Abstract
In recent years, using nanomaterials based on multi-wall carbon nanotubes (MWCNT) through the activation of peroxymonosulfate (PMS) has attracted more attention to the degradation of organic pollutants. This research presented a new route for the synthesis of MWCNT/CoMn2O4 nanocomposite for the degradation of picric acid using advanced oxidation processes (AOPs). Firstly, CoMn2O4 nanoparticles were prepared and then loaded on MWCNT using ultrasonic waves. The results of various analyzes confirmed the successful loading of nanoparticles on carbon nanotubes. As the degradation process proceeds through oxidation processes, the high electronic conductivity of MWCNT and the active sites of Mn and Co in the nanocomposite play an essential role in activating PMS to generate reactive oxygen species (ROS). An investigation of the reaction mechanism in different conditions showed that the highest speed of picric acid decomposition in the presence of nanocomposite (98%) was in 47 min. However, the scavenger test showed that HO· and SO4·− radicals are more important in the degradation process. Meanwhile, the results showed that removing picric acid using MWCNT/CoMn2O4 was more effective than CoMn2O4 alone and confirmed the interaction effect of MWCNT nanotubes with AB2O4 nanocatalyst.
Similar content being viewed by others
Introduction
The rapid technological developments, the growing world population, and the increased diversification of industries have made the issue of supplying clean water one of the most challenging issues around1. Water is contaminated by different substances such as antibiotics, dyes, herbicides, etc., which severely threatens the health of humans, animals, and plants2,3. Dyes are one of the most critical water pollutants due to their use in various industries, such as leather, paper, and food4,5. Picric acid, or 2,4,6-trinitrophenol (TNP), is extensively employed in various industries such as pharmaceuticals, leather, explosives, chemicals, and dyes. Due to its high solubility in water, picric acid is one of the most significant water pollutants6. Due to the harmful effects of picric acid on the human body, especially the skin, kidneys, eyes, liver, and lungs, it is necessary to remove it from water7.
Diverse methods have been reported for removing contamination from water, such as ozonation8, photocatalytic treatment9, degradation10,11, adsorption/separation12, and the Fenton process13. In recent years, the use of advanced oxidation procedures to remove pollutants from the water has received much attention14,15,16. Pollutants are removed using the progressive oxidation method by converting them into active radicals, such as sulfate (SO4·−) and hydroxyl radicals (·OH). The activation of persulfate and peroxymonosulfate (PMS), as well as covering peroxodisulfate (PDS), is one of the most effective sources for the production of SO4·− radical17.
However, we used PMS to degrade picric acid due to its availability, high activity, and cheapness. In recent decades, the spinel oxide AB2O4 (A/B = transition metal ions) catalysts and their composites have been utilized extensively because of their excellent synergistic effect between two kinds of metallic ions for removing water pollutants18,19. Using carbon nanotubes, due to their unique properties, such as high thermal conductivity and mechanical strength, as a substrate for the stabilization and stability of nanoparticles has received much attention from researchers20,21. Many reports have been presented in the field fabrication of CoMn2O4 nanoparticles based on various carbon materials, including pg-C3N4/CoMn2O422, CoMn2O4@N-rGA23, CoMn2O4/NC24, and CoMn2O4/C hollow spheres25. Our findings show that no research has been reported on utilizing MWCNT/CoMn2O4 nanostructure in the removal reaction of picric acid through activation PMS. In this study, the prepared nanocomposite performed excellently in activating PMS for the degradation of picric acid as an organic contamination. The combination of cobalt and manganese nanoparticles with carbon nanotubes is due to the high activity in removing pollutants and electron transfer on carbon nanotubes. This research proposes an easy and new method for preparing of MWCNT/CoMn2O4 nanocomposite. Also, the synergistic effect CoMn2O4 of nanoparticles on multi-walled carbon nanotubes for the degradation of picric acid via the activation of peroxymonosulfate was investigated. Evaluation of picric acid degradation reaction using MWCNT/CoMn2O4 of nanocomposite in different conditions obtained good results. As far as we know, the economic preparation of nanocomposite that can activate peroxymonosulfate for the degradation of picric acid has not been presented.
Experimental
Chemicals
Multi-wall carbon nanotube (MWCNT), cobalt(II) chloride, manganese(II) acetate, oxalic acid, sodium hydroxide (NaOH), benzoquinone (BQ), ethanol (EtOH), potassium dichromate (K2Cr2O7), sodium azide (NaN3), and isopropyl alcohol (IPA) were procured from Sigma-Aldrich, )Germany( and Merck (Germany). Distilled water and ethanol were used as solvents in all processes. Meantime, picric acid (96% purity) was procured from Alvan Sabet Company (Iran).
Instrumentation
Infrared spectra were obtained utilizing a (Shimadzu IR-640 spectrometer, Japan). XRD diffraction patterns were identified with a (Philips-PW 1730, Germany). Field Emission Scanning Electron Microscope (FE-SEM) and sample mapping were prepared using a (TESCAN MIRA3 microscope, Czech Republic). Transmission electron microscopy (TEM) was recorded using (Philips EM 208S, Netherlands). The images of the surface roughness of the nanostructure were obtained using the Atomic Force Microscope (AFM, Brisk, Germany). X-ray Photoelectron Spectroscopy (XPS, EA 10 analysis system, Germany) determined the type of bonds and chemical composition. The electronic vibrations of the nanocomposite and the degradation efficiency were investigated with (Labmann LMSP UV-1200, Canada).
Fabrication of CoMn2O4 nanoparticles
The CoMn2O4 nanoparticles were fabricated in conformity with Wang et al.'s report with a few corrections22. To form a pure solution, 1.74 g of Mn(CH3CO2)2 and 650 mg of CoCl2 were dissolved in a mixture of water–ethanol with a ratio (1:1). Then, 1 g of oxalic acid was added to the medium reaction under vigorous stirring. After a while, to adjust the pH of the solution to 7 that 1 M of sodium hydroxide was used slowly dropwise, and the resulting solution was stirred at 80 °C for 10 h. After cooling down naturally, the black color as prepared was filtered, rinsed many times with ethanol/water, and dried at 80 °C. Eventually, the compound prepared was calcined for 3 h at 500 °C.
Fabrication of MWCNT/CoMn2O4 nanocomposite
In the fabrication of this nanocomposite, 20 mg of MWCNT was added in 10 mL of ethanol to prepare a suspension solution via sonication for 60 min (Fig. 1). Then, 20 mg of as-obtained CoMn2O4 was added to the reaction mixture and sonicated (30 min) to significant adsorption of CoMn2O4 nanoparticles on the multi-wall carbon nanotubes. Next, the process admixture was often filtered and rinsed with ethanol/water. Eventually, it was dried at 80 °C for 12 h to produce MWCNT/CoMn2O4 nanocomposite26.
Degradation test
The degradation process of picric acid was investigated in the presence of various dosages of MWCNT/CoMn2O4 nanocomposite as a PMS activator. The removal reaction of picric acid was checked under different conditions and using various nanocomposite dosages as a PMS activator. In the optimum condition, the removal reaction of picric acid by adding 0.05 g/L MWCNT/CoMn2O4 was put into 100 mL of picric acid solution (10 mg/L) in 30 min until it reached the adsorption–desorption equilibrium. After a while, to generate reactive species oxygen (ROS), PMS (0.3 g/L) was added to the primal process admixture. In the end, the degradation reaction was investigated via a UV–Vis spectrophotometer. The prominent absorbance peak of picric acid was observed at 354 nm, and due to the following relation, the removal efficiency was calculated:
Co and Ct are the final and initial picric acid concentrations, respectively.
Results and discussion
In addition to the scientific findings in developing nanoparticles to degrade organic pollutants27,28,29,30,31, in the present research, a new strategy for the preparation of MWCNT/CoMn2O4 nanocomposite for the degradation of picric acid through the activation of peroxymonosulfate has been presented. The strategy of MWCNT/CoMn2O4 of nanocomposite synthesis consists of 2 steps, in which CoMn2O4 nanoparticles were prepared in step 1. In step 2, for the synthesis of MWCNT/CoMn2O4 nanocomposite, CoMn2O4 nanoparticles prepared using ultrasonic waves were placed on the walls of carbon nanotubes. The unique properties of carbon nanotubes have caused changes in the performance of nanoparticles and increased catalytic power. Therefore, the performance of the synthesized nanocomposite was evaluated in the process of picric acid degradation as an organic pollutant through peroxymonosulfate activation. In comparing this nanocomposite with other nanocomposites32,33,34,35,36, we can point out its distinctive features such as easy preparation, short degradation time, structural checking via various analyses, especially XPS and AFM spectrum, high degradation efficiency, excellent performance by few dosages of the nanocomposite in the degradation process, degradation with the easy method without the need to sunlight and radiation, and investigating the removal picric acid as a vital contamination in drug and industries.
Characterization
The X-ray diffraction pattern has been used to identify and distinguish the crystal structure and crystal size of the synthesized CoMn2O4 nanocatalyst and MWCNT/CoMn2O4 nanocomposite. As shown in Fig. 2b, the different peaks emerged in 18.67\(^\circ\), 29.37\(^\circ\), 31.57\(^\circ\), 33.32\(^\circ\), 44.82\(^\circ\), 61.12\(^\circ\), and 65.53\(^\circ\) were related to (102), (110), (202), (213), (222), (225), and (402) in CoMn2O4 nanoparticles surfaces (JCPDS No. 29-1487), respectively. Among various peaks, 36.72\(^\circ\)(104) was obtained, corresponding to the CoMn2O4 nanocatalyst37. In addition, a peak at 26.78\(^\circ\) can belong to multi-wall nanocarbon nanotubes38. Based on the findings from XRD patterns, both CoMn2O4 and MWCNT/CoMn2O4 nanocomposites were successfully prepared.
The FT-IR spectrum was investigated to find the functional groups in CoMn2O4 and MWCNT/CoMn2O4 nanocomposite from 400 to 4000 cm−1. As indicated in Fig. 2a, a strong peak near 3445 cm−1 corresponding to O–H stretching appeared due to the absorption of H2O on the nanocomposite surface. However, there are various peaks at 1126, 1390, 1627, and 2930 cm−1, which were related to C–C, C–O, C=O, and C–H, respectively. MWCNT/CoMn2O4 nanocomposite has two peaks at 626 and 543 cm−1 because of Co–O and Mn–O bonds, respectively39,40. Based on the peaks in the nanoparticle and nanocomposite structure (related to each of the functional groups) that are characteristic of the pure and correct form of the products after synthesis, it may be concluded that the compounds were produced adequately.
The electronic states and elemental composition of the CoMn2O4 and MWCNT/CoMn2O4 nanostructure were assessed by XPS, as shown in Fig. 3. The full scan XPS spectrum of the nanostructure (Fig. 3a) displayed the existence of elements C, Co, Mn, and O in the CoMn2O4 and MWCNT/CoMn2O4 nanostructure. The high-resolution spectrum of C 1s is divided into four peaks (Fig. 3b), which involving energy bands of 291.40 eV are related to the 284.7 eV to the C=C bond 285.3 eV to the C−C bond, 286.3 eV to the C–O bond, and 287.7 eV to the C=O bond. Figure 3c indicates the high-resolution XPS spectrum of Co 2p. The Co atom was demonstrated in two oxidation states, Co+2 and Co+3, in the structure of the MWCNT/CoMn2O4 compound. Two peaks correspond to Co 2p1/2 and Co 2p3/2. The peak at 932.15 eV is assigned to Co2+, while two peaks at 936.4 and 954.17 eV can attributed to Co3+. Peaks at 529.7, 531.5, and 533.6 eV in the O 1s XPS spectra (Fig. 3d) can be linked to hydroxyl groups, metal–oxygen bonds, and absorbed water, respectively. High-resolution XPS spectrum of Mn appeared in Mn2+, Mn3+, and Mn4+ oxidation states (Fig. 3e). The peak related to Mn2+ appeared at 640.6, while the two peaks linked to Mn3+ were revealed at 642.2 and 653.4 eV. In addition, peaks belonging to Mn4+ were observed at 644.2 and 655 eV.
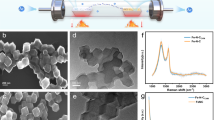
FESEM, TEM, and AFM investigated the structure and surface morphology of CoMn2O4 and MWCNT/CoMn2O4 nanocomposite. As displayed in Fig. 4a, the CoMn2O4 nanoparticles have a symmetrical geometry morphology with cuboid microcrystals. Also, the thickness of nanoparticles was evaluated between 0.1 and 1 µm. Meanwhile, the MWCNT appeared in tubular morphology, corroborating the loading of CoMn2O4 nanostructures on the MWCNT (Fig. 4b). Elemental distribution maps and EDX spectra were utilized to measure the elements′ distribution, and nanocomposite purity. Both analyses' results acknowledged the high purity of carbon, cobalt, manganese, and oxygen elements in the nanocomposite structure (Fig. 4c). The result obtained from elemental mapping analysis is exhibited in Fig. 4d.
An atomic force microscope (AFM) is an essential and helpful instrument for checking the topography of the surface on a nanometer scale. This microscope is used to image the roughness of the nanostructure surface and provide two- and three-dimensional images. The results obtained from the AFM analysis of the nanocomposite structure are indicated in Fig. 5. As shown in Fig. 5, the CoMn2O4 nanoparticles have mountain-like morphology with white edges. In contrast, MWCNT has a non-intersecting surface on which CoMn2O4 nanoparticles were placed. In the meantime, the surface roughness of the MWCNT/COMn2O4 nanocomposite was estimated at 25.62 nm.
TEM is applied to clarify the structure and the morphology of MWCNT/CoMn2O4 nanocomposite even more. The results of the images can be seen in the Fig. 6. These images depict nanoparticles correctly placed on multi-wall carbon nanotubes with high magnification. CoMn2O4 nanoparticles are loaded in black aggregates similar to those between the walls of carbon nanotubes. Compared to SEM, the TEM analysis gives a detailed look at the nanocomposite.
Degradation efficiency
Due to the severe water scarcity in many places, the destruction of organic pollutants, particularly picric acid, which is detrimental to the human body, animals, and plants, has come to notice. Hence, MWCNT/CoMn2O4 nanocomposite, which is totally synthesized and characterized afterward, is employed in this study to act as the PMS activator to degrade picric acid efficiently. The efficiency of the nanocomposite in the production of picric acid hydrolysis was determined by performing the test in the presence of various factors, and the results are shown in Fig. 7. The consequences displayed that utilization of the CoMn2O4 caused degradation of picric acid with yield degradation of 24% within 120 min. Without PMS, MWCNT/CoMn2O4 nanocomposite can only remove about 12% of picric acid in 80 min. The reaction of the picric acid degradation using both PMS (0.3 g/L) and MWCNT/CoMn2O4 nanocomposite (0.05 g/L) went well as expected. Also, the degradation of the picric acid takes place within 47 min. In the degradation of picric acid from wastewater, the offered nanocomposite can compete with the other nanocomposites (Table 1).
Impact of various factors
The effect of picric acid concentration was investigated from 10 to 30 ppm in the picric acid degradation, and the degradation yield of picric acid reduced from 98 to 58% (Fig. 7a). This can be related to the saturation of reactive levels in the nanocomposite, which reduces ROS. Also, the effect of the MWCNT/CoMn2O4 nanocomposite dosage was measured in dosages of 0.05, 0.1, 0.15, and 0.2 g/L in the picric acid removal. As indicated in Fig. 7b, because of efficient nanoparticles of cobalt, manganese and the high electrical conductivity of carbon nanotubes on the level of the nanocomposite, the picric acid removal increased between 79 and 98% within 47 min. Nonetheless, the degradation process of picric acid was evaluated at PMS concentrations of 0.1, 0.2, 0.3, and 0.4 g/L, and picric acid removal was enhanced from 64 to 98% (Fig. 7c). The good impact of this factor is because of the delivery of reactive oxygen species, including HO·, O2·−, 1O2, and SO4·−. The degradation reactions of picric acid using MWCNT/CoMn2O4 nanostructure at different pHs from 3 to 10 were studied and displayed in Fig. 7d. The findings acquired from the impact of pH on the removal reactions specified that the removal of picric acid occurred more successfully in alkaline than in acidic pH. After that, the scavenger experiment was utilized to investigate the radical species included in the removal reaction. We conducted studies on the removal reaction of picric acid by use of various scavengers such as sodium azide (NaN3), ethanol, benzoquinone (BQ), isopropyl alcohol (IPA), and potassium dichromate (K2Cr2O7). According to Fig. 7e, the species HO· and SO4·− are mostly noticed as the significant radicals in degradation reactions, and the catalytic performance must be a low weaken using of EtOH for scavenging HO· and SO4·−. As provided in Fig. 7e, when EtOH was used as a scavenger, the picric acid degraded by efficacy 77% after 47 min. In conjunction with it, the yield removal of picric acid using IPA was 59%. In conformity with the finding, it was determined that EtOH and IPA have an excellent scavenging action compared to sulfate and hydroxyl species. To check the activity of nanostructure for practical applications in the removal reaction, we performed the process on a large scale. For this reason, we utilized picric acid (10 mg/L) of MWCNT/CoMn2O4 nanostructure (0.05 g/L) and PMS (0.3 g/L) to carry out the removal reaction on a larger scale, which caused a more effective remove of picric acid in 47 min. The outcomes indicated that this process is practically stable and manageable.
Proffered mechanism of picric acid removal
Based on the findings, a possible mechanism was presented for the degradation of picric acid via radicals and electron transforms (Fig. 8). The results indicated that placing CoMn2O4 nanoparticles onto the multi-wall carbon nanotubes caused an increase in transform electron and degradation yield. Meanwhile, there are many methods for producing free radicals, including ozone, hydrogen peroxide, and persulfate41. In this study, MWCNT/CoMn2O4 nanocomposite removed the organic pollutant by using PMS to produce ·OH, SO4·−, 1O2, and O2·− prepared radicals. However, the SO4·− radical reacts highly with organic contaminations because of its excellent oxidative potential42. Also, the scavenging test was utilized to show the roles of ROS in the degradation reaction. In the removal process, different scavengers, such as sodium azide (NaN3), potassium dichromate (K2Cr2O7), isopropyl alcohol (iPrOH), ethanol (EtOH), and benzoquinone (BQ), have been checked to trap radicals. According to the findings, the degradation process happened with a higher yield in ethanol than in the presence of other scavengers. The prominent radicals in the reaction are OH· and SO4·− due to the ethanol being a trapper of OH· and SO4·−. The manganese and cobalt on the surface of MWCNT were reacted by PMS (HSO5−) to generate SO4·− and ·OH with Eqs. (1) and (2)43. Then, to create ·OH, water was reacted with SO4·− radicals (Eq. 3). In the next step, the Co+ ion and SO5·− radical were prepared for the reaction of Co2+ ions on the surface MWCNT/CoMn2O4 by PMS (Eq. 4). Since the Co1+ ion converted to Co2+ (Eq. 5). Meantime, Co2+, SO42-, and OH· were prepared from the reaction Co1+ ions with HSO5− (Eq. 6)44. Respectively, the SO4·− and SO5·− were prepared from the reaction of the Mn2+ and Mn3+ ions on the surface MWCNT/CoMn2O4 with HSO5− (Eqs. 7 and 8)45. However, multi-wall carbon nanotubes can have an essential obligation in electron transfer to PMS because of their distinctive properties (Eq. 9). In the end, to complete the degradation process of organic contaminant, the OH· and SO4·− radicals with the picric acid were turned into H2O, CO2, and intermediate (Eq. 10).
Reusability investigations
Compared to previous works, one of the distinguishing features of this work is the ability to reuse the nanocomposite during several cycles. In this research, to investigate the reusability of the nanocomposite after performing the desired reaction, we separated the nanocomposite via a centrifuge (2800 rpm for 15 min) from the reaction mixture, several times rinsed with water/ethanol and next dried it at 100 °C during 7 h. According to Fig. 9, the MWCNT/CoMn2O4 nanocomposite was recycled during four cycles, and no significant change in its activity was observed during the recycling process. Also, the structure of the recycled nanocomposite was studied through different analyses, such as FESEM (Fig. 9b) and TEM (Fig. 9c). Investigations showed that its structure remained almost constant.
Conclusions
In this research, a new generation of MWCNT/CoMn2O4 nanocomposite was designed and successfully made to degrade picric acid. The results obtained through different analyzes confirmed the growth and stabilization of CoMn2O4 nanoparticles on MWCNT. The presence of cobalt and manganese active sites on nanoparticles and the growth of CoMn2O4 nanoparticles on MWCNT as a substrate showed a significant increase in catalytic activity towards the degradation of picric acid. In addition, the investigation of the reaction mechanism and various tests, especially the scavenger test, confirmed that SO4·− and ·OH radicals play an essential role in the degradation of picric acid. Finally, the current study provides new insight into designing and fabricating nanoparticles on carbon nanotubes to improve water treatment.
Data availability
All data have been given in the article.
References
Shang, Y., Xu, X., Gao, B., Wang, S. & Duan, X. Single-atom catalysis in advanced oxidation processes for environmental remediation. Chem. Soc. Rev. 50, 5281–5322 (2021).
Alharbi, O. M., Khattab, R. A. & Ali, I. Health and environmental effects of persistent organic pollutants. J. Mol. Liq. 263, 442–453 (2018).
Balkanloo, P. G., Sharifi, K. M. & Poursattar Marjani, A. Graphene quantum dots: Synthesis, characterization, and application in wastewater treatment: A review. Mater. Adv. 4, 4272–4293 (2023).
Xin, Z. et al. Novel nanozyme Ag/Fe3O4@h-BN with peroxidase-mimicking and oxidase-mimicking activities for dye degradation, As(V) removal and detection. Chem. Eng. J. 461, 141589 (2023).
Sarreshtehdar Aslaheh, H., Poursattar Marjani, A. & Gozali Balkanloo, P. Pelargonium as a cost-effective additive in bio-composite adsorbent in removing dyes from wastewater: Equilibrium, kinetic, and thermodynamic studies. J. Polym. Environ. 31, 3230–3247 (2023).
Hussain, S., Malik, A. H., Afroz, M. A. & Iyer, P. K. Ultrasensitive detection of nitroexplosive-picric acid via a conjugated polyelectrolyte in aqueous media and solid support. Chem. Commun. 51, 7207–7210 (2015).
Kumar, R. et al. Fe-doped ZnO nanoellipsoids for enhanced photocatalytic and highly sensitive and selective picric acid sensor. Mater. Res. Bull. 102, 282–288 (2018).
Hassani, A., Khataee, A., Fathinia, M. & Karaca, S. Photocatalytic ozonation of ciprofloxacin from aqueous solution using TiO2/MMT nanocomposite: Nonlinear modeling and optimization of the process via artificial neural network integrated genetic algorithm. Process. Saf. Environ. Protect. 116, 365–376 (2018).
Alamgholiloo, H. et al. Facile fabrication of Z-scheme TiO2/ZnO@MCM-41 heterojunctions nanostructures for photodegradation and bioactivity performance. J. Mol. Liq. 364, 119990 (2022).
Matzek, L. W., Tipton, M. J., Farmer, A. T., Steen, A. D. & Carter, K. E. Understanding electrochemically activated persulfate and its application to ciprofloxacin abatement. Environ. Sci. Technol. 52, 5875–5883 (2018).
Farajollahi, A., Poursattar Marjani, A., Noroozi Pesyan, N. & Alamgholiloo, H. Efficient degradation of crystal violet by GO/CuMn2O4 nanocomposite via peroxymonosulfate activation. Appl. Surf. Sci. 622, 156903 (2023).
Konwar, A., Gogoi, A. & Chowdhury, D. Magnetic alginate-Fe3O4 hydrogel fiber capable of ciprofloxacin hydrochloride adsorption/separation in aqueous solution. RSC Adv. 5, 81573–81582 (2015).
Shoorangiz, M., Nikoo, M. R., Salari, M., Rakhshandehroo, G. R. & Sadegh, M. Optimized electro-Fenton process with sacrificial stainless steel anode for degradation/mineralization of ciprofloxacin. Process. Saf. Environ. Protect. 132, 340–350 (2019).
Liu, Y. & Wang, J. Multivalent metal catalysts in Fenton/Fenton-like oxidation system: A critical review. Chem. Eng. J. 466, 143147 (2023).
Liu, Y., Zhao, Y. & Wang, J. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J. Hazard. Mater. 404, 124191 (2021).
Wang, J. & Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 701, 135023 (2020).
Wang, J. & Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 334, 1502–1517 (2018).
Zhao, L., Yang, D., Ma, L., Feng, X. & Ding, H. An efficient heterogeneous catalyst of FeCo2O4/g-C3N4 composite for catalytic peroxymonosulfate oxidation of organic pollutants under visible light. Colloids Surf. A Physicochem. Eng. Asp. 610, 125725 (2021).
Qiu, S., Gou, L., Cheng, F., Zhang, M. & Guo, M. An efficient and low-cost magnetic heterogenous Fenton-like catalyst for degrading antibiotics in wastewater: Mechanism, pathway and stability. J. Environ. Manag. 302, 114119 (2022).
Shoukat, R. & Khan, M. I. Carbon nanotubes: A review on properties, synthesis methods and applications in micro and nanotechnology. Microsyst. Technol. 27, 4183–4192 (2021).
Chen, C., Ali, A., Toufouki, S., Jiang, W. & Yao, S. A hybrid electro-driven adsorption and degradation system for antibiotics and dyes based on composite tablets composed of deep eutectic solvent-functionalized nanomaterials. Chem. Eng. J. 471, 144776 (2023).
Wang, Y., Nie, Z., Li, X., Zhao, Y. & Wang, H. Highly sensitive and selective electrochemical sensor based on porous graphitic carbon nitride/CoMn2O4 nanocomposite toward heavy metal ions. Sens. Actuators B Chem. 346, 130539 (2021).
Ren, F. et al. CoMn2O4 nanoparticles embed in graphene oxide aerogel with three-dimensional network for practical application prospects of oxytetracycline degradation. Sep. Purif. Technol. 259, 118179 (2021).
Ho, J. et al. Ionothermal synthesis of N-doped carbon supported CoMn2O4 nanoparticles as ORR catalyst in direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 46, 20503–20515 (2021).
Ma, Y. et al. Sandwich-shell structured CoMn2O4/C hollow nanospheres for performance-enhanced sodium-ion hybrid supercapacitor. Adv. Energy Mater. 12, 2103820 (2022).
Katowah, D. F. et al. Selective fabrication of an electrochemical sensor for Pb2+ based on poly (pyrrole-co-o-toluidine)/CoFe2O4 nanocomposites. ChemistrySelect 4, 10609–10619 (2019).
Mohammed Mohammed, H. A. et al. A novel biosynthesis of MgO/PEG nanocomposite for organic pollutant removal from aqueous solutions under sunlight irradiation. Environ. Sci. Pollut. Res. 30, 57076–57085 (2023).
Davarazar, M. et al. Activation of persulfate using copper oxide nanoparticles for the degradation of Rhodamine B containing effluents: Degradation efficiency and ecotoxicological studies. Chem. Eng. J. 453, 139799 (2023).
Akter, J., Hanif, M. A., Islam, M. A., Sapkota, K. P. & Hahn, J. R. Selective growth of Ti3+/TiO2/CNT and Ti3+/TiO2/C nanocomposite for enhanced visible-light utilization to degrade organic pollutants by lowering TiO2-bandgap. Sci. Rep. 11, 9490 (2021).
Zhang, Y. et al. Construction of TiO2/Ag3PO4 nanojunctions on carbon fiber cloth for photocatalytically removing various organic pollutants in static or flowing wastewater. J. Colloid Interface Sci. 571, 213–221 (2020).
Kumar, I., Mondal, M., Meyappan, V. & Sakthivel, N. Green one-pot synthesis of gold nanoparticles using Sansevieria roxburghiana leaf extract for the catalytic degradation of toxic organic pollutants. Mater. Res. Bull. 117, 18–27 (2019).
Sharifi, A. et al. Investigation of photocatalytic behavior of modified ZnS: Mn/MWCNTs nanocomposite for organic pollutants effective photodegradation. J. Environ. Manag. 247, 624–632 (2019).
Chahal, S. et al. Enhanced photocatalytic degradation of organic dye by CeO2/CNT/GO hybrid nanocomposites under UV light for wastewater treatment. Environ. Sci. Pollut. Res. 30, 124964–124975 (2023).
Altowyan, A. S. et al. Removal of methylene blue dye from aqueous solution using carbon nanotubes decorated by nickel oxide nanoparticles via pulsed laser ablation method. Radiat. Phys. Chem. 198, 110268 (2022).
Al-Musawi, T. J., Rajiv, P., Mengelizadeh, N., Arghavan, F. S. & Balarak, D. Photocatalytic efficiency of CuNiFe2O4 nanoparticles loaded on multi-walled carbon nanotubes as a novel photocatalyst for ampicillin degradation. J. Mol. Liq. 337, 116470 (2021).
Liu, S. et al. Anchoring Fe3O4 nanoparticles on carbon nanotubes for microwave-induced catalytic degradation of antibiotics. ACS Appl. Mater. Interfaces 10, 29467–29475 (2018).
Pattanayak, B. et al. Role of precursors mixing sequence on the properties of CoMn2O4 cathode materials and their application in pseudocapacitor. Sci. Rep. 9, 16852 (2019).
Al-Musawi, T. J., Mengelizadeh, N., Ganji, F., Wang, C. & Balarak, D. Preparation of multi-walled carbon nanotubes coated with CoFe2O4 nanoparticles and their adsorption performance for bisphenol A compound. Adv. Powder Technol. 33, 103438 (2022).
Sandosh, T. A. & Simi, A. Morphology controlled synthesis of one-dimensional CoMn2O4 nanorods for high-performance supercapacitor electrode application. Chem. Pap. 75, 2295–2304 (2021).
Vigneshwaran, P. et al. A study on the synthesis and characterization of CoMn2O4 electrode material for supercapacitor applications. J. Mater. Sci. Mater. Electron. 27, 4653–4658 (2016).
Lu, Y. et al. Impacts of soil and water pollution on food safety and health risks in China. Environ. Int. 77, 5–15 (2015).
Gao, Y. et al. Activity trends and mechanisms in peroxymonosulfate-assisted catalytic production of singlet oxygen over atomic metal–N–C catalysts. Angew. Chem. Int. Ed. 60, 22513–22521 (2021).
Shah, N. S. et al. Hydroxyl and sulfate radical mediated degradation of ciprofloxacin using nano zerovalent manganese catalyzed S2O82−. Chem. Eng. J. 356, 199–209 (2019).
Yao, Y. et al. Sulfate radicals induced from peroxymonosulfate by cobalt manganese oxides (CoxMn3−xO4) for Fenton-like reaction in water. J. Hazard. Mater. 296, 128–137 (2015).
Chen, Z., Bi, S., Zhao, G., Chen, Y. & Hu, Y. Enhanced degradation of triclosan by cobalt manganese spinel-type oxide activated peroxymonosulfate oxidation process via sulfate radicals and singlet oxygen: Mechanisms and intermediates identification. Sci. Total Environ. 711, 134715 (2020).
Acknowledgements
We gratefully acknowledge Urmia University for financially supporting this research project.
Author information
Authors and Affiliations
Contributions
Ayda Farajollahi: Data curation, Investigation, and Writing-original draft. Ahmad Poursattar Marjani: Project administration, Supervision, Investigation, Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farajollahi, A., Poursattar Marjani, A. Preparation of MWCNT/CoMn2O4 nanocomposite for effectual degradation of picric acid via peroxymonosulfate activation. Sci Rep 14, 11475 (2024). https://doi.org/10.1038/s41598-024-62351-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62351-1
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.