Abstract
The coordination of immunity across organs is fundamental to cancer development and progression. It is well known that the hostile metabolic microenvironment in the tumour is a major obstacle to effective anti-tumour immunity. However, whether metabolic alterations in secondary lymphoid tissues beyond the tumour can affect anti-tumour immunity remains elusive. Using positron-emission tomography–computed tomography, we show that the spleens of humans and mice with breast cancer are metabolically reprogrammed to a glycolytic state. Such an increase in glucose consumption in the spleen primarily occurs in neutrophils generated by extramedullary haematopoiesis and recruitment from the bone marrow. These neutrophils in the white pulp create a glucose-deprived microenvironment, which, in turn, induces T cell anergy by impairing pyruvate kinase M2 and its action on STAT5, thus compromising their anti-tumour activities. Furthermore, CCL9 chemokine produced by splenic stromal cells is central to splenic neutrophil accumulation, and blockade of the CCR1 receptor favours tumour eradication. Thus, neutrophils metabolically influence the spleen microenvironment and control anti-tumour T cell responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data generated and/or analysed in this study are included in the article as supplementary files. Raw data from RNA sequencing are available at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA848433. Single-cell RNA-sequencing data of spleens from MMTV-PyMT mice can be downloaded from the Gene Expression Omnibus with accession number GSE139125. Source data are provided with this paper.
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Philip, M. & Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 22, 209–223 (2022).
Gros, A. et al. Recognition of human gastrointestinal cancer neoantigens by circulating PD-1+ lymphocytes. J. Clin. Invest. 129, 4992–5004 (2019).
Gros, A. et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat. Med. 22, 433–438 (2016).
Kubli, S. P., Berger, T., Araujo, D. V., Siu, L. L. & Mak, T. W. Beyond immune checkpoint blockade: emerging immunological strategies. Nat. Rev. Drug Discov. 20, 899–919 (2021).
Hiam-Galvez, K. J., Allen, B. M. & Spitzer, M. H. Systemic immunity in cancer. Nat. Rev. Cancer 21, 345–359 (2021).
den Haan, J. M., Mebius, R. E. & Kraal, G. Stromal cells of the mouse spleen. Front. Immunol. 3, 201 (2012).
Wu, C., Hua, Q. & Zheng, L. Generation of myeloid cells in cancer: the spleen matters. Front. Immunol. 11, 1126 (2020).
Zhao, L. et al. Late-stage tumors induce anemia and immunosuppressive extramedullary erythroid progenitor cells. Nat. Med. 24, 1536–1544 (2018).
Han, Y. et al. Tumor-induced generation of splenic erythroblast-like Ter-cells promotes tumor progression. Cell 173, 634–648 (2018).
Wu, C. et al. Spleen mediates a distinct hematopoietic progenitor response supporting tumor-promoting myelopoiesis. J. Clin. Invest. 128, 3425–3438 (2018).
Cortez-Retamozo, V. et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl Acad. Sci. USA 109, 2491–2496 (2012).
Rice, C. M. et al. Tumour-elicited neutrophils engage mitochondrial metabolism to circumvent nutrient limitations and maintain immune suppression. Nat. Commun. 9, 5099 (2018).
Lim, A. R., Rathmell, W. K. & Rathmell, J. C. The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy. eLife https://doi.org/10.7554/eLife.55185 (2020).
Schrörs, B. et al. Multi-omics characterization of the 4T1 murine mammary gland tumor model. Front. Oncol. 10, 1195 (2020).
Llaó-Cid, L. et al. EOMES is essential for antitumor activity of CD8+ T cells in chronic lymphocytic leukemia. Leukemia 35, 3152–3162 (2021).
Pearce, E. L. et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302, 1041–1043 (2003).
Li, B. & Yang, L. Creatine in T cell antitumor immunity and cancer immunotherapy. Nutrients https://doi.org/10.3390/nu13051633 (2021).
Di Biase, S. et al. Creatine uptake regulates CD8 T cell antitumor immunity. J. Exp. Med. 216, 2869–2882 (2019).
Zheng, Y., Delgoffe, G. M., Meyer, C. F., Chan, W. & Powell, J. D. Anergic T cells are metabolically anergic. J. Immunol. 183, 6095–6101 (2009).
Ross, S. H. & Cantrell, D. A. Signaling and function of interleukin-2 in T lymphocytes. Annu. Rev. Immunol. 36, 411–433 (2018).
Verdeil, G., Puthier, D., Nguyen, C., Schmitt-Verhulst, A. M. & Auphan-Anezin, N. STAT5-mediated signals sustain a TCR-initiated gene expression program toward differentiation of CD8 T cell effectors. J. Immunol. 176, 4834–4842 (2006).
Hand, T. W. et al. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc. Natl Acad. Sci. USA 107, 16601–16606 (2010).
Evrard, M. et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity 48, 364–379 (2018).
Cowland, J. B. & Borregaard, N. Granulopoiesis and granules of human neutrophils. Immunol. Rev. 273, 11–28 (2016).
Margraf, A., Ley, K. & Zarbock, A. Neutrophil recruitment: from model systems to tissue-specific patterns. Trends Immunol. 40, 613–634 (2019).
Alshetaiwi, H. et al. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aay6017 (2020).
Li, B. et al. CCL9/CCR1 induces myeloid‑derived suppressor cell recruitment to the spleen in a murine H22 orthotopic hepatoma model. Oncol. Rep. 41, 608–618 (2019).
Dvorak, H. F. Tumors: wounds that do not heal—redux. Cancer Immunol. Res. 3, 1–11 (2015).
Powell, D. R. & Huttenlocher, A. Neutrophils in the tumor microenvironment. Trends Immunol. 37, 41–52 (2016).
Liu, L., Liu, Y., Yan, X., Zhou, C. & Xiong, X. The role of granulocyte colony‑stimulating factor in breast cancer development: a review. Mol. Med. Rep. 21, 2019–2029 (2020).
Alshamrani, M. A., Al-Foheidi, M. & Abdulrahim, A. H. Granulocyte colony stimulating factor (G-CSF) induced splenic infarction in breast cancer patient treated with dose-dense chemotherapy regimen. Case Rep. Oncol. Med. 2019, 8174986 (2019).
Leonard, W. J., Lin, J. X. & O’Shea, J. J. The γc family of cytokines: basic biology to therapeutic ramifications. Immunity 50, 832–850 (2019).
Rahim, M. K. et al. Dynamic CD8+ T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell 186, 1127–1143 (2023).
Ren, G. et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell 11, 812–824 (2012).
Yu, P. F. et al. TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils. Oncogene 36, 482–490 (2017).
Acknowledgements
This study was supported by grants from the National Key R&D Program of China (2022YFA0807300, Ying Wang) and the National Natural Science Foundation of China (81930085, Y.S.; 31961133024, Y.S.; 31771641, Ying Wang; 81571612, Y.S.; 32070872, Ying Wang; 82072923, Jian Sun (Fudan University); 32000550, X.W.; and 32000626, L.L.), a start-up fund from Soochow University and a research fund from the Department of Science and Technology of Jiangsu Province (BE2016671, Y.S.).
Author information
Authors and Affiliations
Contributions
Conceptualization: Yu Wang, M.X., Ying Wang and Y.S.; methodology: Yu Wang, M.X., Jian Sun (Shanghai Universal Cloud Medical Imaging Diagnostic Center), X.L., H.S., X.W., B.L., T.Z., X.J., L.L., Q.L., Y.H., Y.L., M.H., F. Zheng, F. Zhang and Jian Sun (Fudan University); investigation: Yu Wang, M.X., X.W., B.L. and T.Z.; visualization: Yu Wang and M.X.; funding acquisition: Ying Wang, Y.S., L.L., Jian Sun (Fudan University) and X.W.; project administration: Ying Wang and Y.S.; writing—original draft: Yu Wang and Ying Wang; writing—review and editing: Yu Wang, M.X., Ying Wang and Y.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Limin Zheng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Metabolic properties of spleen in 4T1 tumor-bearing mice.
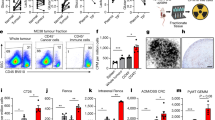
(a) Left, schematic representation of key enzymes in the glycolysis pathway (Created with BioRender.com). Right, protein levels of HK1, HK2, GADPH, PKM1/2, PDH, and LDHα in the spleen of 4T1 tumor-bearing mice and healthy controls (Ctrl); n = 3 mice in healthy control and n = 4 mice in 4T1 tumor-bearing mice. (b) Rate of glucose consumption and lactate acid production in total splenocytes of 4T1 tumor bearing mice and healthy controls. Splenocytes were isolated from 4T1 tumor-bearing mice or healthy controls and cultured in complete RPMI 1640 medium for 3 hours. Glucose and lactic acid were determined, and glucose consumption or lactic production was calculated; n = 3 mice per group, mean ± SD. (c) Immunohistochemistry staining showing the expression of HK2 and LDHα in the spleen of 4T1 tumor-bearing mice. (d, e) Statistic data (d) and representative images (e) show the abundance of mitochondria in lymphocytes and neutrophils detected by transmission electron microscope. White arrows indicate mitochondria; n = 7 individual cells examined by TEM, mean ± SD. Data are representative of at least two independent experiments. All replicates are biological replicates. P values were determined by unpaired two-tailed Student’s t-test [(b), (d)].
Extended Data Fig. 2 Impairment of splenic T cell immunity in 4T1 tumor-bearing mice.
(a) Immunofluorescent staining of Ly6G+ neutrophils in the spleen of 4T1 tumor-bearing mice. (b) Immunohistochemistry staining of Ly6G+ neutrophils in para-tumor and tumor necrotic region. (c) Transmission electron microscopy images of neutrophils in the spleen red pulp of 4T1 tumor-bearing mice. N: neutrophil, L: Lymphocyte. (d) Immunofluorescent staining of Ly6G+ neutrophils in tumor-draining lymph nodes. (e) Spleen weight (n = 3) and number of splenocytes (n = 6) during 4T1 tumor progression; mean ± SD. (f) CD4+, CD8+, and CD19+ lymphocytes number per gram (left) or total spleen (right) during 4T1 tumor progression; n = 6 mice per time point; mean ± SD. (g) Il2 and Il15 expression in the spleen of tumor-bearing mice (n = 6) and healthy control (n = 5); mean ± SD. (h) Ki67 expression in splenic CD4+ and CD8+ T cells; n = 3 mice for control and n = 4 mice at each time point, mean ± SD. (i) Ki67 expression in CD4+ and CD8+ T cells of tumor-draining or control lymph node; n = 4 mice per group, mean ± SD. (j, k) Percentage (j) and number (k) of Ki67+CD4+ and Ki67+CD8+ T cells in peripheral blood of 4T1 tumor-bearing mice with or without splenectomy; n = 4 mice per group, mean ± SD. (l) Purity of Ly6G antibody (clone 1A8) was determined by Coomassie brilliant blue staining. 1A8 hybridoma culture supernatant was subjected to saturated ammonium sulfate cut, dialyzed in 30 kDa dialysis membrane, and purified by affinity chromatography. (M - N) Neutrophil depletion efficiency. Neutrophils in the blood of BALB/c mice intraperitoneally injected with 80 μg purified Ly6G antibody were determined at indicated time points (m). Neutrophils in the blood and spleen of tumor-bearing mice injected with 200 μg Ly6G antibody were detected 24 hours later (n). Data are representative of at least two independent experiments. All replicates are biological replicates. P values were determined by unpaired two-tailed Student’s t-test [(f), (g), (i) – (k)].
Extended Data Fig. 3 Transcriptional analysis of splenic neutrophils.
(a) Neutrophils were isolated from the blood, bone marrow, lung, spleen, and primary tumor of mice bearing 4T1 tumor and analyzed by flow cytometry. The purity of neutrophils was detected before and after cell sorting. Neutrophils are pooled from 6 individual mice. (b) KEGG enrichment analysis of differentially expressed genes in neutrophils from the lungs or tumor versus spleen. (c) Heatmap showing the expression of immunoregulating factors in neutrophils from different tissues. (“N1” neutrophil markers: Fas, CCl3, Nos2; “N2” neutrophil markers: Ccl2, Arg2, Mrc1). (d) Highly purified T cells and neutrophils were isolated from the spleen of 4T1 tumor-bearing mice and subjected to metabolomics analysis. Differential metabolites in neutrophils and T cells were used for enrichment analysis by an online toolkit at https://www.metaboanalyst.ca/. (e) Pan T cells were isolated from naïve mice and co-cultured with splenic neutrophils from 4T1 tumor-bearing mice at a 2:1 ratio, in the presence of titrated glucose concentrations. After anti-CD3/CD28 stimulation for 3 days, CCR7 expression was determined by flow cytometry. (f) Comparison of total ROS production by neutrophils from the bone marrow, spleen, and blood with or without PMA stimulation. Data are representatives of two [(e) - (f)] independent experiments.
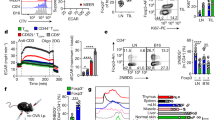
Extended Data Fig. 4 Glucose insufficiency impairs T cell activation through PKM2.
(a) Western blotting analysis of AMPKα and its phosphorylation in T cells cocultured with splenic neutrophils of 4T1 tumor-bearing mice, with high or low concentrations of glucose addition. The co-culture of T cells and neutrophils and T cell activation were performed according to the methods described in Fig. 4a. (b) Schematic representation showing the inhibitors used and their targets in the glucose metabolism pathway. (c) The inhibition of STAT5 phosphorylation by PKM2 inhibitors exhibited a dose dependent pattern. T cells were collected from the spleen and then activated by anti-CD3/CD28 in the presence of IL2 for 3 days. T cells were then washed and incubated for 30 minutes in HBSS supplemented with 1 g/L glucose with the treatment of Shikonin or PKM2-IN-1 at the indicated concentrations. T cells were re-stimulated by crosslinking CD3 and CD28 for 10 minutes and then subjected to lysis for western blotting analysis. (d) Schematic representation showing the difference in mouse Pkm1 and Pkm2 transcripts. ShRNAs were designed by targeting the alternative exon 8 specifically spliced in PKM2. Data are representative of at least two independent experiments [(a), (c)].
Extended Data Fig. 5 Characteristics of neutrophils of mice bearing 4T1 tumor.
(a) Pearson correlation analysis of neutrophil transcriptome among the blood, bone marrow, spleen, primary tumor, and lung of mice bearing 4T1 tumor. (b) Volcano plot showing the differentially expressed genes in neutrophils isolated from bone marrow and spleen. (c) mRNA expressions of Mpo and Elane in neutrophils isolated from bone marrow, spleen, blood, and lung of mice bearing 4T1 tumor; n = 4 mice, mean ± SEM. (d) Transmission electron microscopy showing the morphology and granular formation of splenic neutrophils. Data are representatives of at least two individual microscopic fields. (e) MPO activities in neutrophils from the bone marrow of healthy mice (Ctrl), as well as neutrophils from the bone marrow and spleen of mice bearing 4T1 tumor (4T1); n = 3 mice, mean ± SEM. (f) Gating strategy in identifying circulating c-Kit+Ly6G+ neutrophils. (g) Giemsa staining showing the morphology of sorted c-Kit+Ly6G+ neutrophils (Created with BioRender.com). Data are representative of at least two [(c), (e) - (g)] independent experiments. All replicates are biological replicates. P values were determined by unpaired two-tailed Student’s t-test [(e)].
Extended Data Fig. 6 The role of the CCR1-CCL9 axis in regulating splenic neutrophils during 4T1 tumor progression.
(a) Chemokine receptor expression in neutrophils from RNA-seq data; n = 5, mean ± SD. (b) The expression of CCR1, CXCR2, and CXCR4 in splenic CD45+Ly6G+ and CD45+Ly6G− cells of 4T1 tumor bearing mice. (c) CXCR2 expression on CXCR4lowCCR1high and CXCR4highCCR1low neutrophils. (d) CCR1 expression on Ly6G+c-Kit+ neutrophils of tumor bearing mice. Histogram shows CCR1 expression in splenic Ly6G+ or Ly6G+c-Kit+ neutrophils of mice bearing 4T1 tumor. (e) scRNA-seq data from GSE139125 were downloaded and analyzed following the guideline of Seurat. Featured genes from subpopulation 2 with CCR1 high expression were used for KEGG enrichment analysis. (f) Chemokine expression in the spleen of healthy and 4T1 tumor-bearing mice; n = 4 mice, mean ± SD. (g) Ccl6 expression in the spleen of healthy (n = 3), 4T1-bearing mice (n = 4) or tumor bearing mice with neutrophil depletion (α-Ly6G, n = 4); mean ± SEM. (h) Relative Ccl9 expression in the bone, spleen, and draining lymph nodes (LN) of 4T1 tumor bearing mice and healthy controls; n = 6 mice, mean ± SEM. (i) Transwell assay showing the role of CCL9 in neutrophil chemotaxis; n = 3, mean ± SD. (j) Neutrophil distribution in the spleen of 4T1 tumor-bearing mice with or without CCL9 neutralizing antibody administration (Left) and statistical analysis of the distance of neutrophils to lymphoid nodules in the spleen (Right); n = 37–51 fields from 4 individual mice, mean ± SD. (k) Cleaved CCL9 stimulates HL60 proliferation. Cleaved CCL9 was over-expressed in 293T cells; n = 6, mean ± SD. (l) The expressions of C/EBPα, C/EBPβ, and MYC (m) in HL60 cells treated with cleaved CCL9. Data are representative of at least two independent [(d), (f) - (l)] experiments. All replicates are biological replicates. P values were determined by unpaired two-tailed Student’s t-test [(f) – (k)].
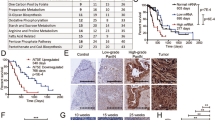
Extended Data Fig. 7 Production of CCL9 by splenic stromal cells.
(a) Changes of splenic CCL9+ cells during 4T1 tumor progression; n = 5 mice for day 0 and 28, n = 6 mice for day 7 and 14, mean ± SEM. (b, c) Immunohistochemistry staining showing the distribution of CCL9+ cells in the spleen of mice bearing 4T1 tumor (b). The distance of CCL9+ cells to a lymphoid nodule was calculated (c); n = 3 field, mean ± SD. (d) Expression of Ccl9 in the spleen, isolated splenic CD45+, and CD45− cells of mice bearing 4T1 tumor; n = 4 mice, mean ± SD. (e) Flow cytometry showing the percentages of CD31−CD45−Ter119−cells in spleen cells of 4T1 tumor-bearing mice and healthy control mice. (f) Percentage of EdU+ cells in CD45−Ter119−CD31− splenic stromal cells of control (n = 3 mice) and 4T1 tumor-bearing mice (n = 4 mice) at indicated time; mean ± SD. (g) Flow cytometry showing the expression of CCL9 in each population of immune cells. Cells were gated for live CD45+Ter119−. (H - I) Clodrosome medicated splenic macrophages depletion efficacy (h), and the mRNA expression of Ccl9 in the spleen (i); n = 2 mice for healthy control, n = 3 mice for LipoControl, n = 4 mice for Clodrosome group, mean ± SD. (j) Identification of splenic stromal cells cultured in vitro. The adhesive cells were cultured and passaged continuously and identified as CD45−CD31−Pdpn+CD44+Sca1+/−CD140a+Nestin+. (k) Flow cytometry showing the expression of CCL9 in isolated splenic stromal cells with or without TNFα (10 ng/ml) stimulation; n = 3 mice, mean ± SD. Data are representative of at least two independent experiments. All replicates are biological replicates. P values were determined by unpaired two-tailed Student’s t-test [(a), (d), (f), (k)].
Supplementary information
Supplementary Information
Supplementary Tables 1–3 including lists of antibodies, reagents, primers and shRNAs.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data and unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data and unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data and unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data and unprocessed western blots.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Xu, M., Sun, J. et al. Glycolytic neutrophils accrued in the spleen compromise anti-tumour T cell immunity in breast cancer. Nat Metab 5, 1408–1422 (2023). https://doi.org/10.1038/s42255-023-00853-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00853-4
This article is cited by
-
Neutrophil diversity is associated with T-cell immunity and clinical relevance in patients with thyroid cancer
Cell Death Discovery (2024)