Abstract
Since the detection of phosphine in the wastewater treatment plants in 1988, more and more investigations revealed that phosphine is closely related to ecological activities on a global scale. Here, we present perspectives on the whole dynamic cycles of phosphorus, particularly in terms of phosphine and its interactions with natural ecosystems, as well as the impacts from human activities. It may conclude that the phosphine-driving cycles of phosphorus depend on the coordination of human activities with natural ecosystems. Most importantly, the extensive recovery of phosphorus in numerous urban wastewater treatment plants may seriously obstruct its global cycles to catch up with the ecological needs in natural ecosystems. Phosphine gas plays an important role in the biogeochemical phosphorus cycle. Phosphorus might be one of the important elements participating in the global climate change together with carbon and nitrogen.
Similar content being viewed by others
Introduction
In the millions of years’ evolution of the global natural ecosystem, the sustainability of this ecological system formed by many element cycles is self-regulated and complex. Most studies focused on the carbon, nitrogen, and sulfur cycles because phosphorus was considered not to exist in gaseous forms. Actually, as one of the critical elements, phosphorus, is cycling in a specific form, i.e., gaseous phosphine (PH3), and getting involved actively in ecological interactions. Now phosphorus might be one of the important elements participating in the global climate change together with carbon and nitrogen.
Occurrence of phosphine
For a long time, the distribution of phosphorus in the cycle of the hydrosphere was thought to be ambiguous and mass-unbalanced, until the detection of phosphine in wastewater treatment plants by Dévai et al. in 19881. With the development of chromatography techniques and sample pretreatment methods, phosphine has been shown to exist universally in the environment. A large number of investigations on the global scope of phosphine have been conducted all over the world1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55. Phosphine exists universally in the natural environment with two different forms: free gaseous phosphine and matrix-bound phosphine (MBP). The former has been detected in marsh gas36, sludge biogas1, upper troposphere37, and the atmosphere around the world33. MBP has been detected in various types of soils and sediments14,45,50,54, sewage sludge5, and feces55. It is worth noting that MBP is defined as the non-gaseous reduced phosphorus compounds that are transformed into phosphine gas by acid or alkaline digestion45. Thus, MBP does not necessarily refer to the pre-existence of phosphine in the matrix. It generally comprises of phosphine adsorbed by the media, phosphine gas in the interstice, and solid phosphides.
The phosphine concentrations in the atmosphere and the sediments demonstrate significant seasonal variations24,56,57. The phosphine concentrations display higher values in the summertime because the high temperature in summer might induce more microbial activity4. Besides, the thunderstorm weather during summer create lightning, which strikes the phosphate-containing organic matter (e.g., soil, dust) to form a local phosphate reducing condition58, favoring the production of phosphine. Since the agricultural practice, specifically fertilization with phosphate-rich fertilizer, is likely to greatly increase phosphine production59, the impact of the seasonal agriculture activities on the phosphine emission should be analyzed in the future. In addition to the seasonal trend, phosphine concentrations in the atmosphere show an obvious diurnal trend24. That is, atmospheric PH3 levels peak during the early morning because phosphine accumulates in the night atmosphere and degraded gradually until noon due to the effects of increasing light intensity that promotes air oxidation60.
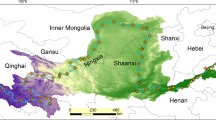
From the detection of free phosphine gas (Fig. 1) and MBP concentrations (Fig. 2) in different environments, it can be concluded that the phosphine levels are seriously impacted by human activities. Generally, the concentrations of phosphine in urbanized and populated areas, are higher than those in the natural ecosystems in rural areas. Glindemann et al. found that the phosphine concentration in the air above the urban areas (e.g., Berlin, Hamburg, Beijing) is 0.62–157 ng/m3 while 0.04–2.03 ng/m3 in rural air, indicating emission by concentrated human activities33. Anthropogenic PH3 production by industry can interfere with the natural cycling of PH3. For example, the exhaust gas from the PH3 fumigation of grain foods in the harbor resulted in high atmospheric PH3 concentrations in Shanghai Harbor28. Besides, high PH3 levels are found in paddy fields and eutrophic lakes13,35,36. The increased PH3 liberating biological activity in polluted ecosystems with agricultural nutrients, such as the excessive P fertilizer, results in the high PH3 values59. Unexpectedly high phosphine concentrations were found in air samples from the poles and the main sources were assumed to be the penguin colonies, guano, and tundra ecosystem26,61.
(Urban air33: 1. Berlin, 2. Leipzig, 3. East Leipzig, 4. Hamburg, 5. Buenos Aires; Rural air33: 6. Leipzig, 7. Hammamet, 8. Israel, 9. Namibia; Pole air7,27,34: 10. Arctic Yellow River station, 11. Arctic New Oldsson region, 12. Antarctica Milo Peninsula; Freshwater area24,35,36: 13. Lake Taihu (Year 2005), 14. Lake Taihu (Year 2011), 15. Beijing Reservoir; Sea area28,37,38: 16. Southwest Yellow Sea, 17. Shanghai Harbor, 18. North-Atlantic Sea, 19. North Sea, 20. Southern Ocean; Marsh Wetland13,15,36: 21. Yancheng Reserve, 22. Beijing paddy fields, 23. Jiangsu Paddy fields, 24. Guangzhou paddy fields; 25. Sludge biogas in Hungary1; Landfill gas39: 26. Beijing, 27. Berlin, 28. Belgium).
(Sludge5,40,41: 1. WWTP in Beijing, 2. WWTPs in Louisiana, USA, 3. Sewage plant in Beijing; Soil14,24,34,42,43,44,45,46: 4. Ardley Island, Antarctica, 5. Arctic Tundra, 6. Beijing paddy, 7. Southern China paddy, 8. Jiangsu paddy, 9. Germany industrial area, 10. Germany rural area, 11. Louisiana marsh, 12. Tropical forest, Mahe, Seychelles; Freshwater sediment14,24,25,40,47,48,49: 13. Hamburg Harbor, 14. Elster River, 15. Elbe River, 16. Lake Illawarra, 17. Taihu Lake, 18. Wulongtang Lake, 19. Shisanling Reservoir, 20. Ming tombs reservoir; Marine Sediment4,11,47,50,51,52,53: 21. Changjiang River Estuary, 22. South Yellow Sea, China, 23. Southwest Yellow Sea, China, 24. Yellow Sea, China, 25. Coastal agriculture area, Jiaozhou Bay, China, 26. Coastal areas, Jiaozhou Bay, 27. Offshore areas, Jiaozhou Bay, 28. Hamburg, Germany, 29. North Sea, Germany; Ornithogenic sediment42,54: 30. Lake Solvatnet, Arctic, 31. Ardley Island, Antarctica, 32. Lake Mochou, Antarctica, 33. Zolotov Island, Antarctica; Feces55: 34. Cattle manure, 35. Swine manure, 36. Feces of man).
Formation and transformation of phosphine in ecosystems
Numerous biological and abiological mechanisms of phosphine formation have been proposed in the literature2,20,23,58,62,63,64,65. Significant evidence demonstrates that the production of phosphine is associated with the microbial reduction of P-containing substances and most of the results have been summarized in a review by Roels and Verstraete62. Biological phosphine formation from mixed bacterial cultures in the lab has been reported in several works2,20,23,63,64. This is consistent with the detection of high phosphine levels in natural environments with significant anaerobic biosphere areas, such as eutrophic lakes, coastal areas, and wetlands35,36,52,53. Non-biological pathways for phosphine formation include corrosion of P-containing metals66, reduction of phosphate by lightning strike67, and mechanochemical reduction of phosphate in minerals65. For example, phosphine is produced when atmospheric lightning strikes the aerosol or soils that contain oxidized forms of phosphorus and chemical reductants58.
In previous studies, phosphorus usually is thought not to undergo redox reactions and the removal of phosphorus in water occurs only from adsorption, complexation, and precipitation68. However, the reduction of phosphate to phosphine occurs commonly in wetlands and paddy fields. It is well-established that the formation of phosphine in nature follows thermodynamics in terms of ORP (oxidation-reduction potential) levels59. Anaerobic microorganisms use a sequence of terminal electron acceptors instead of oxygen during their respiration under the anaerobic condition69. With decreasing redox potential, they reduce nitrates to nitrogen or N2O, sulfates to sulfides, and carbonate to methane62,70. Similarly, the production of PH3 occurs under the reducing conditions while requires more energy and higher reducibility59. When redox potential falls below −300 mV, the phosphate may act as an electron receptor and finally get reduced to phosphine19.
Though the thermodynamics of the production of phosphine by reduction of phosphate is exergonic71, Bains et al. reported that the phosphine production from phosphite is thermodynamically favored in specific ecosystems59. Pasek et al. presented a comprehensive review on the redox chemistry in the phosphorus biogeochemical cycle and proposed that the source of phosphine in the atmosphere is the reduced P compounds, such as phosphite and hypophosphite72,73. As shown in Fig. 2, high concentration of phosphine is detected in Taihu Lake and paddy fields, where relatively high levels of phosphite in basal sediments are observed as well74,75. Consistently, Sun et al. reported that more phosphine was produced from anaerobic activated sludge with hypophosphite as the inorganic phosphorus source than that with phosphate63.
About 10% of the phosphorous in the atmosphere exists as phosphine59,73. Most phosphine is formed in soil, sediments, sludge, or landfill, and prefers to adsorb in the media at a matrix-bound status. Then most phosphine may transform back to phosphates for use by natural plants or microbes76, and the rest may emit from lower layers to surface layers and eventually into the atmosphere.
The living plants in wetlands transfer atmospheric oxygen through aerenchyma to the rhizosphere, keeping a high ORP level in their root areas77. The rhizospheric ORP ranges from 130 to 350 mV in daily time, creating an aerobic microenvironment that is more oxidizing than bulk water (−220 ± 22 mV)78,79. Though the oxygen transportation of plant to roots increases the rhizospheric ORP80 and barely changes the total anaerobic environment in the bulk water, this favors the uptake of P by the plant. Such aerobic conditions may allow phosphine to transform back to phosphates and promote the utilization of phosphorous by plants. On the other hand, if the plants become dead, with no oxygen transporting to the rhizosphere, there will be an anaerobic condition with ORP <−300 mV. In this case, some available phosphates will be reduced back to phosphine, which may emit into the atmosphere.
It is estimated that there are about 40,000 ton/year of phosphine released to the atmosphere81. At certain temperature and under lightning conditions, most of the gaseous phosphine will be oxidized to phosphorus oxides26,82, which might be deposited in clouds or rainwater58,83, becoming a major source of phosphorus for the ecosystems that are poor in phosphorus84.
From Fig. 3, it is found coincidentally that the concentrations of PH3, which are in the sediments, bottom water, and surface water from Taihu Lake of China and in the atmosphere above it, as well as the phosphorus deposition rate, have almost the same monthly variation patterns25,85. The positive correlation between the phosphine and Chl-a has been reported in several studies35,50. It is speculated that phosphine plays an important role in the algal bloom or eutrophication in the lake. Phosphine is produced in sediments, then releases into water and sequentially emits into the atmosphere. Later, mainly in spring and autumn, phosphine may return to the lake water as dry deposition of phosphorus. Consequently, the release of phosphorus from sediments and the dry deposition of phosphorus into the water body would result in algal blooming as indicated by the Chl-α distribution pattern in Fig. 335. In this regard, phosphorus becomes a renewable resource. When phosphorus is not needed, it transforms to phosphine under anaerobic conditions and emits into the atmosphere; in reverse, phosphorus goes back to the ecosystem via dry deposition or rainfall.
Impact of engineering activities on climate change in terms of phosphine
Another issue that is overlooked by scientists is the influence of regional engineering activities on the phosphorus cycle in terms of phosphine. It was reported that Gobi Desert in the northwestern of China is gradually turning green, mainly attributed to the foundation of Three Gorges Reservoir project. This project transports massive amounts of water vapor to the northwestern areas86, probably carrying phosphine, phosphorus oxides as well as plant seeds in the troposphere. Several studies demonstrate that the foundation of Three Gorges Reservoir affect the precipitation, drought, heat wave, local temperature, humidity, and even induce the extreme weather in reservoir regions86,87,88. Therefore, large-scale hydraulic engineering projects may dramatically influence the regional even global climate change. Another grand hydraulic project under planning in China is the Hongqi River water transfer proposal. As shown in Fig. 4, the 6188-km-long Hongqi River will be starting from southeastern Tibet Plateau89, which is going to supply 60 billion m3 water per year to the northwestern inland of China. It could be expected that this grand project will cause an inevitable and marked impact on the phosphorous cycle and the ecosystems.
Phosphine is a reactive atmospheric trace gas, which competes with methane and other greenhouse gases for hydroxyl radicals that are produced by the light degradation of ozone22. Therefore, the presence of phosphine in the atmosphere induces the consumption of ozone, extends the residence time of greenhouse gases, and indirectly enhances the greenhouse effect90. Moreover, phosphine is easily converted into phosphoric acid or phosphate ions in the presence of oxygen and solar radiation60. The phosphoric acid could provide condensation nuclei for cloud formation in the upper troposphere and subsequently influence the global climate37,58. The phosphate ions will deposit to the land and lakes, playing an important role in the biomass growth in phosphorus-limited areas.
Perspectives on future researches
Currently, phosphorus is still considered as a rare resource to be recovered from engineering facilities, such as municipal wastewater treatment plants91. However, the concentration of total phosphorus in raw wastewater is around 10 mg/L, and recovering such highly dissipated phosphorus to pure phosphate requires concentrating for over 105 times. This is an irreversible process that costs more than gains according to the thermodynamic law. Thus recovering phosphorus directly from wastewater is not feasible92. Undoubtedly, the regional recycle of phosphorus as happened in municipal wastewater treatment plants may benefit local farmlands or gardens. However, the accumulation of phosphorus in local areas might result in a runoff of phosphorus during the rainfall season and endanger the local water body due to eutrophication93.
In global ecosystems, the wastewater treatment plant plays a role as a decomposer. If the researchers turn it to be a producer for phosphorus recovery or energy production, the loss will outweigh the gain. Instead, modifying the conventional wastewater treatment processes into a mode that transforms more phosphates into phosphine would benefit the ecological cycle of phosphorus. In this way, all the wastewater treatment plants would become an essential part of the global phosphorus cycle.
The removal of phosphorus from wastewater by phosphine production is accomplished by the phosphate reducing microorganisms in the inoculum (e.g., animal manure, paddy soil) under anaerobic conditions. In general, PH3 emission from conventional biological wastewater treatment is less effective (ng-mg/m3)20. Scholars tried to improve PH3 yield in different wastewater treatment process via artificially strengthened anaerobic digestion systems, such as the anaerobic sequencing batch reactor (ASBR)94, sequencing biofilm batch reactor (SBBR)95, and microbial electrolysis cell (MEC)96. Yang et al. achieved 83% total phosphorus (TP) removal via oxygen-limited membrane bioreactor (OLMBR) and about 19.4% phosphorus was removed by the production of PH3 gas97. In order to find a breakthrough of increasing the phosphine yield, researchers have conducted comparative experiments on the best inoculum sources (e.g., animal manure, paddy soil)95,98, electron donors (i.e., carbon sources)2, sources of phosphorus (e.g., phosphate, hypophosphite, lecithin, phosphonoacetic acid)99,100, investigation of the influencing factors20,101, as well as isolating the microbial functional bacteria64,102,103. The recovery of phosphorus by gaseous phosphine is proposing a different direction for the removal of phosphorus from wastewater. The potential benefits and differences for doing so are illustrated as follows.
First, phosphorus removal in wastewater treatment plants is generally rather problematic and the significant drawback is the absence of phosphorus redox processes. Phosphorus was wrongly assumed not to undergo redox reactions in transformation and migration processes. As discussed above, when redox potential falls below −300 mV, reduction of phosphate to phosphine can take place. If we take advantage of the conversion of more phosphorus to phosphine, it is believed to be a qualitative change of P removal in the wastewater treatment plants (WWTPs) and reduce the amount of phosphorus-rich sludge, saving the cost for the post-treatment of sludge. Second, instead of recovering P as the anthropogenic fertilizers, the redistribution of phosphorus via PH3 transport from rich phosphorus sources could fertilize areas that are poor in phosphorus26. Though the atmospheric phosphine is a small contribution to the phosphorus cycle, PH3 might not be insignificant for areas where P limits the biomass stock or phosphine is the main or only source of phosphorus. Through this work, we aim to raise the research attention on the impact of enormous recovery of P from wastewater on P cycle in natural ecosystems and the production of phosphine when designing the removal processes of phosphorus in the wastewater.
Moreover, according to the global budget of atmospheric phosphorus balance, the total global emissions of atmospheric phosphorus was estimated to be 3.5 Tg/yr, of which 2.7 Tg/yr fell down to the land and 0.8 Tg/yr into the ocean104. However, the successive cycling activities through phosphine or other pathways remain unexplored. The tentative estimation of the global phosphine budget is provided in Supplementary Table 1. Since the emission of phosphine demonstrates significant spatial variations and the survey about phosphine levels has not been carried out globally yet, it is impossible to estimate the global budget so far. For example, the surface area of global oceans is about 3.6 × 108 km2 but the existing survey of phosphine levels in the marine environments is focused on the coastal and offshore areas28,47,53, which are seriously affected by the human activities. The emission flux data in these areas can barely represent the immense ocean areas. It is anticipated that this work will stimulate more research on the role of phosphine in the global phosphorus cycle.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information file.
Code availability
This study does not use any unreported custom computer code or mathematical algorithm that is deemed central to the conclusions.
References
Devai, I., Felföldy, L., Wittner, I. & Plósz, S. Detection of phosphine: new aspects of the phosphorus cycle in the hydrosphere. Nature 333, 343 (1988).
Cao, J. et al. Study on effects of electron donors on phosphine production from anaerobic activated sludge. Water 9, 563 (2017).
Zhang, C., Zhang, K., Wei, W., Rong, H. & Liu, T. Release rule of phosphine in anaerobic sequencing batch process. China Water Wastewater 26, 53–55 (2010).
Hong, Y. et al. Distribution of phosphine in the offshore area of the Southwest Yellow Sea, East Asia. Mar. Chem. 118, 67–74 (2010).
Liu, Z., Jia, S., Wang, B. & Liu, S. Differences in phosphine contents of various environment samples and the effecting factors. Acta Scien. Circum. 5, 852–857 (2004).
Zhu, R., Liu, Y., Sun, J., Sun, L. & Geng, J. Stimulation of gaseous phosphine production from Antarctic seabird guanos and ornithogenic soils. J. Environ. Sci. 21, 150–154 (2009).
Zhang, R., Wu, M., Wang, Q., Geng, J. & Yang, X. The determination of atmospheric phosphine in Ny-Ålesund. Sci. Bull. 55, 1662–1666 (2010).
Zhu, R., Ding, W., Hou, L. & Wang, Q. Matrix-bound phosphine and phosphorus fractions in surface sediments of Arctic Kongsfjorden, Svalbard: effects of glacial activity and environmental variables. Chemosphere 103, 240–249 (2014).
Wang, Q., Geng, J.-j., Jin, H.-m., Shi, H.-h. & Wang, X.-r. Temporal and spatial distributions of microbes and phosphine in Lake Taihu sediments. China Environ. Sci. 26, 350–354 (2006).
Ding, L. et al. Sources of matrix-bound phosphine in advanced wastewater treatment system. Sci. Bull. 50, 1274–1276 (2005).
Li, J.-B. et al. Matrix bound phosphine in sediments of the Changjiang Estuary and its adjacent shelf areas. Estuar. Coast. Shelf Sci. 90, 206–211 (2010).
You, L. et al. Distribution of matrix-bound phosphine in surface sediments of Jinpu Bay. Environ. Sci. 34, 3804–3809 (2013).
Niu, X. et al. Phosphine in paddy fields and the effects of environmental factors. Chemosphere 93, 1942–1947 (2013).
Glindemann, D., Edwards, M., Liu, J.-A. & Kuschk, P. Phosphine in soils, sludges, biogases and atmospheric implications—a review. Ecol. Eng. 24, 457–463 (2005).
Han, S.-H., Zhuang, Y.-H., Liu, J.-A. & Glindemann, D. Phosphorus cycling through phosphine in paddy fields. Sci. Total Environ. 258, 195–203 (2000).
Han, C., Geng, J., Zhang, R., Wang, X. & Gao, S. Matrix-bound phosphine and phosphorus fractions in paddy soils. J. Environ. Monit. 13, 844–849 (2011).
Han, C. et al. Production and emission of phosphine gas from wetland ecosystems. J. Environ. Sci. 22, 1309–1311 (2010).
Glindemann, D., Stottmeister, U. & Bergmann, A. Free phosphine from the anaerobic biosphere. Environ. Sci. Pollut. Res. 3, 17–19 (1996).
Wang, J., Niu, X., Ma, J. & Lu, M. Conversion of phosphorus to phosphine by microbial deoxidization under anaerobic conditions. Microbiol. China 1, 34–41 (2015).
Ding, L. et al. Effect of pH on phosphine production and the fate of phosphorus during anaerobic process with granular sludge. Chemosphere 59, 49–54 (2005).
Zhang, R. et al. Effects of free-air CO2 enrichment on phosphine emission from rice field. Environ. Sci. 30, 2694–2700 (2009).
Ma, J., Chen, W., Niu, X. & Fan, Y. The relationship between phosphine, methane, and ozone over paddy field in Guangzhou, China. Glob. Ecol. Conserv. 17, 1–7 (2019).
Zhang, C., Zhang, K., Sun, L., Rong, H. & Liu, T. Effect of carbon sources on phoshpine production from anaerobic activated sludge. China Water Wastewater 29, 103–106 (2013).
Liu, J.-A. et al. Phosphine in the urban air of Beijing and its possible sources. Water Air Soil Pollut. 116, 597–604 (1999).
Niu, X. et al. Temporal and spatial distributions of phosphine in Taihu Lake, China. Sci. Total Environ. 323, 169–178 (2004).
Zhu, R. et al. Tropospheric phosphine and its sources in coastal Antarctica. Environ. Sci. Technol. 40, 7656–7661 (2006).
Zhu, R., Kong, D., Sun, L., Geng, J. & Wang, X. The first determination of atmospheric phosphine in Antarctica. Sci. Bull. 52, 131–135 (2007).
Zhu, R. et al. Phosphine in the marine atmosphere along a hemispheric course from China to Antarctica. Atmos. Environ. 41, 1567–1573 (2007).
An, S. et al. Mechanism of matrix-bound phosphine production in response to atmospheric elevated CO2in paddy soils. Environ. Pollut. 239, 253–260 (2018).
Zhang, K., Zhang, C., Wei, W., Rong, H. & Liu, T. Phosphine release in aerobic sequencing reactor process and anaerobic/aerobic sequencing reactor process. Environ. Eng. 5, 127–129 (2011).
Ding, L. et al. Distribution of phosphine and phosphorus balance in a full scale UASB system. J. Nanjing Uni. Nat. Sci. 41, 620–626 (2005).
Hou, L. et al. Emission of phosphine in intertidal marshes of the Yangtze Estuary. Appl. Geochem. 26, 2260–2265 (2011).
Glindemann, D., Bergmann, A., Stottmeister, U. & Gassmann, G. Phosphine in the lower terrestrial troposphere. Naturwissenschaften 83, 131–133 (1996).
Feng, Y., Wang, Q., Yao, Z. & Geng, J. Research on distribution of phosphine in the natural environment and its environmental factors. Chin. High. Technol. Lett. 19, 650–655 (2009).
Geng, J. et al. Simultaneous monitoring of phosphine and of phosphorus species in Taihu Lake sediments and phosphine emission from lake sediments. Biogeochemistry 76, 283–298 (2005).
Han, C. et al. Free atmospheric phosphine concentrations and fluxes in different wetland ecosystems, China. Environ. Pollut. 159, 630–635 (2011).
Glindemann, D., Edwards, M. & Kuschk, P. Phosphine gas in the upper troposphere. Atmos. Environ. 37, 2429–2433 (2003).
Gassmann, G., Van Beusekom, J. & Glindemann, D. Offshore atmospheric phosphine. Naturwissenschaften 83, 129–131 (1996).
Roels, J. & Verstraete, W. Occurrence and origin of phosphine in landfill gas. Sci. Total Environ. 327, 185–196 (2004).
Han, S.-h., Wang, Z.-j., Zhuang, Y.-h., Yu, Z.-m. & Glindemann, D. Phosphine in various matrixes. J. Environ. Sci. (China) 15, 339–341 (2003).
Devai, I., DeLaune, R., Devai, G., Patrick, J. W. H. & Czegeny, I. Phosphine production potential of various wastewater and sewage sludge sources. Anal. Lett. 32, 1447–1457 (1999).
Zhu, R. et al. Matrix-bound phosphine in Antarctic biosphere. Chemosphere 64, 1429–1435 (2006).
Niu, X. et al. Matrix-bound phosphine in the paddy soils of South China and its relationship to environmental factors and bacterial composition. J. Soils Sediment. 16, 592–604 (2016).
Zhang, J., Geng, J., Zhang, R., Ren, H. & Wang, X. Matrix-bound phosphine in paddy fields under a simulated increase in global atmospheric CO2. Environ. Chem. 7, 287–291 (2010).
Eismann, F., Glindemann, D., Bergmannt, A. & Kuschk, P. Soils as source and sink of phosphine. Chemosphere 35, 523–533 (1997).
Devai, I. & Delaune, R. Evidence for phosphine production and emission from Louisiana and Florida marsh soils. Org. Geochem. 23, 277–279 (1995).
Gassmann, G. Phosphine in the fluvial and marine hydrosphere. Mar. Chem. 45, 197–205 (1994).
Gassmann, G. & Schorn, E. Phosphine from harbor surface sediments. Naturwissenschaften 80, 78–80 (1993).
Song, X. et al. Matrix-bound phosphine in sediments from Lake Illawarra, New South Wales, Australia. Mar. Pollut. Bull. 62, 1744–1750 (2011).
Feng, Z., Song, X. & Yu, Z. Seasonal and spatial distribution of matrix-bound phosphine and its relationship with the environment in the Changjiang River Estuary, China. Mar. Pollut. Bull. 56, 1630–1636 (2008).
Feng, Z., Song, X. & Yu, Z. Distribution characteristics of matrix-bound phosphine along the coast of China and possible environmental controls. Chemosphere 73, 519–525 (2008).
Yu, Z. & Song, X. Matrix-bound phosphine: a new form of phosphorus found in sediment of Jiaozhou Bay. Sci. Bull. 48, 31–35 (2003).
Mu, Q., Song, X. & Yu, Z. Matrix-bound phosphine (PH3) distribution characteristics in the sediments of Jiaozhou Bay. China Environ. Sci. 26, 135–138 (2005).
Zhu, R. et al. Occurrence of matrix-bound phosphine in polar ornithogenic tundra ecosystems: effects of alkaline phosphatase activity and environmental variables. Sci. Total Environ. 409, 3789–3800 (2011).
Eismann, F., Glindemann, D., Bergmann, A. & Kuschk, P. Balancing phosphine in manure fermentation. J. Environ. Sci. Health B 32, 955–968 (1997).
Li, J.-B., Zhang, G.-L., Zhang, J., Liu, S.-M. & Ren, J.-L. Matrix bound phosphine in sediments of the yellow sea and its coastal areas. Cont. Shelf Res. 30, 743–751 (2010).
Han, C., Geng, J., Zhang, J., Wang, X. & Gao, S. Phosphine migration at the water–air interface in Lake Taihu, China. Chemosphere 82, 935–939 (2011).
Glindemann, D., Edwards, M. & Schrems, O. Phosphine and methylphosphine production by simulated lightning—a study for the volatile phosphorus cycle and cloud formation in the earth atmosphere. Atmos. Environ. 38, 6867–6874 (2004).
Bains, W., Petkowski, J. J., Sousa-Silva, C. & Seager, S. New environmental model for thermodynamic ecology of biological phosphine production. Sci. Total Environ. 658, 521–536 (2019).
Cao, H., Liu, J., Zhuang, Y. & Dietmar, G. Emission sources of atmospheric phosphine and simulation of phosphine formation. Sci. China, Ser. B: Chem. 43, 162 (2000).
Zhu, R. et al. Penguins significantly increased phosphine formation and phosphorus contribution in maritime Antarctic soils. Sci. Rep. 4, 1–9 (2014).
Roels, J. & Verstraete, W. Biological formation of volatile phosphorus compounds. Bioresour. Technol. 79, 243–250 (2001).
Sun, L., Zhang, C., Zhang, K., Rong, H. & Liu, T. Effects of different phosphorus sources on phosphine production from anaerobic sludge. China Water Wastewater 28, 89–91 (2012).
Jenkins, R., Morris, T.-A., Craig, P. J., Ritchie, A. & Ostah, N. Phosphine generation by mixed-and monoseptic-cultures of anaerobic bacteria. Sci. Total Environ. 250, 73–81 (2000).
Glindemann, D., Edwards, M. & Morgenstern, P. Phosphine from rocks: mechanically driven phosphate reduction? Environ. Sci. Technol. 39, 8295–8299 (2005).
Glindemann, D., Eismann, F., Bergmann, A., Kuschk, P. & Stottmeister, U. Phosphine by bio-corrosion of phosphide-rich iron. Environ. Sci. Pollut. Res. 5, 71 (1998).
Glindemann, D., De Graaf, R. & Schwartz, A. W. Chemical reduction of phosphate on the primitive Earth. Orig. Life Evol. Biosphere 29, 555–561 (1999).
Hammer, D. A. Constructed Wetlands for Wastewater Treatment: Municipal, Industrial and Agricultural (CRC Press, 1989).
Whitmire, S. L. & Hamilton, S. K. Rates of anaerobic microbial metabolism in wetlands of divergent hydrology on a glacial landscape. Wetlands 28, 703–714 (2008).
Picek, T., Čížková, H. & Dušek, J. Greenhouse gas emissions from a constructed wetland—plants as important sources of carbon. Ecol. Eng. 31, 98–106 (2007).
Roels, J., Huyghe, G. & Verstraete, W. Microbially mediated phosphine emission. Sci. Total Environ. 338, 253–265 (2005).
Pasek, M. A role for phosphorus redox in emerging and modern biochemistry. Curr. Opin. Chem. Biol. 49, 53–58 (2019).
Pasek, M. A., Sampson, J. M. & Atlas, Z. Redox chemistry in the phosphorus biogeochemical cycle. Proc. Nat. Acad. Sci. USA 111, 15468–15473 (2014).
Han, C. et al. Phosphite in sedimentary interstitial water of Lake Taihu, a large eutrophic shallow lake in China. Environ. Sci. Technol. 47, 5679–5685 (2013).
Han, C. et al. Determination of phosphite in a eutrophic freshwater lake by suppressed conductivity ion chromatography. Environ. Sci. Technol. 46, 10667–10674 (2012).
Wang, J., Li, L., Niu, X. & Zou, D. Phosphine-induced phosphorus mobilization in the rhizosphere of rice seedlings. J. Soils Sediment. 16, 1735–1744 (2016).
Zhang, C.-B. et al. Responses of dissimilatory nitrate reduction to ammonium and denitrification to plant presence, plant species and species richness in simulated vertical flow constructed wetlands. Wetlands 37, 109–122 (2017).
Li, H., Chen, Z. & Chen, Z. Daily variation of the rhizosphere redox potential of six wetland plants. Acta Ecologica Sin. 34, 5766–5773 (2014).
Fu, R., Zhu, Y. & Yang, H. DO and ORP conditions and their correlation with plant root distribution in a continuous-flow constructed wetland treating eutrophic water. Acta Scien. Circum. 28, 2036–2038 (2008).
Colmer, T. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 26, 17–36 (2003).
Liu, S., Li, T., Ning, P., Wu, M. & Yu, S. Research progress of the release, distribution and transformation of phosphine in environment. Chem. Ind. Eng. Prog. 38, 1085–1096 (2019).
Wang, D., Leng, B., An, X. & Lu, Q. Effects of UV light wave, temperature and humidity on phosphine concentration degrading. Plant Quar. 27, 45–49 (2013).
Chen, L., Arimoto, R. & Duce, R. A. The sources and forms of phosphorus in marine aerosol particles and rain from northern New Zealand. Atmos. Environ. 19, 779–787 (1985).
Chen, H. Y. & Chen, L. D. Importance of anthropogenic inputs and continental‐derived dust for the distribution and flux of water‐soluble nitrogen and phosphorus species in aerosol within the atmosphere over the East China Sea. J. Geophys. Res. Atmos. 113, D11303 (2008).
Xu, Z. et al. Dry and wet atmospheric deposition of nitrogen and phosphorus in Taihu Lake. Environ. Monit. Forewarning 119, 37–42 (2019).
Ma, Z., Zhang, Q. & Qin, Y. Numerical simulation and analysis of the effect of Three Gorges reservoir project on the regional climate change. Res. Environ. Yangtze Basin 19, 1044–1052 (2010).
Wang, M., Zhou, Y., Ren, Y. & Fang, S. Spatial-temporal change characteristics of precipitation over the key region of Three Gorges Reservoir. Meteorol. Environ. Sci. 40, 40–46 (2017).
Zhang, S., Lu, Z. & Zhang, N. Analysis of influence of Three Gorges Dam storage on reservoir region precipitation. Water Resour. Power 31, 21–24 (2013).
Zhang, H. Potential pitfalls in “Hongqi River” water transfer proposal and drought management in Northwest China. Water Resource Prot. 2, 8–11 (2018).
Prinn, R. G. The interactive atmosphere: global atmospheric-biospheric chemistry. Ambio 23, 50–61 (1994).
Kleemann, R. et al. Evaluation of local and national effects of recovering phosphorus at wastewater treatment plants: Lessons learned from the UK. Resour. Conserv. Recycl. 105, 347–359 (2015).
Pradel, M. & Aissani, L. Environmental impacts of phosphorus recovery from a “product” Life Cycle Assessment perspective: allocating burdens of wastewater treatment in the production of sludge-based phosphate fertilizers. Sci. Total Environ. 656, 55–69 (2019).
Humphrey, C. P., Anderson-Evans, E., O’Driscoll, M., Manda, A. & Iverson, G. Comparison of phosphorus concentrations in coastal plain watersheds served by onsite wastewater treatment systems and a municipal sewer treatment system. Water Air Soil Pollut. 226, 2259 (2015).
Ding, L.-L. et al. Distribution of phosphine and phosphorus balance in a full-scale UASB system. J. Nanjing Uni. Nat. Sci. 41, 620–626 (2005).
Yang, Z., Zhou, J., Li, J., Han, Y. & He, Q. Pre-processing of raw wastewater in a septic tank leads to phosphorus removal by phosphine production in a sequencing batch biofilm reactor (SBBR). Desalination Water Treat. 57, 810–818 (2016).
Liu, W., Niu, X., Chen, W., An, S. & Sheng, H. Effects of applied potential on phosphine formation in synthetic wastewater treatment by Microbial Electrolysis Cell (MEC). Chemosphere 173, 172–179 (2017).
Yang, S. & Yao, G. Simultaneous removal of concentrated organics, nitrogen and phosphorus nutrients by an oxygen-limited membrane bioreactor. PLoS ONE 13, e0202179 (2018).
Zhang, P., Rong, H., Zhang, K., Liu, T. & Cao, Y. Effect of diverse mud and phosphorus sources on total phosphorus removal efficiencies. Guangdong Chem. Ind. 56, 118–119 (2011).
Han, S., Zhuang, Y., Zhang, H., Wang, Z. & Yang, J. Phosphine and methane generation by the addition of organic compounds containing carbon–phosphorus bonds into incubated soil. Chemosphere 49, 651–657 (2002).
Rutishauser, B. V. & Bachofen, R. Phosphine formation from sewage sludge cultures. Anaerobe 5, 525–531 (1999).
Wan, J., Deng, M., He, H. & Tang, A. Factors influencing release of phosphine in piggery wastewater. China Water Wastewater 23, 117–120 (2013).
Fan, Y., Lv, M., Niu, X., Ma, J. & Song, Q. Evidence and mechanism of biological formation of phosphine from the perspective of the tricarboxylic acid cycle. Int. Biodeterior. Biodegrad. 146, 104791 (2020).
Fan, Y. et al. Analysis of the characteristics of phosphine production by anaerobic digestion based on microbial community dynamics, metabolic pathways, and isolation of the phosphate-reducing strain. Chemosphere 262, 128213 (2021).
Wang, R. et al. Significant contribution of combustion-related emissions to the atmospheric phosphorus budget. Nat. Geosci. 8, 48 (2015).
Author information
Authors and Affiliations
Contributions
X.Z. proposed the concept and supervised. W.F. created the first draft of the article. The authors contributed in revising and finalizing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fu, W., Zhang, X. Global phosphorus dynamics in terms of phosphine. npj Clim Atmos Sci 3, 51 (2020). https://doi.org/10.1038/s41612-020-00154-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41612-020-00154-7
This article is cited by
-
Water activity in Venus’s uninhabitable clouds and other planetary atmospheres
Nature Astronomy (2021)