Abstract
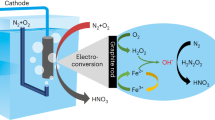
Hydroxylamine is an important nitrogenous feedstock for the chemical industry. Conventional hydroxylamine synthesis methods utilize ammonia as the nitrogen source and require harsh reaction conditions, leading to unfavourable environmental footprint. Here we develop a plasma-electrochemical cascade pathway (PECP) powered by electricity for sustainable hydroxylamine synthesis directly from ambient air and water at mild conditions. In the first step, the plasma treatment of ambient air and water delivers a nitric acid solution with a concentration of up to 120.1 mM. Subsequently, the obtained nitric acid is selectively electroreduced to hydroxylamine using a bismuth-based catalyst. The faradaic efficiency for hydroxylamine reached 81.0% at −1.0 V versus reversible hydrogen electrode. As a result, this PECP method achieves a high hydroxylamine yield rate of 713.1 μmol cm−2 h−1 with a selectivity of 95.8%. Notably, both steps of the PECP method are operated at room temperature. Overall, our work provides a viable approach for efficient hydroxylamine synthesis from simpler feedstock at milder conditions, contributing to the sustainability transformation of the chemical industry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data in this work are available in the text and Supplementary Information. The relevant raw data for each figure or table (in the text and Supplementary Information) are listed in Excel documents and provided as source or supplementary data files. Source data are provided with this paper.
References
Rosca, V., Duca, M., de Groot, M. T. & Koper, M. T. M. Nitrogen cycle electrocatalysis. Chem. Rev. 109, 2209–2244 (2009).
Tabolin, A. A. & Ioffe, S. L. Rearrangement of N-oxyenamines and related reactions. Chem. Rev. 114, 5426–5476 (2014).
Huang, H., Ji, X., Wu, W. & Jiang, H. Transition metal-catalyzed C–H functionalization of N-oxyenamine internal oxidants. Chem. Soc. Rev. 44, 1155–1171 (2015).
Zhao, F. et al. A simple and efficient approach for preparation of hydroxylamine sulfate from the acid-catalyzed hydrolysis reaction of cyclohexanone oxime. Chem. Eng. J. 272, 102–107 (2015).
Manente, F., Pietrobon, L., Ronchin, L. & Vavasori, A. Trifluoroacetic acid hydroxylamine system as organocatalyst reagent in a one-pot salt free process for the synthesis of caprolactam and amides of industrial interest. Catal. Lett. 151, 3543–3549 (2021).
Tursun, M. & Wu, C. Single transition metal atoms anchored on defective MoS2 monolayers for the electrocatalytic reduction of nitric oxide into ammonia and hydroxylamine. Inorg. Chem. 61, 17448–17458 (2022).
Tauszik, G. R. & Crocetta, P. Production of hydroxylamine from nitrogen oxide: a short review. Appl. Catal. 17, 1–21 (1985).
Benson, R. E., Cairns, T. L. & Whitman, G. M. Synthesis of hydroxylamine. J. Am. Chem. Soc. 78, 4202–4205 (1956).
Shelef, M. & Graham, G. W. Why rhodium in automotive three-way catalysts? Catal. Rev. 36, 433–457 (1994).
Murray, R. W. & Singh, M. A high yield one step synthesis of hydroxylamines. Synth. Commun. 19, 3509–3522 (1989).
Semon, W. L. The preparation of hydroxylamine hydrochloride and acetoxime. J. Am. Chem. Soc. 45, 188–190 (1923).
Mantegazza, M. A. & Leofanti, G. A one-step rapid synthesis of TS-1 zeolites with highly catalytically active mononuclear TiO6 species. Stud. Surf. Sci. Catal. 82, 541–550 (1994).
Tsegaw, Y. A. et al. Formation of hydroxylamine in low-temperature interstellar model ices. J. Phys. Chem. A 121, 7477–7493 (2017).
Zecchina, A. et al. Structural characterization of Ti centres in Ti-silicalite and reaction mechanisms in cyclohexanone ammoximation. Catal. Today 32, 97–106 (1996).
Wu, P., Komatsu, T. & Yashimal, T. Ammoximation of ketones over titanium mordenite. J. Catal. 168, 400–411 (1997).
Wu, Y., Jiang, Z., Lin, Z., Liang, Y. & Wang, H. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nat. Sustain. 4, 725–730 (2021).
Liu, X., Jiao, Y., Zheng, Y., Jaroniec, M. & Qiao, S. Z. Mechanism of C–N bonds formation in electrocatalytic urea production revealed by ab initio molecular dynamics simulation. Nat. Commun. 13, 5471 (2022).
Zheng, T. et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering. Nat. Catal. 5, 388–396 (2022).
Chen, G.-F. et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper-molecular solid catalyst. Nat. Energy 5, 605–613 (2020).
Chen, F.-Y. et al. Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst. Nat. Nanotechnol. 17, 759–767 (2022).
Liu, Y. et al. A highly efficient metal-free electrocatalyst of F-doped porous carbon toward N2 electroreduction. Adv. Mater. 32, e1907690 (2020).
Winter, L. R. & Chen, J. G. N2 fixation by plasma-activated processes. Joule 5, 300–315 (2021).
Li, L. et al. Efficient nitrogen fixation to ammonia through integration of plasma oxidation with electrocatalytic reduction. Angew. Chem. Int. Ed. 60, 14131–14137 (2021).
Li, D. et al. Direct conversion of N2 and O2: status, challenge and perspective. Natl Sci. Rev. 9, nwac042 (2022).
Ding, J. et al. Sustainable ammonia synthesis from air by the integration of plasma and electrocatalysis techniques. Inorg. Chem. Front. 10, 5762 (2023).
Kwedi-Nsah, L.-M. & Kobayashi, T. Sonochemical nitrogen fixation for the generation of NO2− and NO3− ions under high-powered ultrasound in aqueous medium. Ultrason. Sonochem. 66, 105051 (2020).
Chen, S. et al. Direct electroconversion of air to nitric acid under mild conditions. Nat. Synth. 3, 76–84 (2024).
Yu, Y. et al. A nitrogen fixation strategy to synthesize NO via the thermally-assisted photocatalytic conversion of air. J. Mater. Chem. A 8, 19623–19630 (2020).
Daems, N., Sheng, X., Alvarez-Gallego, Y., Vankelecom, I. F. J. & Pescarmona, P. P. Iron-containing N-doped carbon electrocatalysts for the cogeneration of hydroxylamine and electricity in a H2-NO fuel cell. Green Chem. 18, 1547–1559 (2016).
Sheng, X. et al. Carbon-supported iron complexes as electrocatalysts for the cogeneration of hydroxylamine and electricity in a NO-H2 fuel cell: a combined electrochemical and density functional theory study. J. Power Sources 390, 249–260 (2018).
Kim, D. H. et al. Selective electrochemical reduction of nitric oxide to hydroxylamine by atomically dispersed iron catalyst. Nat. Commun. 12, 1856 (2021).
Zhang, X. et al. Direct electro-synthesis of valuable C=N compound from NO. Chem. Catal. 2, 1807–1818 (2022).
Wang, Y. et al. Enhanced nitrate-to-ammonia activity on copper–nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc. 142, 5702–5708 (2020).
Song, Z. et al. Efficient electroreduction of nitrate into ammonia at ultralow concentrations via an enrichment effect. Adv. Mater. 34, 2204306 (2022).
Ye, S. et al. Elucidating the activity, mechanism and application of selective electrosynthesis of ammonia from nitrate on cobalt phosphide. Energy Environ. Sci. 15, 760 (2022).
Kong, X. et al. Enhancing CO2 electroreduction selectivity toward multicarbon products via tuning the local H2O/CO2 molar ratio. Nano Lett. 22, 8000–8007 (2022).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 32, 1456 (2011).
Henkelman, G., Uberuaga, B. P. & Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
Z.G. acknowledges the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0450401), the National Natural Science Foundation of China (22322901), the CAS Project for Young Scientists in Basic Research (YSBR-022), and the National Key Research and Development Program of China (2022YFC2106000). J. Zeng acknowledges the National Key Research and Development Program of China (2021YFA1500500), the CAS Project for Young Scientists in Basic Research (YSBR-051), the National Science Fund for Distinguished Young Scholars (21925204), the National Science Fund for Distinguished Young Scholars (21925204), the National Natural Science Foundation of China (22221003, 22250007 and 22361162655), the Fundamental Research Funds for the Central Universities, the Provincial Key Research and Development Program of Anhui (202004a05020074), the Collaborative Innovation Program of Hefei Science Center, CAS (2022HSC-CIP004), and the USTC Research Funds of the Double First-Class Initiative (YD2340002002). X.K. acknowledges the National Natural Science Foundation of China (22209163). This work was partially carried out at the USTC Center for Micro and Nanoscale Research and Fabrication, and the Instruments Center for Physical Science, University of Science and Technology of China.
Author information
Authors and Affiliations
Contributions
J. Zeng, Z.G. and X.K. conceived the idea and co-wrote the paper. X.K. and J.N. synthesized catalysts, conducted structural characterizations and performed electroreduction tests with the help of J. Zheng and Z.X. Z.S. and X.K. designed the plasma discharge device and performed the N2 plasma fixation tests with the help of L.Q. Z.Y. performed the theoretical calculations. H.L. made suggestions on the paper. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Chang Hyuck Choi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–37, Tables 1 and 2, and Notes.
Supplementary Video 1
30 s absorption of NOx in the aqueous absorbent containing 0.02 mM methyl orange
Supplementary Data 1
All source data for supplementary figures.
Source data
Source Data Fig. 2
Experimental source data.
Source Data Fig. 3
Experimental source data.
Source Data Fig. 4
Experimental source data.
Source Data Fig. 5
Experimental source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kong, X., Ni, J., Song, Z. et al. Synthesis of hydroxylamine from air and water via a plasma-electrochemical cascade pathway. Nat Sustain (2024). https://doi.org/10.1038/s41893-024-01330-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41893-024-01330-w