Abstract
The tumor immune composition influences prognosis and treatment sensitivity in lung cancer. The presence of effective adaptive immune responses is associated with increased clinical benefit after immune checkpoint blockers. Conversely, immunotherapy resistance can occur as a consequence of local T-cell exhaustion/dysfunction and upregulation of immunosuppressive signals and regulatory cells. Consequently, merely measuring the amount of tumor-infiltrating lymphocytes (TILs) may not accurately reflect the complexity of tumor-immune interactions and T-cell functional states and may not be valuable as a treatment-specific biomarker. In this work, we investigate an immune-related biomarker (PhenoTIL) and its value in associating with treatment-specific outcomes in non-small cell lung cancer (NSCLC). PhenoTIL is a novel computational pathology approach that uses machine learning to capture spatial interplay and infer functional features of immune cell niches associated with tumor rejection and patient outcomes. PhenoTIL’s advantage is the computational characterization of the tumor immune microenvironment extracted from H&E-stained preparations. Association with clinical outcome and major non-small cell lung cancer (NSCLC) histology variants was studied in baseline tumor specimens from 1,774 lung cancer patients treated with immunotherapy and/or chemotherapy, including the clinical trial Checkmate 057 (NCT01673867).
Similar content being viewed by others
Introduction
Adaptive immune responses to cancer include the local accumulation of tumor-infiltrating lymphocytes (TILs) at the tumor site comprising both B-cell and T-cell subsets. The interaction of these cells occurs within lymphoid organs as well as at sites of inflammation and tumor microenvironment; often, these structures are referred to as immune cell niches1,2. These TILs can initiate, recognize, and destroy tumor cells thus propagating and maintaining anti-cancer immune responses3,4. Despite increasing evidence in the clinical scenarios and the novel design of combination of different therapy strategies (e.g., chemotherapy + immunotherapy), there is a need to understand the effect of said therapies on the tumor microenvironment5. For instance, immunotherapy-based treatment has yielded limited response rates and unclear underlying biological mechanisms6, a phenomenon also observed in the context of chemotherapy7. To date, there continue to be gaps in the comprehensive and systematic characterization of the tumor milieu, limiting our ability to predict treatment response for chemotherapy and immunotherapy.
The challenges deepen in the context of tumor-immune characterization and association with treatment response between tumor histology sub-types such as adenocarcinoma (AD) and squamous cell carcinoma (SCC). These differences are likely on account of the tumor immune milieu, which is extremely diverse on account of unique and distinct cellular phenotypes (immune deserts, immune-excluded, and inflamed tumors)8. Further, the presence of specific immune cells such as cancer-specific T-cells is required for effective anti-cancer immunity. Yet, evidence indicates that only a small fraction of T-cells is cancer-specific with most acting as ‘bystanders’ (non-tumor antigens)9. Others are identified as exhausted or dysfunctional and have the potential to be reinvigorated by immune checkpoint blockers10. These cells are not homogeneous groups but highly diverse in their specificities and effector functions.
A number of computational-based approaches have been proposed to measure the total number of TILs and their density on immunofluorescence (IF) images to assess the association of these measures with prognosis and response to therapy11,12. However, the preparation procedures not only requires a fluorescence microscope, but the specific antibodies and fluorochromes preparation can take up to 5 h (Leica Microsystems- Immunofluorescence protocol), compared to H&E (Hematoxylin & eosin) which is performed in minutes. Further, these approaches tend to be very expensive, complex, and involve tissue destruction. On the other hand, studies using H&E images use computational enumeration of TIL count and density12,13. Other works14,15,16,17 have employed spatial arrangement-based approaches to capture the spatial patterns of TILs on H&E tissue images, with some studies showing association with response to treatment;15,18 however, these approaches have considered TILs as a single entity, which assumes that all the TILs contribute equally to the outcome. This assumption has been refuted by several studies11,19 that have identified activated immune cells (as opposed to exhausted T-cells or bystander immune cells) as being responsible for engendering an active response to therapies such as chemotherapies or immune checkpoint inhibitors (ICI) blockade. This is only possible on the IF scope, due to the difficulty in identifying subtypes of immunes cells on H&E.
In this study, we present PhenoTIL, a computational pathology approach that utilizes H&E-stained images to first identify immune cell niches, and subsequently capture quantitative metrics relating to the spatial interplay of TILs and cancer cells within these immune cell niches. PhenoTIL assumes that the TILs within the tumor microenvironment have different roles in relation to the outcome of the patients and enable characterization of patient risk based off composition of the constituent clusters. The quantitative PhenoTIL metrics describe each of the TILs in reference to their color, shape, texture and convey spatial architectural information about the neighboring TILs and non-TILs at different length scales. The main hypothesis is aimed to investigate the value of PhenoTIL as the immune-related biomarker for treatment-specific outcomes in NSCLC. An important factor of PhenoTIL is the ability to identify distinct TIL clusters on H&E images that bear a resemblance to activated immune ‘hotspots’ associated with tumor rejection or immune ‘cold spots’ associated with dysfunctional features and contributing to adverse outcomes. We evaluated this approach in its ability to prognosticate overall survival (OS) on pre-treatment H&E-stained samples of 1774 patients with lung AD (n = 1189) and SCC (n = 585) treated with different types of chemotherapy and immunotherapy agents (Fig. 1); the study also included patients from a completed clinical trial of immunotherapy in lung AD patients (Checkmate 057)20. Kaplan–Meier (KM) survival curves were generated to evaluate the association of the phenoTIL signature with outcome. The PhenoTIL signature was also evaluated in terms of the molecular composition of immune cell subtypes (Fig. 2) by identifying single cells molecularly using co-registered quantitative immunofluorescence image (QIF) information on a subset of H&E images. To identify which biological pathways were associated with the PhenoTIL signature, gene-set enrichment analysis (GSEA) was performed. Separate analyses were carried out for AD and SCC cases considering their documented differences in their pathophysiology, clinical features, immunogenicity, prognosis, and treatment sensitivity21,22,23,24,25.
The inclusion criteria for the study. In total 1774 patients who satisfied all the inclusion criteria and who did not meet any of the exclusion criteria were identified. Images with low quality, blurry effects, and significant artifacts were considered for all the datasets, and images that presented them were excluded from the analysis. For D6, D7, and D8 the additional inclusion criteria invoked included the availability of histologic subtype AD. Inclusion criteria for D7 were as follows: from the initial 211 patients, those patients were considered who either underwent surgical resection after neoadjuvant therapy or had a primary resection at a locally advanced stage, which qualified them for neoadjuvant therapy. Three patients had more than one WSI scanned due to the following reasons: Firstly, the patient had a sample with neoadjuvant lung SCC with small AD as an incidental finding, second was a patient with neoadjuvant adenosquamous carcinoma and the third patient had a primary lung AD with three regions with quite different growth patterns within the primary. After invoking the inclusion and exclusion criteria for this study, 71 cases for D1 (49 excluded), 71 for D2 (35 excluded), 79 for D3 (57 excluded), 231 for D4 (49 excluded), 850 for D5 (239 excluded), 21 for D6 (49 excluded), 93 for D7 (118 excluded) and 358 for D8 (224 excluded) were included.
A Tissue preparation: The cohorts were digitized and represented in the form of patches extracted from whole slide images (WSIs) and tissue microarray (TMA) punches. B Image preprocessing: A subset composed of H&E-stained TMA and corresponding immunofluorescence (IF) images were utilized to analyze tumor-infiltrating lymphocyte (TIL) subtypes. C Cell Identification: Corresponding TILs from the H&E samples were associated with IF molecular labels (CD4+, CD8+, CD20+). D Feature extraction: Phenotyping features were extracted from the TIL cells patches extracted from the WSI and TMAs. E Single-Cell Clustering: An unsupervised clustering approach was applied to the phenotyping features of TILs. F Molecular Assessment: RNA-seq-based transcriptome data is obtained from each WSI-TCGA sample. TIL clusters were used as the input matrix to build a model that associate with the clinical outcome overall survival, using a Cox proportional hazards regression model with elastic net regularization. Associations between cluster conformation, molecular, morphological and genomics composition were studied. Minor components of the figure were obtained from BioRender.
Results
Description of patients cohorts
Eight datasets (D1–D8) were used in this multi-institutional study, including seven retrospective institutional cohorts and one from a completed clinical trial. Figure 1 illustrates the inclusion and exclusion criteria for patient selection. Out of the 2594 patients available, a total of 1774 (68.4%) patients, who satisfied all the inclusion criteria and did not meet any of the exclusion criteria, were incorporated into this study. A total of 820 (31.6%) patients were excluded from the study due to various reasons related to the image samples. A total of 562 (21.7%) images were removed due to insufficient quality (faulty image format, artifacts such as complete image blurriness, color corruption, no tissue sample available, etc.). In addition, 258 (9.9%) images were removed due to missing medical information or were not classified as either adenocarcinoma or squamous cell carcinoma. The quality control was performed utilizing HistoQC26. The first three datasets (D1–D3) consisted of pre-treatment formalin-fixed paraffin-embedded tumor sections from 44 AD and 27 SCC patients (D1), 43 AD and 28 SCC patients (D2), and 58 AD and 21 SCC patients (D3) provided by the Department of Pathology at Yale University (D2 were collected at Sotiria General Hospital and Patras University General Hospital at Greece, but were made available from Yale Pathology)12. Corresponding QIF images from D3 had been previously acquired and prepared via pan-cytokeratin staining, CD4+, CD8+, and CD20+ using the sequential multiplexed immunofluorescence protocol (See an example in the Supplementary fig. 1(A)), with isotype-specific primary antibodies being employed for staining T lymphocytes (CD3+ IgG, 1:100, clone E272, Novus biologicals, CO), cytotoxic T-cells (CD8 + IgG1, 1:250, clone C8 + /144B, DAKO), and B lymphocytes (CD20 + IgG2a, 1:150, clone L26, DAKO)12. D4 comprised 145 AD and 86 SCC patients obtained from the Cleveland Clinic (CCF). D1-D3 were represented in tissue microarrays (TMAs) with cores of size 0.6 mm from each paraffin block (digitally scanned at 20×). D4 was represented in TMAs with cores of size 1.2 mm from each paraffin blocks (digitally scanned at 40×)27. D5 comprised whole slide images (WSIs) of pre-treatment H&E-stained pathology slides (scanned at 40×) of 427 AD and 423 SCC patients (D5) obtained from The Cancer Genome Atlas (TCGA). D6 comprised diagnostic WSI samples (scanned at 40×) of 21 AD patients who had received ICI-based immunotherapy at the University of Pennsylvania Hospital (UPenn).
D7 (acquired at 20× resolution; ratio of 0.2431 μm/px) comprised 93 AD patients (from a larger cohort of 211 patients with 215 available WSIs); these patients had no neuroendocrine histology and had a sufficient amount of primary tumor. A subset of 43 patients (out of the 93) were treated with chemotherapy prior to resection with neoadjuvant intention. 50 patients (out of the 93) were primary resected LUAD with pathologically confirmed infiltration of lymph nodes of at least the mediastinal level. The patients which were neoadjuvantly treated, received platinum-based chemotherapy combinations. Ultimately, slides corresponding to 93 patients were included, along with corresponding tumoral programmed cell death 1 ligand 1 (PD-L1) expression values assessed on the resection samples, for these patients28,29. PD-L1 expression was assessed by an expert pathologist as the tumor proportional score (TPS), i.e., the proportion of PD-L1 positive tumor cells to all tumor cells. PD-L1 positive tumor cells were defined as showing membranous staining of any intensity. TPS was assessed as a continuous parameter (1% increments up to 10 and 5% increments in cases showing >10% expression). For statistical analyses, each patient was categorized as high or low expression based on the intensity of immunohistochemistry staining of the lung resection samples. Patients with over 50% PD-L1 expression were classified as high PD-L1 and patients with less than 50% expression were classified as low PD-L1. The data was provided by the University of Bern in Switzerland (UBern).
D8 comprised WSIs (scanned at 20×) of 358 AD patients provided by Bristol-Myers Squibb (BMS), the clinical trial CA209-05716 (ClinicalTrials.gov identifier: NCT01673867). The patients from D8 were randomized from an open-label phase-3 study (international) of non-squamous non-small cell lung cancer (NSCLC), also referred to as CheckMate 057. Patients whose lung cancer progressed during or after a platinum-based doublet chemotherapy regimen received nivolumab or docetaxel. The actual enrollment for the study was 782 participants from 115 international study locations16. A total of 204 patients received nivolumab (at a dose of 3 mg per kilogram of body weight every 2 weeks) and 154 patients received docetaxel (at a dose of 75 mg per square meter of body-surface area every 3 weeks). The primary endpoint was OS. The validation on this dataset was carried out in a blinded fashion in which we had access to whole slide images but not to clinical data. Our algorithms were applied to the images to predict risk scores and patient labels, and then these values were sent to the BMS team, who carried out the statistical analysis and provided us with the corresponding results.
Table 1 provide a summary of the datasets and images employed in this study along with the corresponding clinicopathology and outcome information (Supplementary Table 1 shows the clinicopathology information in more detail for each cohort).
Cohorts D1 (n = 71) and D2 (n = 71) were employed for feature discovery and model training as they were previously employed in studies12,30 that demonstrated association between TILs and patient survival.
PhenoTIL identified immune cell niches from pretreatment H&E samples are associated with overall survival in lung adenocarcinoma and squamous cell carcinoma patients treated with chemotherapy
Figure 3(A) illustrates the Kaplan Meier (KM) survival curves corresponding to the model using the training sets (DAD1, n = 44 and DAD2 = 43) with cluster TIL-based measures (MAD). MAD was statistically significantly prognostic of OS on the training sets (DAD1 and DAD2) with a hazard ratio (HR) of 1.73 (95% confidence interval (95% CI) = 1.04–2.87, p value (p) = 0.035, concordance-index (C-index) = 0.711 (standard error (SE) = 0.070), n = 87). MAD was also prognostic of OS in D3 (p = 0.04, HR = 2.24, 95% CI 1.03–4.88, C-index: 0.705 (SE 0.091), n = 58), D4 (p = 0.015, HR = 1.82; 95% CI, 1.12–2.94, C-index: 0.689 (SE 0.051), n = 145), D5 (p = 0.02, HR = 5.88; 95% CI, 1.51–2.15, C-index: 0.645 (SE 0.07), n = 427), and D7 (p = 0.037, HR = 1.89; 95% CI, 1.03–3.46, C-index: 0.645 (SE 0.07), n = 93) as illustrated in Fig. 2(B)–(E). Further, the models (MAD and MSCC) were evaluated merging all the patients from the testing cohorts (See Supplementary Fig. 3(A–B)). MAD was evaluated across different lung AD stages (IA, IB, IIA, IIB, IIIA, IIIB, and IV) for patients on datasets D3, D4, D5, D6 and D7; results are shown in Supplementary Fig. 1(C–H) and Supplementary Fig. 2(A–F). MAD yielded a statistically significant separation between low and high-risk groups (n = 97, p = 0.038, HR = 1.96, 95% CI: 1.03–3.76) in Stage III patients. For Stages IB (n = 127, p = 0.05, HR = 1.95, 95% CI: 0.99–3.83) and IIIA (n = 80, p = 0.057, HR = 1.97, 95% CI: 0.97–4.0), MAD showed association with OS but did not reach statistical significance.
A Training set (DAD1 and DAD2) applied to (B) chemotherapy treated cohorts D3, (C) D4, (D) D5 and (E) D7. KM curves for the TIL cluster model (MSCC (F) training (DSCC1 and DSCC2) set applied to (G) D3, (H) D4 and (I) D5. KM of TIL cluster model (MAD) applied to immunotherapy treated cohorts (J) D6 and (K) D8. L Bar plot displaying the correlation between the dichotomous risk groups determined by TIL cluster model (MAD) for cohort D6 on Adenocarcinoma patients and the manual assessment of percentage of TILs. The high-risk group contains 63.6% of Low TIL and the low-risk group contains 80% of High TIL content. M Bar plot displaying the correlation between the risk groups determined by TIL cluster model (MAD) on D7 and the PD-L1 expressions (High expression >50%, low expression ≤50%, Positive >1% and Negative expression ≤1%). The low-risk group contains 79.5% of the low PD-L1. The high-risk group contains 30% of the high PD-L1.
Figure 3(F) illustrates the KM curves corresponding to the model using the training sets (DSCC1, n = 27 and DSCC2 = 28) with cluster TIL-based measures (MSCC). MSCC was statistically significantly prognostic of OS on the training sets (DSCC1 and DSCC2) with HR of 6.19 (95% CI: 2.08–18.4, p = 0.001, C-index: 0.664 (SE 0.059), n = 55). MSCC was also prognostic of OS in D3 (p = 0.04, HR = 3.36; 95% CI, 1.08–10.5, C-index: 0.910 (SE 0.061), n = 21), D4 (p = 0.01, HR = 2.34; 95% CI, 1.21–4.5, C-index: 0.654 (SE 0.07), n = 86), and D5 (p = 0.07, HR = 1.38; 95% CI, 1.05–1.82, C-index: 0.590 (SE 0.039), n = 423) as illustrated in Fig. 2(G)–(I). MSCC was evaluated using different lung SCC stages (I, II, III, and IV) across the cohorts D3–D7; results are shown in Supplementary Fig. 3(C–H). MSCC yielded a statistically significant separation between low and high-risk groups (n = 279, p = 0.007, HR = 1.53, 95% CI: 1.12–2.09) in Stage I patients. Similarly, the trend is seen for Stage III (n = 73, p = 0.05, HR = 1.97, 95% CI: 0.99–3.92) and slight separation can be seen for Stage II (n = 167, p = 0.772, HR = 1.06, 95% CI: 0.72–1.55) but the differences did not rise to the level of statistical significance. MSCC showed association with OS for Early-Stage disease (combined Stage I and II) (n = 446, p = 0.018, HR = 1.33, 95% CI: 1.05–1.7). For Late-Stage disease, the separation of the risk groups (n = 79, p = 0.064, HR = 1.84, 95% CI: 0.96–3.53) can be visually appreciated but the differences did not rise to the level of statistical significance. Interestingly, when MAD and MSCC were evaluated on SCC and AD patients respectively, neither model yielded a significant association with OS (See Supplementary Fig. 2(G–H)). A multivariable analysis was performed on the cohorts, after adjusting for the effects of clinicopathological variables (Table 2). Only the clinco-pathologic variables that were available across all cohorts were considered for this analysis. Gender was found to be significant for D1, D2, D3 and D7. Histological subtype was also found to be significant for D1, D2 and D3. Only partial clinical information for D8 was available. Supplementary Table 3 shows the multivariable survival analysis performed using the training models (MAD and MSCC) for some clinical variables (age, gender, and stage). In D7, MAD was able to identify high and low risk groups in patients treated with Docetaxel plus Cisplatin or Carboplatin (p = 0.002, HR = 4.1, 95% CI: 1.55–10.82) (Supplementary Fig. 2(I–L)). A similar trend was observed for patients who received Cisplatin plus Navelbine or Docetaxel or Pemetrexed (p = 0.041, HR = 2.04, 95% CI: 1.02–4.08). For patients in D8 treated with single monotherapy Docetaxel, MAD did not show to be prognostic of OS with an HR of 0.91 (95% CI: 0.66–1.26) (See Supplementary Fig. 2(M)). Further, MAD was significantly associated with OS for those patients who received radiotherapy but not radiochemotherapy from D5 and D7 (n = 208, p = 8.7e-07, HR = 2.94, 95% CI: 1.88–4.62) (See Supplementary Fig. 6 (A–B)).
For D7, patients were divided into low (≤50%) and high (>50%) PD-L1. Also, patients were represented into positive (>1%) and negative (≤1%) PD-L1 expression groups. MAD was also found to be associated with OS for the low PD-L1 arm, with HR of 2.41 (n = 69, p = 0.013, 95% CI: 1.17–4.94, C-index: 0.689 (SE 0.074)) (See Fig. 3(M)). Conversely, for the high PD-L1 arm, MAD did not show an association with OS (See Supplementary Fig. 6(C–F)).
PhenoTIL comparison with density and spatial aspects of TIL
For comparison purposes, the prognostic ability of two models based on density of TILs (denTILSCC and denTILAD) was assessed on datasets D3, D4, D5, and D7. These models, however, were not able to distinguish between patients treated with chemotherapy at low- and high- risk of death (p > 0.05) (Supplementary Fig. 4). Validation of the PhenoTIL models on D8 was done blindly in which we had access to whole slide images but not clinical data. Our algorithms were applied to the images to predict risk scores and patient labels, and then these values were sent to the BMS team, who carried out the statistical analysis and provided us with the corresponding results. Therefore, it was not possible to validate the current density of TILs models (denTILSCC and denTILAD)”.
“In addition, the prognostic ability of two models based on density of TILs (denTILSCC and denTILAD) was assessed on dataset D6. These models, however, were not able to distinguish between patients treated with immunotherapy at low and high risk of death (p > 0.05) (Supplementary Fig. 5).
The prognostic ability of the two models based on spatial location of TILs (spaTILSCC and spaTILAD) was assessed on datasets D3, D4, D5, and D7. These models, however, were not able to distinguish between patients treated with chemotherapy at low and high-risk of death (p > 0.05) (Supplementary Fig. 5).
PhenoTIL identified immune cell niches from pretreatment H&E samples were associated with overall survival in lung adenocarcinoma and squamous cell carcinoma patients treated with immunotherapy
For D6, patients who received nivolumab and were identified by MAD as having a “low risk of death” had significantly longer survival time (p = 0.004, HR = 5.83, 95% CI: 1.53–22.26) compared to patients identified as high risk, (see Fig. 3(J)). An expert pathologist manually assessed and categorized the quantity of TILs as low TIL (1–33%), moderate TIL (34–66%), and high TIL (67–99%) (for more details see Fig. 3(L)). A total of 70% of the patients labeled as “high risk” by MAD were found to have a low TIL count (1–33% of TILs) while 63.6% of the patients labeled as “low risk” by MAD had a high TIL count (34–99% of TILs). For D8, MAD showed an association with OS but did not reach statistical significance within the trial arm of patients who received nivolumab with an HR of 1.27 (n = 204, p = 0.15, 95% CI: 0.93–1.7), see Fig. 3(K)).
Identified cluster patterns of TILs in H&E images are different morphologically and molecularly between lungs adenocarcinoma and squamous cell carcinoma
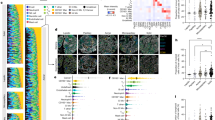
A qualitative and quantitative evaluation of the TIL niches identified by PhenoTIL from H&E images was performed on images from D3. Differences in composition, spatial behavior, and intercellular communication of TIL subtypes (i.e., CD4, CD8, CD20) were analyzed for the different PhenoTIL clusters. Since TIL subtypes are not discernible on standard H&E samples, this analysis was carried out only on dataset D3 as it was the only one with subtype information available via QmIF. An illustration of the molecularly identified TIL subtypes (CD4+, CD8+ and CD20+) on the QmIF image is shown alongside the corresponding TIL cluster identified by MAD on the co-registered H&E image (See Fig. 4(A)). A similar illustration was constructed for MSCC (See Fig. 4(B)). A two-dimensional UMAP representation of the cell’s features was performed for the AD and SCC samples (See Fig. 4(D) and 4(H)), with corresponding QmIF labels (See Fig. 4(C) and 4(G)). The percentage of the QmIF subtype concentration among the MAD and MSCC clusters can be seen in Fig. 4(E) and 4(I) respectively.
TIL subtype single-cell visual representation on an (A) lung AD and (B) SCC samples from cohort D3. The IF channels are displayed highlighting the spatial distribution of CD4+, CD8+, and CD20+ cells. Cluster labels are overlaid with corresponding H&E images. Smaller patches are shown to visualize the magnitude of complexity of cell detection and identification, depicting niche structures. C UMAP representation of TIL subtype composition for the lung AD. D UMAP of a lung AD sample cluster. E TIL subtype composition of clusters lung AD sample clusters has some concentrations of C1: CD4+ 10%, CD8+ 86%, CD20+ 4%; C4: CD4 + 25%, CD8+ 50%, CD20+ 25%; C6: CD4+ 9%, CD8+ 72%, CD20+ 19%. F The β coefficients (C1: 4.0, C2: −3.2, C3: −10.2, C4: 15.4, C5: 0.2, C6: 0.5, C7: 0.2, C8: −12.7) of the TIL cluster model (MAD). G UMAP representation of TIL subtype composition for the lung SCC. H UMAP of SCC sample clusters. I TIL subtype composition of SCC sample cluster. J The β coefficients (C1: −2.5, C2: 9.9, C3: 0.2, C4: 5.2, C5: 1.8, C6: 8.9, C7: −2.6, C8: 2.1) of the TIL cluster model (MSCC). K Chord diagram representing the cluster composition for lung AD. L Interconnection between the TIL subtypes and (M) between ‘constructive’ clusters. N Chord diagram representing the cluster composition for lung SCC. O Interconnection between the TIL subtypes and (P) ‘constructive’ clusters. CD4+ has a broader influence among clusters for SCC compared to AD. CD20+ plays a bigger role on the AD clusters.
By using the Elastic Net31 coefficients, major differences in the cluster involvement on survival for both the AD (MAD) and SCC (MSCC) can be observed in Fig. 3(F) and 3(J). When a cluster contribution toward a patients’ survival is found to be ‘constructive,’ it is interpreted as patients having longer survival times. For instance, for AD, clusters C1, C4, C5, C6, and C7 play constructive roles (Fig. 4(F)) while C2, C3, and C8 play obstructive roles. On the other hand, for SCC, clusters C2, C3, C4, C5, C6, and C8 play constructive roles while C1 and C8 play obstructive roles (See Methods or more details). Intercellular communication is examined using a chord diagram, which interrogates the inter- and intra-relationship between TIL subtypes and the clusters. The influence of the TIL subtypes among clusters for AD (See Fig. 4(K)–4(M)) and SCC (See Fig. 4(N)–4(P)) varies greatly.
In Fig. 5, the density of cells for two low- and high-risk samples (two AD and two SCC) is illustrated. The density plots are illustrated next to their corresponding H&E and IF images. This is performed for both models (MAD and MSCC). The clusters on MAD were grouped based on their roles as either ‘constructive’ (C (+)) or ‘obstructive’ (C (−)). They were represented by a bivariate histogram (hexagonal bin plot), useful for visualizing the structure of multiple clusters of cells. The spatial arrangement of the TIL clusters is illustrated at the WSI level for a PhenoTIL identified low- and high-risk AD tumor (See Fig. 6). In addition, the spatial cell interaction of the ‘constructive’ and ‘obstructive’ clusters is shown across two SCC and AD tumors (See Fig. 7).
The density plots based on the cluster’constructive’ C (+) and’obstructive’ C (−) are shown, depicting the concentration of each cluster group concentration. The TIL density plots also display concentration of TIL cells at the same position for each subtype, CD4+, CD8+ and CD20+. H&E samples are displayed at the outer layer of the figure and IF samples in the inside layer. The plots are shown for low- and high-risk groups assigned by the trained models (MAD and MSCC). For lung AD, CD20+ had a few cells spatially located to CD4+ pockets. high-risk TIL density plot (HRTD) sample displays CD8+ pockets to be sparse. CD20+ was found to be less frequent around CD8+ pockets. The ‘constructive’ clusters are seen to be forming highly concentrated pockets, compared to the ‘obstructive’ clusters, for both AD and SCC low-risk samples. Similarly, on the low-risk TIL density plot (LRTD), CD8+ can be seen forming concentrated pockets for AD. For SCC, the CD8+ pockets are sparser.
Two adenocarcinoma samples, labeled as low and high-risk by the trained model (MAD) from cohort D6. A It displays the H&E WSI for a sample labeled as low-risk. The overlaid grid describes patches of 2000 × 2000 pixel size which colors indicates tissue content and usefulness (green = High amount of tissue content, Yellow = Medium amount of tissue, and red = Low to no tissue content). Tiles allocated into the green grid were used for this representation. It depicts a zoomed-in region of interest at (B) 250 µm and (C) 50 µm. For each tile, TIL features and clusters were generated for the (D) for the low-risk sample. The colored-bar size indicates the log10 scale of the quantity of cells for the sample. The quantity of cells for each cluster is shown next to each colored dot and label identifier. A zoom-in patch is displayed alongside, highlighting the conformation of different cluster labels at (E) 250 µm and (F) 50 µm. Density estimation plot is further shown. The density estimation is performed through a probability density function in 2-D by a kernel density estimate, generating a binning grid area. The higher the quantity of data points (cells) that fall within the binning grid area, the higher the color intensity. This is performed for (G) individual clusters, (H) the ‘constructive’ clusters labeled as C (+) and ‘obstructive’ as negative C (−) and (I) density estimation of all the detected lymphocyte cells, highlighting the densest areas in red tonality (‘High’). Similarly, for high-risk samples, the samples were utilized from (J) H&E images, zoomed-in (K, L), (M) TIL clusters at WSI level and (N, O) zoom-in. Density plots for (P) clusters, (Q) ‘constructive’ and ‘obstructive,’ and (R) lymphocyte density plot.
The first two columns are squamous cell carcinoma cases (First and second columns are low and high-risk respectively). The last two columns are adenocarcinoma cases (Third and fourth columns are low and high-risk respectively). The first row represents the H&E WSI samples for lung AD (A, B) and SCC (C, D). Second row represents the plotting of cluster position that were assigned as ‘obstructive’ by the TIL model (MSCC) for (E, F) lung SCC and TIL model (MAD) for (G, H) lung AD. Third row represents the plotting of cluster position that were assigned as ‘constructive’ by the TIL model (MSCC) for (I, J) lung SCC and TIL model (MAD) for (K, L) lung AD model. For the low-risk samples (I, K), the influence of ‘constructive’ Cluster C4 around other clusters, is highlighted by the dotted-line region.
The distribution of the clusters across the risk groups revealed that for MAD, cluster C1 is more abundant for the low-risk cases. A similar trend is seen for TIL subtype CD8+. For MSCC, the trend is less clear, showing that the combination of clusters C2 and C6 participate in the low-risk cases (See Supplementary Fig. 7 (B–E)).
The morphometric measures used to construct TIL clusters by the models (MAD and MSCC), are also shown across the risk groups for lung AD (Supplementary Fig. 8(A–D)) and SCC (Supplementary Fig. 8(E–H)). Further, the morphometric features identified as part of the model MAD, found to be associated with cluster C1, are mostly related to the texture of the TIL. For the low-risk groups, the feature shows a low variation in texture and color intensity, as opposed to the high-risk groups in which there is significantly high variation. Another feature implicated in MAD is the distance between a TIL and non-TIL cell, which was significantly different, being shorter for tumors identified in the low-risk group and longer for the tumors in the high-risk group. This can be seen in the cluster of TILs associated with cluster C1 (See more details in the Supplementary Data 15).
Biological pathway association of PhenoTIL clusters with Immune activation, regulation, and antigen presentation
To understand the underlying biological processes associated with the PhenoTIL identified clusters, a gene enrichment analysis was performed. RNA sequencing of 20,531 genes was available for D5. For lung AD (n = 427), a functional profile of 1159 sets of genes were found to be significantly associated with the risk scores derived using the PhenoTIL-derived cluster measurements (MAD). From the identified set of genes for lung AD, the enrichment analysis identified 342 biological pathways (results with false discovery rate (FDR) p < 0.05), obtained from the Gene Ontology (GO) analysis platform32,33. Out of the 242 identified for AD, 24 pathways were found to be immune-related, from which 8 were shown to be associated directly with each PhenoTIL cluster for ADs. Pearson’s correlation coefficient was used to measure the strength and direction of the linear association between the immune-related pathways and the AD TIL clusters. The AD TIL clusters C3, C6, C7, and C8 were correlated with NRF2-mediated Oxidative Stress Response, C4 with IL-6 and IL-9 pathways. A heatmap illustrating the association between the most associated GO class and the individual TIL clusters determined by the TIL model (MAD) was constructed and illustrated in Fig. 8(A). The top 20 immune-related pathways, such as P13K signaling in B lymphocytes (B cell development) and TNFR2 signaling on regulatory T-cells (Increment on T-reg stability) are illustrated in Fig. 8(B).
Heatmap representation of the significant gene expression for (A) lung AD. Each row represents an overly expressed gene. Each column indicates the cluster. The colors of the heatmap represent the Pearson correlation coefficient. The grouped genes are color-coded with a GO term representation. B The biological and molecular pathways are described in box plots. The most significant (FDR adjusted p value) terms are illustrated. The number of total associated gene signatures is shown as number of regulatory genes (nRG). Similarly, for lung SCC is shown (C) the gene expression heatmap and the (D) biological and molecular pathways is also included.
For lung SCC (n = 423), 2710 genes were found to be significantly associated with the risk scores derived using MSCC. A total of 413 biological pathways (results with FDR p < 0.05) were found from which 30 pathways were immune-related. 10 were shown to be associated directly with each TIL cluster. TIL cluster C4 was found to negatively correlate with mTOR signaling pathway, which regulates T cell proliferation and orchestrates T cell quiescence34, and tRNA charging and was positively correlated with an antigen presentation pathway. Cluster C1 had a negative correlation with CD28 T-helper signaling. C7 positively correlates with CDK5 and apoptosis signaling. Cluster C6 had a positive correlation with cell cycle regulation by GTB1 and GTB2. Figure 8(C) illustrates the heatmap of the GO class associated with each TIL cluster from lung SCC determined by the model (MSCC). Figure 8(D) shows the top 20 immune-related pathways, such as natural killer cell signaling, T helper cell differentiation, ICos-ICosL signaling in T helper cells, and mTOR signaling identified for SCC tumors. The complete biological process, cellular component, molecular function, and gene pathways results based on the GO analysis are shown in Supplementary Fig. 9(A–B). In general terms, the most significant pathways found in lung AD and SCC were involved in, (a) the immune recognition, (b) the antigen presentation, (c) the antigen response and (d) the antigen regulation.
Discussion
T cells are generated in the thymus and undergo further differentiation in the periphery to become specialized T cells such as CD8+ and CD4 + T cells. These cells effectively navigate to acute viral infection or tumor presence35. The tumor immune microenvironment (TIME) comprises abundant activated effector cytotoxic CD8+ and helper CD4+ tumor-infiltrating lymphocytes (TILs). The function of CD8 + T cells is to suppress tumors, by killing cancer cells with cytotoxic molecules. CD4 + T cells’ primary role is to mediate the anti-tumor immunity by stimulating CD8 + T cells36. Interestingly, a large quantity of cytotoxic CD8+ TILs found in TIME that have not been activated by tumor antigens behave as “bystanders”9. After a period of hyperresponsive state caused by chronic antigen stimulation, an activated CD8 + TIL undergoes ‘exhaustion’, characterized by high inhibitory expression and reduced cytotoxicity10,37. These diverse populations of immune cells form heterogeneous clusters with intricate communications within the TIME38. Due to the TIME complexity, estimating immunological frequency and TIL density may not be sufficient to describe cellular heterogeneity and their spatial distribution. However, capturing the complexity of the TIME could allow the development of prognostic and predictive biomarkers of response to current cancer treatments that target tumors (chemotherapy and radiation) and modulators of immune responses (immunotherapy).
Several studies have explored immune response mechanisms and their role in lung cancer treatment response39. For instance, Nejati et al. 40 demonstrated that high concentrations of intra-tumoral CD8+ and CD4+ TILs were associated with prolonged OS in patients treated with chemotherapy. A similar scenario was seen in lung SCC19 in which elevated levels of CD8+ or CD4+ TILs was associated with a longer disease-specific survival and disease-free survival, compared to patients with a lower concentration. In experiments using H&E slides, Wang et al. 18 explored the interplay between TIL and non-TIL cells in the tumor epithelium area, and identified spatial statistics related to TIL-cancer cell distances that were associated with OS and progression-free survival. Similarly, Azarianpour et al. 15 examined the spatial arrangement of TIL and cancer nuclei on H&E images, demonstrating an association between these spatial distance statistics with clinical benefit to chemotherapy, radiation therapy and nivolumab in gynecological cancers. Furthermore Ding et al. 21 explored both the density and spatial aspects of TILs and their association with outcome in patients treated with chemotherapy and nivolumab for lung AD and SCC. Also, finding the TIL density to be more crucial in patients with SCC and TIL spatial distribution for AD patients.
In this study, we introduced a new computationally-derived TIL phenotyping approach called ‘PhenoTIL’. PhenoTIL is a computational pathology approach that captures and employs the phenotypic and morphologic characteristics and spatial organization of TILs in the tumor microenvironment on H&E-stained tumor samples to identify distinct immune clusters, composition of which are associated with clinically relevant outcomes. In addition, PhenoTIL signature was able to stratify patients based on their risk of death using pre-treatment biopsy H&E-stained samples and a single cohort with post-neoadjuvant sections (D7), in the context of different therapy scenarios such as chemotherapy and targeted immunotherapy. Dedicated PhenoTIL models for characterizing the TIME in SCC and AD tumors were developed using 87 lung AD and 53 lung SCC patients respectively, subsequently evaluated for predicting OS on 1102 lung AD patients and 530 SCC patients. Unique PhenoTIL signatures that were associated with clinically relevant outcomes for squamous cell carcinomas and adenocarcinomas were identified.
In the context of adenocarcinomas, PhenoTIL was able to risk-stratify patients undergoing different therapies including chemotherapy and radiotherapy (Supplementary Fig. 6(A–B)). This trend was seen across dataset D7, in which chemotherapy was used in a neoadjuvant setting (i.e., before surgical resection) and scenarios with a combination of two chemo agents (Taxol plus platinum-based). The PhenoTIL model for adenocarcinomas (MAD) and squamous cell carcinoma (MSCC) were trained with most patients with early-stage (I and II) lung cancer, and the validation cohorts corresponded to patients from a plurality of staging groups (58.45%), indicating that the signatures were not specific to a particular disease stage, but the prognostic trend was observed most emphatically for early-stage disease. Most likely the immune reaction across the stages is highly variable, with late-stage immune landscape predominantly characterized by high levels of T-cell exhaustion41 and early-stage represented by a gradual transition from immune activation to immunosuppression, including the decrease of T-cell clonotypes, increase in regulatory T-cells infiltration and reduced infiltration of anti-tumor helper T cells42. For instance, despite PhenoTIL model MAD not having a statistically significant stratification of risk (p = 0.12, HR = 1.27 (0.93–1.7)) in the phase 3 clinical trial cohort D8 (NCT01673867) of patients who received just Nivolumab, the Kaplan–Meir plots clearly revealed distinct risk groups (Fig. 3(K)). These patients were predominantly late-stage IV, so, surgery was not a viable option due to tumor dissemination. A similar behavior was seen for cohort D6, in which most of the patients were stage III and IV (76%) and were treated with Nivolumab (57%). More importantly, the PhenoTIL model MAD was able to discriminate between groups of patients with a low and high risk of death in a low PD-L1 (<50%) setting, potentially allowing these patients who might otherwise be recommended for ICI-chemo combination therapy, to be candidates for ICI monotherapy; thereby potentially obviating the need for chemotherapy in a subset of LUAD patients with low PD-L1 expression.
At the tumor microenvironment level, we observed differences between the two NSCLC subtypes, with SCC exhibiting higher inter- and intra-tumoral heterogeneity than lung AD, which aligns with previous findings. For instance, some studies found that SCC tissue displayed enriched neutrophils at the cellular level compared to AD and has a more robust interaction with tumor cells43. Another study found that the spatial differences in PD-1 and CD8+ cells were significantly different between AD and SCC, regardless of the infiltration in the tumor proximity. In AD, a higher density of PD-1+ cells in the stroma and tumor islet cells was associated with shorter survival times while, in SCC, it was associated with better outcomes44. Using molecular and genomic analysis, we found that CD4+ and CD8+ T cell interactions played a key role in SCC patients with low risk of death while CD8+ and C20+ T cells were key components for lung AD patients with low risk of death. Regulation of the B-cells activation and proliferation, which play a critical role in recognizing antigens45, were correlated with ‘constructive’ clusters of TIL signature (MAD). Similarly, the ‘obstructive’ clusters were associated with NRF2-mediated oxidative stress response pathways, whose deficiency causes enhanced susceptibility to inflammatory diseases, deterioration, and oxidation;46,47, ultimately correlating with the exhausted state of T-cells. For the SCC PhenoTIL signature, the desirable clusters were found to be associated with mTOR signaling, which influences T-cell differentiation, stimulation, and proliferation, a major regulator of memory antigen-activated CD8+ T-cell differentiation, whose correlates play important roles in the TIME.
PhenoTIL is not the only approach that has characterized and used the spatial interplay of TILs, for instance studies carried out by Park et al. 48, Lopez de Rodas et al. 49, Wang et al. 50 and Ding et al. 21 have been exploring the use of computational imaging and artificial intelligence-based approaches for characterizing the density and spatial architecture of TILs in H&E images and their association with response to ICI and survival. Differences between our approach and these previously published studies include (1) the use of tiles or patches to obtain TIL-related features as opposed to our study which used the TIL immunophenotyping information on a cell-by-cell basis via the use of qmIF images, (2) risk stratification based on phenotypic traits of the TIL clusters, and (3) identifying molecular differences and distinct functions of the stratified TILs groups between lung tumor subtypes AD and SCC. In addition, the underlying hypothesis behind these studies14,16,17,18,21,48,49,50 remains the same, namely that all TILs are treated in a consistent and homogeneous manner.
The PhenoTIL approach identified clusters on the H&E images potentially represent individual aggregates of activated, exhausted, and ‘bystander’ immune cells, this hypothesis will need to be explicitly validated in future work. While we have not explicitly validated that PhenoTIL cluster mimics the functionality of each specific TIL subtype, we assume that the PhenoTIL signature appears to distinguish the unique formation of TIL clusters that resemble the functional aspects of activated TILs, which are prone to have a ‘constructive’ behavior toward tumor regulation and exhausted and bystander T cells, which have a more ‘obstructive’ behavior. The fundamental hypothesis of PhenoTIL, is that not all TILs uniformly contribute toward a prognostic signature for lung AD and SCC subtypes. Even though PhenoTIL does not explicitly identify individual molecular subtypes of TILs, it does appear to cluster the TILs into unique immune cell niches within the TIME, the architecture of which has clinical relevance with outcome for multiple therapy types.
Our study did have its limitations. One limitation was that the study design was retrospective in nature. In addition, although PhenoTIL was found to be prognostic of OS in both lung AD and SCC, the PhenoTIL signature MAD was not associated with OS for patients who received either single-agent platinum or three-agent variations of multiple Taxol plus platinum. This behavior has been seen in patients benefiting from receiving combination compared to single-agent use51. This is probably due to the monotherapy scheme and outcome effects, relating to the biological difference reflected on the survival effect of TILs. Similar trends have been observed in the use of single chemo-agents which could inflict deleterious and inhibitory effects on the immune system and lymphocyte activation52. In addition, since both PhenoTIL signatures MAD and MSCC were trained solely on cohorts of patients treated with different chemotherapy agents, our signatures while associated with OS for individual therapies, were not explicitly validated as predictive for benefit of specific therapies. However, a strength of our approach was that the signature was associated with clinically relevant outcomes across different treatment types, reflecting the ability of the PhenoTIL signature to pick up biological hallmarks of good and poor tumor biology. Further, in this work, we studied the association between patient prognosis and nuclei clusters, built from contextual features (PhenoTIL), on the premise that cells in the tumor tend to act as groups rather than individually53. However, future work may benefit from analysis of the association between patient outcome and PhenoTIL features, independent of clusters.
In summary, we have developed and validated a computational biomarker, ‘PhenoTIL’, an immune-related biomarker associated with treatment-specific outcomes in NSCLC. It is also capable of capturing the immune phenotypes of TILs and their spatial interplay, allocating them into unique ‘immune clusters’ using digitized H&E-stained biopsy samples. PhenoTIL can also identify low-risk lung AD and SCC patients, which would allow clinicians to make adequate changes in their therapy management. Future work will involve prospective validation across independent cohorts and validation of the PhenoTIL signature as predictive of benefit of specific therapies.
Methods
Data and image processing
Figure 2 shows the overall workflow including image preparation, feature extraction, TIL single-cell cluster formation, molecular identification of TIL, and genomic pathway association for the PhenoTIL approach. As part of the inclusion criteria for all the datasets, images with low quality, blurry effects, and significant artefacts were excluded from the analysis. Image quality was checked using HistoQC26, an open-source quality control tool for digital pathology slides. HistoQC analyzes a set of WSIs and generates image masks indicating the regions that are not useful for analysis because of large blurry areas, obstructive dotting pen markings, or sub-coverslip bubbles. Images where the computationally valuable regions were either empty or smaller than 40% of the total real-estate of the slide were discarded. For this project, HistoQC was run using its default parameters26. This was performed for datasets D5, D6, D7, and D8. For cohorts with TMA images, a previous study54 have mentioned that their use for evaluating TILs may be an issue due to TMAs not having substantial cell-related information and small core diameter may not reflect the entire tissue composition, nevertheless further studies as indicated by55 have shown that well-annotated TMAs datasets were optimal for drawing concordant results with TIL-related biomarkers and clinical outcome, indicating TMAs to be good option for this type of study. Regarding the TMA cohorts on our study, D1, D2, D3, and D4, images that were not able to be processed for feature extraction due to lack of tissue (images containing 20% or less percentage of pixels in the tissue area) and sufficient nuclei detection (images containing 20 or less identified TIL and non-TILs) were removed. For D6, D7, and D8 the additional inclusion criteria invoked included the availability of histologic subtype AD (See Fig. 1). Inclusion criteria for D7 were as follows: From the initial 211 patients, the considered patients were those who either underwent surgical resection after neoadjuvant therapy or had a primary resection at a locally advanced stage, which qualified them for neoadjuvant therapy. Three patients had more than one WSI scanned due to the following reasons: the first patient had a sample with neoadjuvant lung SCC with small AD as an incidental finding, the second was a patient with neoadjuvant adenosquamous carcinoma, and the third patient had a primary lung AD with three regions with very different growth patterns within the primary. Only one WSI containing the most tissue sample was selected for each patient. After invoking the inclusion and exclusion criteria for this study, 71 cases for D1 (49 excluded), 71 for D2 (35 excluded), 79 for D3 (57 excluded), 231 for D4 (49 excluded), 850 for D5 (239 excluded), 21 for D6 (49 excluded), 93 for D7 (118 excluded) and 358 for D8 (224 excluded) were included. Tiles of size 2048 by 2048 pixels (at 20× resolution; approx. 990 μm) were extracted from an automatic segmented tumor area18 of each WSI, from cohorts D5, D6, D7, and D8. TMA spots from cohorts D1, D2, and D3 were directly utilized. TMAs from D4 were down-sampled to 20× using nearest-neighbor interpolation. Supplementary Table 2 provides a summary of the image-related information for each cohort.
An inspection for assessing batch effects was performed using the subsequent full set of 288 PhenoTIL features across the different histologic image tiles and TMAs. The set of features were embedded into a two-dimensional feature space and plotted using UMAP56. The embedding shows (See Supplementary Fig. 7(A)) that there were no significant clusters of patients or isolation of samples by institutional site (cohort). This suggests that PhenoTIL features are resilient to batch effects and appear to be reproducible across the multiple sites/cohorts; in other words, no evidence of batch effects was identified.
Nuclei segmentation and TIL identification
In D5, for each patient, 10 tiles of pixel size 2048 × 2048 (~990 μm) were extracted. Similarly, from D6, D7, and D8, 10 tiles were obtained per WSI, outlined from an automatically segmented tumor area. For D7 multiple tiles were obtained from 4 WSIs for visualization purposes. The method developed by Wang et al. 18 was employed for the task of tumor segmentation. This method is a U-Net based convolutional network tuned using adversarial training, and it receives as input a WSI and outputs a tumor heatmap (See Supplementary Fig. 1(B)). Authors of the method report that, in a validation testing set containing 45 WSIs, the detector achieved a 90.6% patch-level accuracy18. The validation set is from the lung TCGA archives (TCGA-LUAD). The same cohort is used in the present study (D5). In addition, this model was used as is, i.e., it was not retrained or adjusted. The model was applied on each WSI and subsequently down-sampled to 20× through nearest-neighbor interpolation. The tumor detector was used to generate the heatmap for WSI to indicate the probability of tumor (90.6% patch-level accuracy) (See Supplementary Fig. 1(B)). D4 TMAs were down-sampled to 20× through nearest-neighbor interpolation. Two nuclei segmentation models were used to detect and segmentate the nuclei. First, the deep learning model introduced by Wang et al. 18 was used “as is” to delineate the boundary pixels of each nucleus. This model is also a U-Net network-tuned model, using adversarial training that receives a patch as input and then generates a mask indicating the location of the nuclei within the patch (and Supplementary Data 2). According to the authors, this model yielded an f-score of 0.88 in a dataset with 8000 nuclei annotated18. A second machine learning model based on morphological transformations and image processing using a watershed-based algorithm was used to further segmentate the nuclei57, resulted in a positive predictive value of 90% (Supplementary Data 3). Both models’ outputs were combined, and a single binary nuclei mask was obtained. Next, the model developed by Corredor et al. 58 is employed, as is, for classifying each segmented nucleus as either a TIL or non-TIL. It receives as input both an image patch and its respective nuclei segmentation mask (obtained using the method previously described), and it outputs the location of TILs and non-TILs within the image. Authors report that this model is a support vector machine trained visual features (texture, shape, and color), which yielded an f-score of 0.86 in validation phase58. Approximately 53,000 TILs were identified by automatically counting the total detected amount from lung AD patients (n = 87) in DAD1 and DAD2, and 40,000 TILs were identified from SCC (n = 55) in DSCC1 and DSCC2. Due to the color variation of images acquired from different scanners, color-normalization was applied to the H&E images using a technique a spectral matching named Macenko’s normalization59 to ensure coherent color representation across all datasets. An expert pathologist performed a manual TIL assessment for cohort D6 and assigned a label of 1–33%, 34–66%, and 67–99%, based on the percentage of infiltrating lymphocytes.
PhenoTIL: TIL-based feature extraction and cluster computation
Characterization of the interaction of TILs within the tumor microenvironment was done by quantifying their spatial interaction with all other neighboring cells30. The algorithm comprises four steps: (1) The rectangular position (two-dimensional coordinate with positions, x, and y) of each TIL is obtained. (2) Iteratively, the algorithm locates each TIL and from its centroid coordinate, three circles are defined with incremental increasing radii of k = dL*10, dL*20, and dL*30 pixels (dL = 20 pixels, the average diameter of lymphocytes at 20× resolution. For a visual representation, see Supplementary Fig. 10(A)). (3) At each circle, a set of cell features were extracted from the neighboring cells including morphological characteristics (e.g., size, orientation, diameter, color intensity), density (e.g., number of surrounding lymphocytes), cell texture (Haralick et al. 60) And graph-based metrics (e.g., distances between lymphocytes and non-lymphocytes) were computed (The complete list can be seen in the Supplementary Table 4 and Supplementary Data 1 and 4). This set of 288 features (See PhenoTIL: TIL-based digital risk score and statistical analysis) characterize each TIL. 4) TILs were then clustered based on the features using a Gaussian Mixture Model61, from which a total of 8 unique TIL clusters were found (For more details of the clustering process, see the Supplementary Data 5). Finally, a patient is represented by eight vectors. These vectors represent the eight unique TIL clusters (C1, C2, C3, C4, C5, C6, C7, C8). The total TIL-related data is represented as a single matrix of size 1774 × 8 (1774 patients and eight TIL vector clusters).
IF and H&E image registration
For D3, two challenges were found when processing the immunofluorescence (IF) images. One of the challenges was the overexposure found across the different IF markers intensities, affecting the comparison between the different images. To address this issue, the quantitative immunofluorescence score calculated by AQUA (Automated quantitative analysis) was used12 for intensity normalization. Another challenge was that the IF and H&E-stained images were generated from consecutive tissue sections. Their alignment is imperfect on account of the partial overlap of different TIL subtypes markers on a single-cell basis and the variation in the degrees of misalignment between the corresponding H&E TMA boundaries. To match both images and address the geometric misalignment of the images, a piece-wise linear transformation62 and spatial transformation via MATLAB function “cp2tform” was applied.
Single-cell molecular pairing of TIL in H&E with IF images
One of the first steps to interrogate the TILs was to assign a unique molecular label to each identified TIL on H&E images. Once the images are co-registered, the identification of the TIL subtypes is performed. The process is based on the comparison on a pixel level, of the intensities of each cell. For each detected cell, the membrane is isolated. The intensity of the immunofluorescence stain of each marker (CD4 + , CD8+ and CD20 + ) is quantified from within the membrane (See the Supplementary Fig. 10(B–D)). These values are then normalized by the AQUA value12 (See Methods section, IF and H&E Image Registration). Finally, the marker with the highest value is taken as the true label for the cell. This process is repeated for each TIL.
Gene enrichment and pathway analysis
The enrichment of genes and pathway analysis is performed for dataset D5. The gene collection was obtained from TCGA. The quantification of gene expression by RNA-seq is obtained for both lung AD (TCGA-LUAD) and SCC (TCGA-LUSC). The data is prepared into a matrix of gene expressions for each patient. A correlation matrix is computed between the gene expression matrix and the TIL clusters obtained from each model (MAD and MSCC). Pearson’s correlation coefficient is calculated to identify the genes highly associated (values higher than 50% of the maximum correlation value). These genes are referred to as ‘activation genes’ or differentially expressed genes. These genes were used to find on the gene ontology (GO) knowledgebase32, pathways of biological processes through enrichment analysis. The pathways for each cluster were identified and visualized using a heatmap and bar plot displaying the canonical pathways significantly overrepresented. The most significant pathways are identified and listed according to their FDR corrected p value, shown as (−) log10(FDR). The analysis was performed using the R package TCGAbiolinks33, an integrative analysis with genomic data commons data.
PhenoTIL: TIL-based digital risk score and statistical analysis
Overall survival was defined as the time interval between the date of diagnosis and the date of death and was censored at the date of the last follow-up for those patients known to be alive. An OS risk score (OSRS) for each ith patient is defined as \({{OSRS}}_{i}={\sum }_{i=1}^{n}{N}_{{ij}}{\beta }_{j}\), where βj is the regularizing coefficients returned by the Elastic Net31 method and Nij is the number of clusters for the ith patient. These steps were performed for developing the PhenoTIL signatures for lung AD using DAD1 and DAD2 and SCC using DSCC1 and DSCC2. The βj coefficients are also indicators of cluster contribution toward patients’ survival, clusters with +βj value is ‘constructive’ and those with −βj values are ‘obstructive.’ In summary an OSRS was computed for each patient as a linear combination of the clusters and their respective coefficients (Supplementary Data 6). The median value of all risk scores of patients was computed and used as a cutoff, so a patient whose risk score value is higher than the median is considered “high risk”, and a patient with a risk score value lower than the median is considered “low risk”. Two different survival Cox proportional hazard regression models63 are obtained for lung AD (MAD) and SCC (MSCC) from (DAD1 and DAD2) and (DSCC1 and DSCC2), respectively, and are validated on D3, D4, D5, D6, D7, and D8. MSCC is validated only on D3, D4, and D5. To evaluate the efficacy of MAD and MSCC to predict OS, the Mantel-Haenszel log-rank test64 was used to assess the difference in OS. P values were two-sided, and values under 0.05 were considered to be statistically significant. The performance of the models MAD and MSCC were evaluated employing Harrell’s concordance-index (C-index)65. Kaplan–Meier survival analysis was utilized to determine the difference of OS across the different patient risk groups.
Three statistical analyses were performed between the TIL clusters across lung AD and SCC on D3. First, the statistical differences across the TIL clusters, e.g., how statistically different is cluster C1 on AD compared to cluster C1 on SCC (See Supplementary Table 5(A)). Second, the difference between AD from SCC regardless of the cluster labels, e.g., how statistically different are the TIL phenotypic features from AD vs SCC samples (See Supplementary Table 5(B)). Third, the statistical differences across the TIL subtypes (CD4+, CD8+, CD20+), e.g., how statistically different is the arrangement of CD8+ across all the clusters on AD compared to all the clusters on SCC (See Supplementary Table 5(C)).
DenTIL: Developing prognostic models using features based on density of TILs
19 features relating to the density of TILs (denTIL)21 were extracted from each patch. These features include descriptors such as ratio between the number of TILs and the tissue area, ratio between the number of TILs and the total number of nuclei, and ratio between the total area covered by TILs to the total area of the tissue. The final feature vector for each patient was obtained by computing the mean, median, skewness and kurtosis for each feature across all its constituent tiles (i.e., four features per patient). Finally, two different survival Cox proportional hazard regression models were trained for lung AD (denTILAD) and SCC (denTILSCC) from (DAD1 and DAD2) and (DSCC1 and DSCC2), respectively, and are validated on D3 to D7.
SpaTIL: Developing prognostic models using features based on TIL architecture
350 features were used to quantify the spatial arrangement of TILs and spatial interaction between TILs and non-TILs (spaTIL)21 as extracted from each patch. These features include the area of TIL clusters and the intermixing of the TIL and non-TIL clusters, among others. The final feature vector for each patient was obtained by computing the mean, median, skewness and kurtosis for each feature across all its constituent tiles (i.e., four features per patient). Finally, two different survival Cox proportional hazard regression models were trained for lung AD (spaTILAD) and SCC (spaTILSCC) from (DAD1 and DAD2) and (DSCC1 and DSCC2), respectively, and are validated on D3 to D7.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Since the cases from the involved institutions are protected through institutional compliance, the clinical repository of cases can only be shared per specific institutional review board (IRB) requirements. Upon reasonable request, a data sharing agreement can be initiated between the interested parties and the clinical institution following institution-specific guidelines. This is applicable for Cohort D1, D2, D3, D4, D6, D7, and D8. D1, D2, D3 were provided by the Department of Pathology at Yale University (D2 were collected at Sotiria General Hospital and Patras University General Hospital at Greece but were made available from Yale Pathology). Clinical information was provided. D4 was obtained from the Cleveland Clinic (CCF). Clinical information was provided. D5 was generated by TCGA Research Network (http://cancergenome.nih.gov/), and they have made them publicly available. The diagnostic slide (H&E slides) as well as the RNA sequencing data (https://portal.gdc.cancer.gov/projects/TCGA-LUAD and https://portal.gdc.cancer.gov/projects/TCGA-LUSC). Clinical information can be found at their portal. D6 was provided by the University of Pennsylvania Hospital (UPenn). Clinical information was provided. D7 was provided by the University of Bern in Switzerland (UBern). Clinical information was provided. D8 was provided by Bristol-Myers Squibb (BMS), from the clinical trial CA209-057 (ClinicalTrials.gov identifier: NCT01673867). No clinical information was provided.
Code availability
All the codes and guidance for MATLAB and Python scripts can be found on GitHub: https://github.com/maberyick/PhenoTIL_V1.
Nuclei Segmentation https://github.com/maberyick/nucleiSegmentationHEDL.
Lymphocyte detection https://github.com/maberyick/LympDetect_HE_ML_V2.
References
Medaglia, C. et al. Spatial reconstruction of immune niches by combining photoactivatable reporters and scRNA-seq. Science. Am. Assoc. Adv. Sci. 358, 1622–1626 (2017).
Qi, H., Kastenmüller, W. & Germain, R. N. Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu. Rev. Cell Dev. Biol. 30, 141–167 (2014).
Matsushita, H. et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482, 400–404 (2012).
Rabinovich, G. A., Gabrilovich, D. & Sotomayor, E. M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 25, 267–296 (2007).
Park, Y. H. et al. Chemotherapy induces dynamic immune responses in breast cancers that impact treatment outcome. Nat. Commun. Nat. Publ. Group 11, 6175 (2020).
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 8, 1069–1086 (2018).
Lattuca-Truc, M. et al. Trends in response rate and survival in small-cell lung cancer patients between 1997 and 2017. Lung Cancer Amst. Neth. 131, 122–127 (2019).
Reck, M. et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. Mass. Med. Soc. 375, 1823–1833 (2016).
Meier, S. L., Satpathy, A. T. & Wells, D. K. Bystander T cells in cancer immunology and therapy. Nat. Cancer Nat. Publ. Group 3, 143–155 (2022).
Sobottka, B., Moch, H. & Varga, Z. Differential PD-1/LAG-3 expression and immune phenotypes in metastatic sites of breast cancer. Breast Cancer Res. 23, 4 (2021).
Hao, J. et al. Infiltration of CD8 + FOXP3 + T cells, CD8 + T cells, and FOXP3 + T cells in non-small cell lung cancer microenvironment. Int J. Clin. Exp. Pathol. 13, 880–888 (2020).
Schalper, K. A. et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J. Natl Cancer Inst. 107, dju435 (2015).
Gataa, I. et al. Tumour-infiltrating lymphocyte density is associated with favourable outcome in patients with advanced non–small cell lung cancer treated with immunotherapy. Eur. J. Cancer 145, 221–229 (2021).
Corredor, G. et al. Spatial Architecture and Arrangement of Tumor-Infiltrating Lymphocytes for Predicting Likelihood of Recurrence in Early-Stage Non-Small Cell Lung Cancer. Clin. Cancer Res. J. Am. Assoc. Cancer Res. 25, 1526–1534 (2019).
Azarianpour, S. et al. Computational image features of immune architecture is associated with clinical benefit and survival in gynecological cancers across treatment modalities. J. Immunother. Cancer 10, e003833 (2022).
Saltz, J. et al. Spatial Organization and Molecular Correlation of Tumor-Infiltrating Lymphocytes Using Deep Learning on Pathology Images. Cell Rep. 23, 181–193.e7 (2018).
Yuan, Y. Spatial Heterogeneity in the Tumor Microenvironment. Cold Spring Harb. Perspect. Med. 6, a026583 (2016).
Wang, X. et al. A prognostic and predictive computational pathology image signature for added benefit of adjuvant chemotherapy in early stage non-small-cell lung cancer. EBioMedicine 69, 103481 (2021).
Hoesli, R. et al. Proportion of CD4 and CD8 tumor infiltrating lymphocytes predicts survival in persistent/recurrent laryngeal squamous cell carcinoma. Oral. Oncol. 77, 83–89 (2018).
Borghaei, H. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. Mass. Med. Soc. 373, 1627–1639 (2015).
Ding, R. et al. Image analysis reveals molecularly distinct patterns of TILs in NSCLC associated with treatment outcome. NPJ Precis Oncol. 6, 33 (2022).
Seo, J.-S., Kim, A., Shin, J.-Y. & Kim, Y. T. Comprehensive analysis of the tumor immune micro-environment in non-small cell lung cancer for efficacy of checkpoint inhibitor. Sci. Rep. Nat. Publ. Group 8, 14576 (2018).
Wang, B.-Y. et al. The comparison between adenocarcinoma and squamous cell carcinoma in lung cancer patients. J. Cancer Res. Clin. Oncol. 146, 43–52 (2020).
Scagliotti, G. et al. The Differential Efficacy of Pemetrexed According to NSCLC Histology: A Review of Two Phase III Studies. Oncologist 14, 253–263 (2009).
Nirmal, A. J. et al. Immune Cell Gene Signatures for Profiling the Microenvironment of Solid Tumors. Cancer Immunol. Res. 6, 1388–1400 (2018).
Janowczyk, A., Zuo, R., Gilmore, H., Feldman, M. & Madabhushi, A. HistoQC: An Open-Source Quality Control Tool for Digital Pathology Slides. JCO Clin. Cancer Inform. 3, 1–7 (2019).
Camp, R. L., Chung, G. G. & Rimm, D. L. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat. Med. 8, 1323–1327 (2002).
Zens, P. et al. A prognostic score for non-small cell lung cancer resected after neoadjuvant therapy in comparison with the tumor-node-metastases classification and major pathological response. Mod. Pathol. Nat. Publ. Group 34, 1333–1344 (2021).
Zens, P. et al. The effect of neoadjuvant therapy on PD-L1 expression and CD8+lymphocyte density in non-small cell lung cancer. Mod. Pathol. 35, 1848–1859 (2022).
Barrera C., et al. Phenotyping tumor infiltrating lymphocytes (PhenoTIL) on H&E tissue images: predicting recurrence in lung cancer. Med Imaging 2019 Digit Pathol [Internet]. SPIE; 2019 [cited 2022 May 12]. 21–28. https://www.spiedigitallibrary.org/conference-proceedings-of-spie/10956/1095607/Phenotyping-tumor-infiltrating-lymphocytes-PhenoTIL-on-HE-tissue-images/10.1117/12.2513048.full.
Zou, H. & Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B Stat. Methodol. 67, 301–320 (2005).
Ashburner, M. et al. Gene Ontology: tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
Colaprico, A. et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 44, e71 (2016).
Zeng, H. & Chi, H. mTOR and lymphocyte cell metabolism. Curr. Opin. Immunol. 25, 347–355 (2013).
Jiang W., et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front Immunol [Internet]. Front Immunol; 2021 [cited 2022 Jun 6];11. https://pubmed.ncbi.nlm.nih.gov/33633741/.
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Martinez-Usatorre A., et al. Enhanced Phenotype Definition for Precision Isolation of Precursor Exhausted Tumor-Infiltrating CD8 T Cells. Front Immunol [Internet]. 2020 [cited 2022 May 12];11. https://www.frontiersin.org/article/10.3389/fimmu.2020.00340.
Fu, T. et al. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J. Hematol. OncolJ Hematol. Oncol. 14, 98 (2021).
Monette, A. et al. Immune-enrichment of non-small cell lung cancer baseline biopsies for multiplex profiling define prognostic immune checkpoint combinations for patient stratification. J. Immunother. Cancer 7, 86 (2019).
Nejati, R. et al. Prognostic Significance of Tumor-Infiltrating Lymphocytes in Patients With Pancreatic Ductal Adenocarcinoma Treated With Neoadjuvant Chemotherapy. Pancreas 46, 1180–1187 (2017).
Saab S., et al. Insights Into Lung Cancer Immune-Based Biology, Prevention, and Treatment. Front Immunol [Internet]. 2020 [cited 2023 Mar 23];11. https://www.frontiersin.org/articles/10.3389/fimmu.2020.00159.
Chen W-W, et al. Deciphering the Immune–Tumor Interplay During Early-Stage Lung Cancer Development via Single-Cell Technology. Front Oncol [Internet]. 2022 [cited 2023 Mar 23];11. https://www.frontiersin.org/articles/10.3389/fonc.2021.716042.
Wu, F. et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat. Commun. Nat. Publ. Group 12, 2540 (2021).
Chen, L. et al. The landscape of immune microenvironment in lung adenocarcinoma and squamous cell carcinoma based on PD-L1 expression and tumor-infiltrating lymphocytes. Cancer Med. 8, 7207–7218 (2019).
Jellusova, J. & Rickert, R. C. The PI3K pathway in B cell metabolism. Crit. Rev. Biochem. Mol. Biol. 51, 359–378 (2016).
Angkasekwinai, P. & Dong, C. IL-9-producing T cells: potential players in allergy and cancer. Nat. Rev. Immunol. Nat. Publ. Group 21, 37–48 (2021).
Neeve, S. C., Robinson, B. W. & Fear, V. S. The role and therapeutic implications of T cells in cancer of the lung. Clin. Transl. Immunol. 8, e1076 (2019).
Park, S. et al. Artificial Intelligence-Powered Spatial Analysis of Tumor-Infiltrating Lymphocytes as Complementary Biomarker for Immune Checkpoint Inhibition in Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off J. Am. Soc. Clin. Oncol. 40, JCO2102010 (2022).
Rodas, M. L. et al. Role of tumor infiltrating lymphocytes and spatial immune heterogeneity in sensitivity to PD-1 axis blockers in non-small cell lung cancer. J. Immunother. Cancer BMJ Specialist J. 10, e004440 (2022).
Wang, X. et al. Spatial interplay patterns of cancer nuclei and tumor-infiltrating lymphocytes (TILs) predict clinical benefit for immune checkpoint inhibitors. Sci. Adv. 8, eabn3966 (2022).
Rigas, J. R. Taxane-platinum combinations in advanced non-small cell lung cancer: a review. Oncologist 9, 16–23 (2004). Suppl 2.
Wimmer, K. et al. Differential effects of epirubicin and docetaxel on the immune system in patients with breast cancer (BC). J. Clin. Oncol. Wolters Kluwer 38, 588–588 (2020).
Friedl, P. et al. Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro. Cancer Res. 55, 4557–4560 (1995).
Lee, A. T. J. et al. The adequacy of tissue microarrays in the assessment of inter- and intra-tumoural heterogeneity of infiltrating lymphocyte burden in leiomyosarcoma. Sci. Rep. 9, 14602 (2019).
Salgado, R. et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. J. Eur. Soc. Med. Oncol. 26, 259–271 (2015).
McInnes L., Healy J., Melville J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction [Internet]. arXiv; 2020 [cited 2023 Feb 26]. http://arxiv.org/abs/1802.03426.
Veta, M. et al. Automatic Nuclei Segmentation in H&E Stained Breast Cancer Histopathology Images. PLOS ONE. Public Libr. Sci. 8, e70221 (2013).
Corredor G., et al. A watershed and feature-based approach for automated detection of lymphocytes on lung cancer images. Med Imaging 2018 Digit Pathol [Internet]. SPIE; 2018 [cited 2023 Feb 26]. 213–218. https://www.spiedigitallibrary.org/conference-proceedings-of-spie/10581/105810R/A-watershed-and-feature-based-approach-for-automated-detection-of/10.1117/12.2293147.full.
Macenko, M. et al. A method for normalizing histology slides for quantitative analysis. 2009 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Boston, MA, USA, pp. 1107-1110 (2009). https://doi.org/10.1109/ISBI.2009.5193250.
Haralick, R. M., Shanmugam, K. & Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. SMC-3, 610–621 (1973).
Görür, D. & Edward Rasmussen, C. Dirichlet Process Gaussian Mixture Models: Choice of the Base Distribution. J. Comput. Sci. Technol. 25, 653–664 (2010).
Goshtasby, A. Piecewise linear mapping functions for image registration. Pattern Recognit. 19, 459–466 (1986).
Fisher, L. D. & Lin, D. Y. Time-dependent covariates in the Cox proportional-hazards regression model. Annu. Rev. Public Health 20, 145–157 (1999).
Kuritz, S. J., Landis, J. R. & Koch, G. G. A general overview of Mantel-Haenszel methods: applications and recent developments. Annu. Rev. Public Health 9, 123–160 (1988).
Harrell, F. E., Califf, R. M., Pryor, D. B., Lee, K. L. & Rosati, R. A. Evaluating the yield of medical tests. JAMA 247, 2543–2546 (1982).
Acknowledgements
Research reported in this publication was supported by the National Cancer Institute under award numbers R01CA268287A1, U01CA269181, R01CA26820701A1, R01CA249992-01A1, R01CA202752-01A1, R01CA208236-01A1, R01CA216579-01A1, R01CA220581-01A1, R01CA257612-01A1, 1U01CA239055-01, 1U01CA248226-01, 1U54CA254566-01, National Heart, Lung and Blood Institute 1R01HL15127701A1, R01HL15807101A1, National Institute of Biomedical Imaging and Bioengineering 1R43EB028736-01, National Center for Research Resources under award number 1 C06 RR12463-01, VA Merit Review Award IBX004121A from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service the Office of the Assistant Secretary of Defense for Health Affairs, through the Breast Cancer Research Program (W81XWH-19-1-0668), the Prostate Cancer Research Program (W81XWH-20-1-0851), the Lung Cancer Research Program (W81XWH-18-1-0440, W81XWH-20-1-0595), the Peer Reviewed Cancer Research Program (W81XWH-18-1-0404, W81XWH-21-1-0345, W81XWH-21-1-0160), the Kidney Precision Medicine Project (KPMP) Glue Grant and sponsored research agreements from Bristol Myers-Squibb, Boehringer-Ingelheim, Eli-Lilly and Astrazeneca. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the U.S. Department of Veterans Affairs, the Department of Defense, or the United States Government.
Author information
Authors and Affiliations
Contributions
C.B., A.M. conceived and designed the study. C.B, G.C., C.L., developed the methodology. C.B., A.M. analyzed and interpreted the data. P.V., P.T., V.V., D.R., K.S., S.B., P.Z. provided biological and clinical insights into the project. K.S. provided the Yale University datasets including TMA (tumor microarrays) and QmIF images. S.B. and P.Z. provided the Bern University dataset. V.B., D.B., M.B. provided the CA209-057 dataset and generated complementary results. All authors have reviewed, edited, and approved the paper.
Corresponding author
Ethics declarations
Competing interests
A.M. is an equity holder in Elucid Bioimaging, Picture Health and in Inspirata Inc. He is also a scientific advisory consultant for Aiforia Inc, Picture Health and SimbioSys. In addition, he has served as a scientific advisory board member for Inspirata Inc, Astrazeneca, Bristol-Myers-Squibb and Merck. He also has sponsored research agreements with Philips, Astrazeneca, Bristol-Myers Squibb, Boehringer-Ingelheim and Eli-Lilly. His technology has been licensed to Elucid Bioimaging and Picture Health. He also consults for Castle Biosciences, Biohme Inc. and SimbioSys. He is also involved in a NIH (National Institutes of Health) U24 grant with PathCore Inc, and 3 different R01 grants with Inspirata Inc. Kurt Schalper received research funding from Genoptix/Navigate (Novartis), Tesaro, Moderna Therapeutics, Takeda, Surface Oncology, Pierre-Fabre Research Institute, Merck, Bristol-Myers Squibb, AstraZeneca, and Eli Lilly. In addition, he has received honoraria for consultant/advisory roles from Celgene, Moderna Therapeutics, Shattuck Labs, Pierre-Fabre, AstraZeneca, EMD Serono, Ono Pharmaceuticals, Clinica Alemana de Santiago, Dynamo Therapeutics, PeerView, Abbvie, Fluidigm, Takeda/Millenium Pharmaceuticals, Merck, Bristol-Myers Squibb, Agenus, and Torque Therapeutics. D.L.R. has served as an advisor for AstraZeneca, Agendia, Amgen, BMS, Cell Signaling Technology, Cepheid, Danaher, Daiichi Sankyo, Novartis, GSK, Konica Minolta, Merck, NanoString, PAIGE.AI, Perkin Elmer, Regeneron, Roche, Sanofi, Ventana and Ultivue. Amgen, Cepheid, Konica Minolta, NavigateBP, NextCure, and Lilly have funded research in his lab. V.B., D.B., and M.B. are currently employees of Bristol-Myers Squibb. S.B. received research funding from Roche and Basilea (to institution) and has served as a scientific advisory board member for Eli Lilly. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barrera, C., Corredor, G., Viswanathan, V.S. et al. Deep computational image analysis of immune cell niches reveals treatment-specific outcome associations in lung cancer. npj Precis. Onc. 7, 52 (2023). https://doi.org/10.1038/s41698-023-00403-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-023-00403-x
This article is cited by
-
Spatial insights into immunotherapy response in non-small cell lung cancer (NSCLC) by multiplexed tissue imaging
Journal of Translational Medicine (2024)
-
Semi-automated approaches for interrogating spatial heterogeneity of tissue samples
Scientific Reports (2024)