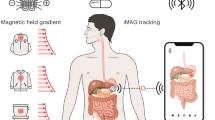
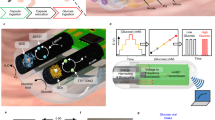
Abstract
The ability to record high-quality electrophysiology data from the gastrointestinal tract and enteric nervous system is of use in understanding a variety of disorders and improving healthcare via early diagnosis. However, such measurements remain challenging because electrodes must be implanted surgically or worn on the skin, which results in a trade-off between signal quality and invasiveness. Here we report an ingestible device for gastric electrophysiology. The non-invasive system, which is termed multimodal electrophysiology via ingestible, gastric, untethered tracking (MiGUT), consists of encapsulated electronics and a sensing electrode ribbon that unrolls in the stomach following delivery to make contact with the mucosa. The device then records and wirelessly transmits biopotential signals to an external receiver. We show that the device can record electrical signals—including the gastric slow wave, respiration signal and heart signal—in a large animal model and can monitor slow wave activity in freely moving and feeding animals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that supports the findings of this study are available at https://github.com/adamgierlach/MiGUT_data_repository.
Code availability
The code that supports the findings of this study is available at https://github.com/adamgierlach/MiGUT_data_repository.
References
Furness, J. B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294 (2012).
Spencer, N. J. & Hu, H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 17, 338–351 (2020).
Camilleri, M. et al. Gastroparesis. Nat. Rev. Dis. Prim. 4, 41 (2018).
Grover, M., Farrugia, G. & Stanghellini, V. Gastroparesis: a turning point in understanding and treatment. Gut 68, 2238–2250 (2019).
Mahadeva, S. Epidemiology of functional dyspepsia: a global perspective. World J. Gastroenterol. 12, 2661 (2006).
Travagli, R. A., Browning, K. N. & Camilleri, M. Parkinson disease and the gut: new insights into pathogenesis and clinical relevance. Nat. Rev. Gastroenterol. Hepatol. 17, 673–685 (2020).
Jones, J. D., Rahmani, E., Garcia, E. & Jacobs, J. P. Gastrointestinal symptoms are predictive of trajectories of cognitive functioning in de novo Parkinson’s disease. Parkinsonism Relat. Disord. 72, 7–12 (2020).
Warnecke, T., Schäfer, K.-H., Claus, I., Del Tredici, K. & Jost, W. H. Gastrointestinal involvement in Parkinson’s disease: pathophysiology, diagnosis, and management. npj Parkinsons Dis. 8, 31 (2022).
Hsiao, E. Y. Gastrointestinal issues in autism spectrum disorder. Harv. Rev. Psychiatry 22, 104–111 (2014).
Saurman, V., Margolis, K. G. & Luna, R. A. Autism spectrum disorder as a brain-gut-microbiome axis disorder. Dig. Dis. Sci. 65, 818–828 (2020).
Farajidavar, A. Bioelectronics for mapping gut activity. Brain Res. 1693, 169–173 (2018).
O’Grady, G. et al. Methods for high-resolution electrical mapping in the gastrointestinal tract. IEEE Rev. Biomed. Eng. 12, 287–302 (2019).
Keller, J. et al. Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat. Rev. Gastroenterol. Hepatol. 15, 291–308 (2018).
Sanders, K. M., Koh, S. D. & Ward, S. M. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu. Rev. Physiol. 68, 307–343 (2006).
Alvarez, W. The electrogastrogram and what it shows. J. Am. Med. Assoc. 78, 1116 (1922).
Lammers, W. J. E. P., Stephen, B., Arafat, K. & Manefield, G. W. High resolution electrical mapping in the gastrointestinal system: initial results. Neurogastroenterol. Motil. 8, 207–216 (1996).
Angeli, T. R. et al. High-resolution electrical mapping of porcine gastric slow-wave propagation from the mucosal surface. Neurogastroenterol. Motil. 29, e13010 (2017).
Paskaranandavadivel, N. et al. Ambulatory gastric mucosal slow wave recording for chronic experimental studies. In 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 755–758 (IEEE, 2017); https://doi.org/10.1109/EMBC.2017.8036934
Paskaranandavadivel, N. et al. Multi-day, multi-sensor ambulatory monitoring of gastric electrical activity. Physiol. Meas. 40, 025011 (2019).
Gharibans, A. A. et al. Artifact rejection methodology enables continuous, noninvasive measurement of gastric myoelectric activity in ambulatory subjects. Sci. Rep. 8, 5019 (2018).
Steiger, C. et al. Ingestible electronics for diagnostics and therapy. Nat. Rev. Mater. 4, 83–98 (2019).
Yang, S.-Y. et al. Powering implantable and ingestible electronics. Adv. Funct. Mater. 31, 2009289 (2021).
Kalantar-Zadeh, K. et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat. Electron. 1, 79–87 (2018).
De la Paz, E. et al. A self-powered ingestible wireless biosensing system for real-time in situ monitoring of gastrointestinal tract metabolites. Nat. Commun. 13, 7405 (2022).
Koziolek, M. et al. Intragastric pH and pressure profiles after intake of the high-caloric, high-fat meal as used for food effect studies. J. Control. Release 220, 71–78 (2015).
Sarker, S., Jones, R., Chow, G. & Terry, B. Design of a soft, self-uncoiling stent for extended retention of drug delivery in the small intestine. In Proc. 2021 Design of Medical Devices Conference V001T12A010 (ASME, 2021); https://doi.org/10.1115/DMD2021-1063
Schostek, S. et al. Pre-clinical study on a telemetric gastric sensor for recognition of acute upper gastrointestinal bleeding: the “HemoPill monitor”. Surg. Endosc. 34, 888–898 (2020).
Hutten, G. J., van Thuijl, H. F., van Bellegem, A. C. M., van Eykern, L. A. & van Aalderen, W. M. C. A literature review of the methodology of EMG recordings of the diaphragm. J. Electromyogr. Kinesiol. 20, 185–190 (2010).
Lokin, J. L., Dulger, S., Glas, G. J. & Horn, J. Transesophageal versus surface electromyography of the diaphragm in ventilated subjects. Respir. Care 65, 1309–1314 (2020).
Sanger, G. J. & Furness, J. B. Ghrelin and motilin receptors as drug targets for gastrointestinal disorders. Nat. Rev. Gastroenterol. Hepatol. 13, 38–48 (2016).
Hopkins, S. Clinical toleration and safety of azithromycin. Am. J. Med. 91, S40–S45 (1991).
Gharibans, A. A. et al. Gastric dysfunction in patients with chronic nausea and vomiting syndromes defined by a noninvasive gastric mapping device. Sci. Transl. Med. 14, eabq3544 (2022).
Ray, T. R. et al. Bio-integrated wearable systems: a comprehensive review. Chem. Rev. 119, 5461–5533 (2019).
Vujic, A. et al. Gut-brain computer interfacing (GBCI): wearable monitoring of gastric myoelectric activity. In 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 5886–5889 (IEEE, 2019); https://doi.org/10.1109/EMBC.2019.8856568
Obioha, C. et al. Effect of body mass index on the sensitivity of magnetogastrogram and electrogastrogram. J. Gastroenterol. Hepatol. Res. 2, 513–519 (2013).
Abramson, A. et al. Ingestible transiently anchoring electronics for microstimulation and conductive signaling. Sci. Adv. 6, eaaz0127 (2020).
Kong, Y. L. et al. 3D‐printed gastric resident electronics. Adv. Mater. Technol. 4, 1800490 (2019).
Abid, A. et al. Wireless power transfer to millimeter-sized gastrointestinal electronics validated in a swine model. Sci. Rep. 7, 46745 (2017).
Javan-Khoshkholgh, A., Sassoon, J. C. & Farajidavar, A. A wireless rechargeable implantable system for monitoring and pacing the gut in small animals. In 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS) 1–4 (IEEE, 2019); https://doi.org/10.1109/BIOCAS.2019.8919125
Yuk, H., Wu, J. & Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 7, 935–952 (2022).
Ramadi, K. B., Srinivasan, S. S. & Traverso, G. Electroceuticals in the gastrointestinal tract. Trends Pharmacol. Sci. 41, 960–976 (2020).
Li, C. et al. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 5, 61–81 (2020).
Abramson, A. et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 363, 611–615 (2019).
Acknowledgements
We are grateful for discussions with R. Langer and the numerous members of the Traverso, Langer and Chandrakasan Laboratories. We are also grateful to G. Liu for suggesting names and acronyms for the MiGUT device. We thank V. E. Fulford, Alar Illustration, for work in Fig. 1. This work was in part supported by the following grants including a grant from Novo Nordisk, Karl van Tassel (1925) Career Development Professorship, the Department of Mechanical Engineering, Massachusetts Institute of Technology (MIT) and the Division of Gastroenterology, Brigham and Women’s Hospital. The research was funded in part by the Advanced Research Projects Agency for Health (ARPA-H) under Award Number D24AC00040-00. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Advanced Research Projects Agency for Health. A.G. is supported by the Natural Science and Engineering Research Council of Canada Postgraduate Scholarship-Doctoral and a Takeda Fellowship, MIT. P.S. was supported by a Rotary Global Grant scholarship. S.-Y.Y. was supported in part by a Mathworks Fellowship, MIT. S.O. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases award 1T32DK135449-01.
Author information
Authors and Affiliations
Contributions
S.S.Y., A.G., P.S., H.-W.H. and G.T. conceived and designed the research. S.S.Y., A.G., G.S., I.M., P.S. and S.-Y.Y. designed, tested and validated electronics. S.S.Y., A.G., P.S., S.-Y.Y., K.I., W.A.M.M., J.J. and A.H. performed animal experiments. S.S.Y., A.G., G.S. and I.M. wrote device firmware. S.S.Y. and A.G. wrote software to analyse data. S.S.Y., A.G., P.S., S.O. and G.T. interpreted results. S.S.Y., A.G., P.S., A.P.C. and G.T. wrote the paper. All authors reviewed and approved the paper.
Corresponding author
Ethics declarations
Competing interests
Financial competing interests for G.T. that may be interpreted as related to the current paper include current and prior funding from Novo Nordisk, Hoffman La Roche, Oracle, Draper Laboratory, MIT Lincoln Laboratory, NIH (NIBIB and NCI), Bill and Melinda Gates Foundation, The Leona M. and Harry B. Helmsley Charitable Trust, Karl van Tassel (1925) Career Development Professor, MIT, the Defense Advanced Research Projects Agency, and the Advanced Research Projects Agency for Health (ARPA-H) as well as employment by the Massachusetts Institute of Technology and Brigham and Women’s Hospital. Personal financial interests include equity/stock (Lyndra Therapeutics, Suono Bio, Vivtex, Celero Systems, Syntis Bio), board of directors member and/or consultant (Lyndra Therapeutics, Novo Nordisk, Suono Bio, Vivtex, Celero Systems, Syntis Bio) and royalties (past and potentially in the future) from licensed and/or optioned intellectual property (Lyndra Therapeutics, Novo Nordisk, Suono Bio, Vivtex, Celero Systems, Syntis Bio, Johns Hopkins, MIT, Mass General Brigham Innovation). Complete details of all relationships for profit and not-for-profit for G.T. can be found in Supplementary Information. The authors S.S.Y., A.G., G.S., S.-Y.Y. and G.T., with R. Langer, report a patent application (US Provisional Patent Application No. 63/589,401) describing the system reported in the paper. The other authors declare no competing interests.
Peer review
Peer review information
Nature Electronics thanks Aydin Farajidavar, Bozhi Tian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–17, Tables 1–4 and a full list of competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
You, S.S., Gierlach, A., Schmidt, P. et al. An ingestible device for gastric electrophysiology. Nat Electron (2024). https://doi.org/10.1038/s41928-024-01160-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41928-024-01160-w