for previously reported miRNAs22. Significant REs were cross-referenced with paternally provided RE lists from Estill et al., 2019, as modified by Swanson, GM et al., 2023 (5 × enriched and 2 × enriched in sperm compared to oocyte)26,30.
Results
Human sperm contain abundant exonic REs
Overall, 4,767,971,832 reads were obtained from NovaSeq sequencing of 44 samples, which was trimmed to 4,711,072,297 reads (average 107 million reads per sample). The mean number of reads did not differ significantly (Welch’s one-way ANOVA) between high, low and average blastocyst groups (75,783,820 vs 77,856,326 vs 73,173,326), indicating sequencing depth was comparable and sufficient. Reads were mapped to several broad categories, the proportions of which were also similar across blastocyst rate groups, summarized in Supplementary Table 2. The most abundant sequences were human or unclassified in all but one sample (LRNA44), which had a top sequence assigned to the Enterobacteriaceae family. The microbes primarily within this bacterial family are Escherichia coli, which is often present in the ejaculate31.
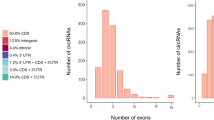
RNA discovery through the REDa identified 435,606 REs across all samples. This number was reduced to 61,549 after filtering for abundant sequences (> 2 RPKM). Their genomic distribution is presented in Table 2. Proportions were highly similar across groups.
Sperm RE and RE-RNA expression differs with blastocyst development rate
Previous reports suggest that sperm-borne REs are associated with male fertility in idiopathic cases. To investigate associations between sperm REs and resulting embryo development rates in a presumably normal population of patients, we leveraged state-of-the-art bioinformatic analyses. Mfuzz clustering identifies patterns of RE expression changes based on the group median RPKM. Based on the number of clusters, alongside required parameters for pattern membership (how closely the RE expression changes follow the pattern), REs are segregated into specific patterns. Here, we identified 8 patterns. A sample-sample membership of 0.4 was used when clustering, indicating REs must closely follow the pattern. A total of six major patterns were identified based on differential abundance across blastocyst rate groups (Supplementary Fig. 1). These patterns are referred to as same-up, up-up, down-same down-down, up-down and down-up, based on how RE abundance changed with increased blastocyst rate. For example, same-up refers to REs following a pattern of similar abundance between low and average groups (same) with increased abundance in the high group (up), while down-down refers to REs following a pattern of highest abundance in the low group with lower abundance in the average group and further decreased abundance in the high group. Of these major patterns, those related to embryo development were comprised of elements with significantly higher abundance in the high blastocyst rate group (same-up and up-up) and elements with significantly higher abundance in the low blastocyst rate group (down-same, down-down), shown in Fig. 1.
RE clusters related to embryo development. Two major patterns were reflective of REs with highest abundance in the high blast rate group (a): REs moving same-up (I) and REs moving up-up (II) across groups. Two major patterns demonstrated the highest abundance in low blast rate samples (b): REs moving down-same (I) and REs moving down-down (II) across groups. Graphs are visualized across blastocyst rate groups based on fold-change in the abundance of the cluster patterns.
A total of 12,519 REs comprised the four Mfuzz patterns most related to blastocyst development, of which 366 were statistically significant (padj < 0.05). Principal component analysis (PCA) of significant REs showed clustering amongst blastocyst rate groups, but more variability with decreasing blastocyst rate (Fig. 2). However, there was overlap between some members of the average with the high group. This suggests that there may be a specific RE signature required to support normal blastocyst development, while variations in the sperm-borne RE complement could reduce blastocyst rates.
The number of REs with significant differences between groups, and the corresponding number of related significant genes are reported in Table 3, with the complete list of significant REs contributing to the major patterns included in Supplementary Table 3.
Sperm RE-RNAs are involved in developmentally important processes
A summary of findings from the gene ontology (GO) enrichment analysis is included in Supplementary Table 4. For gene patterns reflecting highest abundance in the high blastocyst rate group, genes moving same-up are involved in cellular processes such as the peroxisomal oxidation and mitosis, while the RE-RNA for the up-up pattern (PPP4R1), is related to phosphorylation and dephosphorylation. For patterns reflecting highest abundance in the low blastocyst rate group, RE-RNAs moving down-down are involved in processes related to immune cells (e.g., macrophages, neutrophils, microglial, leukocytes), as well as ectoderm development (ITGAM), while RE-RNAs moving down-same are involved in mitotic spindle formation (DLG1, ACTN4, HDAC6, PIF1, and ARHGEF7) and mesoderm development (OVOL1, PRKACA, and TXNRD1).
Sperm RE and RE-RNAs are related to MiRNA enrichment
REs with significant differences between groups of blastocyst development were compared to the differentially expressed miRNAs from overlapping samples as reported previously22. Specifically, lists of miRNAs which were enriched in the low and high blastocyst rate groups were searched against miRNAs using TargetScan and the miRTarBase. Overlapping RE-RNA movement for miRNAs enriched in the high and low blastocyst rate groups is summarized in Table 4.
The PHC3 and AKIRIN1 RE-RNAs, which both move down-same in relationship to blastocyst rate groups, were associated with hsa-miR-181b-5p and hsa-miR-224-5p, respectively, across both databases (Fig. 3). USP37 was associated with hsa-miR-19a-3p in both databases, however this RE-RNA moves up-down in relationship to blastocyst progression, with highest abundance in the average group. A complete list of miRNAs associated with each overlapping gene for individual databases is included in Supplementary Table 5.
Enrichment of miR-associated RE-RNAs Across blastocyst rate groups. The abundance of AKIRIN1 (a) and PHC3 (b) are plotted against miR-224-5p and miR-181b-1-5p, respectively. A proportion of mean abundance for each blastocyst rate group relative to overall mean abundance (across all samples) was used to normalize abundance between the genes and miRNAs of interest. AKIRIN1 includes: AKIRIN1-201; AKIRIN1-202; AKIRIN1-203; and AKIRIN1-204. PHC3 includes: PHC3-215; PHC3-214; and PHC3-209.
Nine RE-RNAs are targets of miRNAs that were enriched in the low blastocyst development group. Interestingly, these 9 RE-RNAs are depleted in these samples, suggestive of miRNA-mediated gene regulation. Further, a corresponding decrease in the significant REs was also noted for 5 of the 9 RE-RNAs (GSE1, MOCS3, NDRG2, NPM1, and ZNF675). Moreover, 18 RE-RNAs are targets of miRNAs that were enriched in the high blastocyst rate group. All significant Mfuzz REs were decreased for all 18 RE-RNAs, and 14 of 18 RE-RNAs were depleted in all samples. Of the remaining four RE-RNAs, two are 5’ depleted in all samples (TMEM63B, USP37) and two are full-length in at least 1 sample, but are depleted in the remaining samples (CIT, QKI). Interestingly, the TMEM63B RE is paternally provided according to previously published datasets. Specifically, TMEM63B is five-fold enriched in sperm compared to the oocyte30. TMEM63B functions to enable calcium-activated cation channels and its knockdown results in preweaning lethality in mice (complete penetrance), suggesting it may have a highly important developmental role32,33. TMEM63B is targeted by hsa-miR-19a-3p and hsa-miR-145-5p (which were enriched in the high blastocyst rate group). Eleven other blastocyst rate-associated RE-RNAs were identical to those defined previously as paternally-provided REs (Table 5). These RE-RNAs are either five-fold or two-fold enriched in sperm, compared to the oocyte. Two of the 12 RE-RNAs, TMEM63B and CCDC9, overlap with the paternally-provided datasets. CCDC9, which is full-length in two samples, but depleted in the remaining samples, is also five-fold enriched in sperm compared to the oocyte and functions to enable RNA binding34.
Discussion
Next-generation sequencing was used to profile RNA extracted from the spermatozoa of males with normal semen parameters, but variable IVF outcomes. We identified 61,549 abundant REs across all human sperm samples through the REDa RNA discovery pipeline, which included exonic, intronic and novel uncharacterized REs. A previous sperm RNA-seq study identified 185,037 REs in boar sperm, with the top 10% of these REs accounting for 65% of the read count35. The 18,504 REs occupying this top decile followed a similar distribution to the REs presented in this study, with 69% exonic REs and 12% intronic REs (compared to 68 and 17%, respectively), though almost 15% were novel orphan REs (compared to 7%)33. While a number of other sperm RNA-seq studies have been conducted in a variety of species, the human sperm transcriptome is comparatively understudied35,36,37,38. Further validation across species with a specific focus on the human sperm transcriptome would be valuable.
Clustering of REs revealed differences between blastocyst development groups. The low blastocyst rate group had the most unique profile of REs, with 304 REs (and 138 common RE-associated RNAs (RE-RNAs)) significantly more abundant compared to the average and high blastocyst rate groups. While the high blastocyst rate group had the most unique small RNA profile in our previous study, the enrichment of REs reported here in the low blastocyst rate group may reflect differences in small RNA regulation (and specifically, RNA interference), which influence the amount of fully intact REs that are present22. Overall small and total RNA variation between groups may be indicative of gene regulation which influences the competence of preimplantation embryos and specifically, their ability to progress to the blastocyst stage. While the mechanisms through which sperm RNA elements may regulate preimplantation embryonic development are unknown, it has been proposed that they may alter retroelement activity upon delivery to the oocyte17,39. For instance, targeted transfer RNA-derived small RNA (tsRNA) depletion through co-injection of antisense sequences (along with sperm) into porcine oocytes led to aberrant cleavage and blastocyst progression whilst altering embryonic expression of retroelements40. Specifically, L1M3b of LINE-1 family, which was previously shown to regulate chromatin accessibility in murine embryos, was downregulated40,41. Interestingly, one unique gene identified here, CHM13_T1000172, which was significantly enriched in the low blastocyst rate group compared to the average and high blastulation groups (down-same pattern), is located on chromosome Y and overlaps with LINE L1 repeat L1PA10. This finding supports the hypothesized involvement of retroelements, either directly or indirectly, in the sperm RNA-mediated modulation of early embryo development.
Gene ontology enrichment analysis of genes associated with the significant REs revealed their involvement in critical developmental processes, including mitotic spindle formation and ectoderm and mesoderm development. These findings suggest that there are developmentally important genes and their associated RNA elements which are differentially represented in the sperm of males with lower rates of blastocyst development, compared to those with higher rates. The association of significant genes with primary germ layer formation supports numerous studies which have suggested that modifying the sperm RNA payload can influence offspring phenotypes beyond blastulation42,43,44. For instance, in a study by Chen et al., not only did mice consuming a high-fat diet demonstrate changes in their RNA payload–specifically tsRNAs–the injection of sperm tsRNAs from mice consuming this diet into normal zygotes altered metabolic gene expression in early embryos and induced metabolic disorders in the offspring44. While the precise mechanisms through which paternally-delivered elements may influence development are unclear, regulation of transcriptional and translational machinery in the oocyte likely involves a complex interplay between numerous factors, including transposable elements, DNA methylation and chromatin structure45.
Further, comparing significant RE-associated RNAs (RE-RNAs) with miRNA target gene abundance across blastocyst rate groups revealed 9 and 18 RE-RNAs that are targets of miRNAs that were enriched in the low and high blastocyst rate groups, respectively. The majority of these gene targets were depleted or 5’ depleted in all samples and many of the significant REs associated with these genes had corresponding decreases. Depletion of these targets could indicate that these RE-RNAs are silenced as they are no longer required, perhaps beyond sperm maturation. Alternatively, these discoveries could indicate a potential miRNA-mediated downregulation of genes that are differentially expressed among males with variable IVF outcomes. Sperm-delivered miRNAs have previously been linked to sperm characteristics and IVF outcomes46,47,48. Importantly, the specific enriched human miRNAs reported here have also been previously reported to influence developmental processes. In particular, hsa-miR-181b-5p, which was enriched in the sperm of the low blastulation group, has previously been shown to inhibit trophoblast migration and invasion49. Interestingly, miR-181b-5p was also previously identified in both mouse spermatozoa and epididymosomes50. Hsa-miR-224, which was also enriched in the low blastocyst rate group, has been functionally-related to the differentiation of spermatogonial stem cells in mice51. Higher expression of miRNA-19a/b-3p has previously been associated with oligoasthenozoospermia and male infertility52. While miRNA-19a/b-3p was indeed down-regulated in the high blastocyst rate group, it was also reduced in the low group, suggesting its activity in normozoospermic males may require further elucidation.
Finally, 12 significant RE-RNAs overlapped with previously published datasets of paternally-provided RNAs, with two of these 12 RE-RNAs as exact overlapping genomic regions between datasets: TMEM63B and CCDC9. With the exact genomic region overlapping, the significance of these RE-RNAs as paternally-derived is highlighted. Importantly, knockdown of TMEM63B results in preweaning lethality, which suggests it may have a significant role in embryo development32. CCDC9 was also previously identified as a novel candidate gene of severe asthenozoospermia and has been reported to regulate sperm motility and spermiogenesis53,54. Moreover, CCDC9 has also previously been associated with ZC3H11A, an mRNA binding protein that is required for mouse embryo development to the peri-implantation stage55.
This study is limited by a relatively small sample size and the analysis of only one ejaculate from each included patient (to limit our analysis to only samples for which direct embryology outcomes were available) and therefore still needs validation to confirm that specific REs were not identified by random chance. However, while these samples were assumed to be reflective of each patient’s overall semen profile, it should be noted that the RNA sequencing data presented does not capture the heterogeneity that may exist across repeated ejaculates or between individual sperm. Additionally, DNA fragmentation indexes were not available for the population studied as DNA fragmentation is not routinely used at this clinic. Thus, this study is also limited by the significant fragmentation that is inherently present within sperm, which may not only influence blastocyst progression itself, but it also influences the number of intact transcripts which may be analyzed (despite cut-offs for sequence quality). Use of the Transcript Integrity Index (TII) algorithm begins to combat the transcript integrity aspect of this limitation as it was specifically designed for RNA quality assessment of samples exhibiting high RNA fragmentation rates29. Using a cut-off optimized in human sperm of a minimum 5 RPM across half the transcript as a measure of transcript integrity, combined with visualization of transcripts on the UCSC Genome browser, enabled identification of full-length RE-RNAs for evaluation. Furthermore, the TII algorithm has the capability of determining whether samples contain similar RNA quality, for downstream analysis. Further study in larger patient populations could corroborate the identified relationship between the presence of the identified significant sperm-borne RNAs and IVF outcomes; including embryo development, and pregnancy and live birth rates. Gene knock-out and other mechanistic studies in sperm and embryos, specifically targeting the RNA elements and genes presented here, could elucidate the functional pathways and genetic underpinnings through which RNAs delivered by sperm may direct blastocyst progression and embryogenesis.
High resolution methods for assessing sperm competence are currently lacking. An RE-based assay for assessing sperm quality, and its ability to generate blastocysts in the absence of female factors, could improve patient counseling and reduce time to pregnancy. With further investigation and clinical validation, modifying important RNAs within sperm or selecting sperm based on their RNA contents could offer novel therapeutic strategies to improve clinical outcomes and ameliorate the burden of infertility.
Data availability
The data used in this analysis can be accessed under accession number GSE262969.
References
Stanhiser, J. & Steiner, A. Z. Psychosocial aspects of fertility and assisted reproductive technology. Obstet. Gynecol. Clin. North Am. 45, 563–574 (2018).
Health, T. L. G. Infertility-why the silence?. Lancet Glob. Health 10, e773 (2022).
Calvert, J. K. et al. The male infertility evaluation still matters in the era of high efficacy assisted reproductive technology. Fertil. Steril. 118, 34–46 (2022).
Pandruvada, S. et al. Lack of trusted diagnostic tools for undetermined male infertility. J. Assist. Reprod. Genet. 38, 265–276 (2021).
Schlegel, P. N. et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. Fertil. Steril. 115, 54–61 (2021).
Schlegel, P. N. et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part II. J. Urol. 205, 44–51 (2021).
Agarwal, A. et al. Male infertility. Lancet 397, 319–333 (2021).
Jodar, M. et al. The presence, role and clinical use of spermatozoal RNAs. Hum. Reprod. Update 19, 604–624 (2013).
Hamilton, M., Russell, S., Moskovtsev, S., Krawetz, S. A. & Librach, C. The developmental significance of sperm-borne ribonucleic acids and their potential for use as diagnostic markers for male factor infertility. F&S Rev. 3, 11–23 (2022).
Santiago, J., Silva, J. V., Howl, J., Santos, M. A. S. & Fardilha, M. All you need to know about sperm RNAs. Hum. Reprod. Update 28, 67–91 (2021).
Johnson, G. D. et al. The sperm nucleus: Chromatin, RNA, and the nuclear matrix. Reproduction 141, 21–36 (2011).
Lismer, A. & Kimmins, S. Emerging evidence that the mammalian sperm epigenome serves as a template for embryo development. Nat. Commun. 14, 2142 (2023).
Conine, C. C., Sun, F., Song, L., Rivera-Pérez, J. A. & Rando, O. J. Small RNAs gained during epididymal transit of sperm are essential for embryonic development in mice. Dev. Cell 46, 470-480.e3 (2018).
Yuan, S. et al. Sperm-borne miRNAs and endo-siRNAs are important for fertilization and preimplantation embryonic development. Development 143, 635–647 (2016).
Guo, L. et al. Sperm-carried RNAs play critical roles in mouse embryonic development. Oncotarget 8, 67394–67405 (2017).
Sharma, U. et al. Small RNAs are trafficked from the epididymis to developing mammalian sperm. Dev. Cell 46, 481-494.e6 (2018).
Sharma, U. et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351, 391–396 (2016).
Estill, M., Hauser, R., Nassan, F. L., Moss, A. & Krawetz, S. A. The effects of di-butyl phthalate exposure from medications on human sperm RNA among men. Sci. Rep. 9, 12397 (2019).
Hoffmann, L. B. et al. Chronically high stress hormone levels dysregulate sperm long noncoding RNAs and their embryonic microinjection alters development and affective behaviours. Mol. Psychiatry. https://doi.org/10.1038/s41380-023-02350-2 (2023).
Champroux, A. et al. Preimplantation embryos amplify stress-induced changes in sperm derived miRNA content to mediate transgenerational epigenetic inheritance. bioRxiv. https://doi.org/10.1101/2023.04.21.537854 (2023).
Jodar, M. et al. Absence of sperm RNA elements correlates with idiopathic male infertility. Sci. Transl. Med. 7, 295re6 (2015).
Hamilton, M., Russell, S., Menezes, K., Moskovtsev, S. I. & Librach, C. Assessing spermatozoal small ribonucleic acids and their relationship to blastocyst development in idiopathic infertile males. Sci. Rep. 12, 20010 (2022).
Burl, R. B., Clough, S., Sendler, E., Estill, M. & Krawetz, S. A. Sperm RNA elements as markers of health. Syst. Biol. Reprod. Med. 64, 25–38 (2018).
World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. (2010).
Goodrich, R. J., Anton, E. & Krawetz, S. A. Isolating mRNA and small noncoding RNAs from human sperm. Methods Mol. Biol. 927, 385–396 (2013).
Estill, M. S., Hauser, R. & Krawetz, S. A. RNA element discovery from germ cell to blastocyst. Nucleic Acids Res. 47, 2263–2275 (2019).
Swanson, G. M. et al. Human chromatin remodeler cofactor, RNA interactor, eraser and writer sperm RNAs responding to obesity. Epigenetics 15, 32–46 (2020).
Conesa, A. et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 17, 13 (2016).
Swanson, G. M., Estill, M. S. & Krawetz, S. A. The transcript integrity index (TII) provides a standard measure of sperm RNA. Syst. Biol. Reprod. Med. 68, 258–271 (2022).
Swanson, G. M. et al. Phthalates impact on the epigenetic factors contributed specifically by the father at fertilization. Epigenet. Chromatin 16, 3 (2023).
Folliero, V. et al. Impact of outer membrane vesicles on sperm function. Pathogens 11, 782 (2022).
Tábara, L. C. et al. TMEM63C mutations cause mitochondrial morphology defects and underlie hereditary spastic paraplegia. Brain 145, 3095–3107 (2022).
Dickinson, M. E. et al. High-throughput discovery of novel developmental phenotypes. Nature 537, 508–514 (2016).
Baltz, A. G. et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol. Cell 46, 674–690 (2012).
Gòdia, M. et al. A RNA-Seq analysis to describe the boar sperm transcriptome and its seasonal changes. Front. Genet. 10, 299 (2019).
Gòdia, M. et al. A technical assessment of the porcine ejaculated spermatozoa for a sperm-specific RNA-seq analysis. Syst. Biol. Reprod. Med. 64, 291–303 (2018).
Matsushima, W., Brink, K., Schroeder, J., Miska, E. A. & Gapp, K. Mature sperm small-RNA profile in the sparrow: Implications for transgenerational effects of age on fitness. Environ. Epigenet. 5, dvz007 (2019).
Fraser, L. et al. Transcriptome analysis of boar spermatozoa with different freezability using RNA-Seq. Theriogenology 142, 400–413 (2020).
Mastora, E., Christodoulaki, A., Papageorgiou, K., Zikopoulos, A. & Georgiou, I. Expression of retroelements in mammalian gametes and embryos. In Vivo 35, 1921–1927 (2021).
Chen, X. et al. Early cleavage of preimplantation embryos is regulated by tRNA-derived small RNAs present in mature spermatozoa. J. Biol. Chem. 295, 10885–10900 (2020).
Jachowicz, J. W. et al. LINE-1 activation after fertilization regulates global chromatin accessibility in the early mouse embryo. Nat. Genet. 49, 1502–1510 (2017).
Gapp, K. et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 17, 667–669 (2014).
Rodgers, A. B., Morgan, C. P., Leu, N. A. & Bale, T. L. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. U. S. A. 112, 13699–13704 (2015).
Chen, Q. et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400 (2016).
Chen, Q., Yan, W. & Duan, E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet. 17, 733–743 (2016).
Abu-Halima, M. et al. MicroRNAs in combined spent culture media and sperm are associated with embryo quality and pregnancy outcome. Fertil. Steril. 113, 970-980.e2 (2020).
Alves, M. B. R., Celeghini, E. C. C. & Belleannée, C. From sperm motility to sperm-borne microRNA signatures: New approaches to predict male fertility potential. Front. Cell Dev. Biol. 8, 791 (2020).
Rahbar, S. et al. MicroRNA-based regulatory circuit involved in sperm infertility. Andrologia 52, e13453 (2020).
Miao, J., Zhu, Y., Xu, L., Huang, X. & Zhou, X. miR-181b-5p inhibits trophoblast cell migration and invasion through targeting S1PR1 in multiple abnormal trophoblast invasion-related events. Mol. Med. Rep. 22, 4442–4451 (2020).
Reilly, J. N. et al. Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci. Rep. 6, 31794 (2016).
Cui, N. et al. MicroRNA-224 regulates self-renewal of mouse spermatogonial stem cells via targeting DMRT1. J. Cell. Mol. Med. 20, 1503–1512 (2016).
Abu-Halima, M., Becker, L. S., Ayesh, B. M. & Meese, E. MicroRNA-targeting in male infertility: Sperm microRNA-19a/b-3p and its spermatogenesis related transcripts content in men with oligoasthenozoospermia. Front. Cell Dev. Biol. 10, 973849 (2022).
Sha, Y. et al. is identified as a novel candidate gene of severe asthenozoospermia. Syst. Biol. Reprod. Med. 65, 465–473 (2019).
Priyanka, P. P. & Yenugu, S. Coiled-coil domain-containing (CCDC) proteins: Functional roles in general and male reproductive physiology. Reprod. Sci. 28, 2725–2734 (2021).
Younis, S. et al. Ablation of ZC3H11A causes early embryonic lethality and dysregulation of metabolic processes. Proc. Natl. Acad. Sci. U S A. 120(23), e2216799120 (2023).
Acknowledgements
The CReATe research team, especially the BioBank and Genetics teams, were essential to the completion of this project. We also thank all study participants who generously consented to donate their materials and information for this project.
Funding
Funding was provided by Canadian Fertility and Andrology Society and Charlotte B. Failing Professorship.
Author information
Authors and Affiliations
Contributions
M. H. completed the experimental protocols (RNA isolation, cDNA library preparation and next-generation sequencing), assisted with data analysis, and completed the first draft of the manuscript. S. R. directed the investigation, completed bioinformatics and other analyses, and assisted with manuscript composition. G. M. S. and S. A. K. completed deep bioinformatics analyses and assisted with manuscript composition and S. A. K. directed the investigation. K. M. assisted with experimental protocols and completed optimization experiments. S. M. and C. L. led patient recruitment, provided project direction, and aided in manuscript composition. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamilton, M., Russell, S., Swanson, G.M. et al. A comprehensive analysis of spermatozoal RNA elements in idiopathic infertile males undergoing fertility treatment. Sci Rep 14, 10316 (2024). https://doi.org/10.1038/s41598-024-60586-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60586-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.