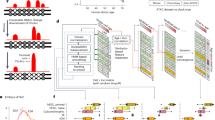
Abstract
Age-related changes in DNA methylation (DNAm) form the basis of the most robust predictors of age—epigenetic clocks—but a clear mechanistic understanding of exactly which aspects of aging are quantified by these clocks is lacking. Here, to clarify the nature of epigenetic aging, we juxtapose the dynamics of tissue and single-cell DNAm in mice. We compare these changes during early development with those observed during adult aging in mice, and corroborate our analyses with a single-cell RNA sequencing analysis within the same multiomics dataset. We show that epigenetic aging involves co-regulated changes as well as a major stochastic component, and this is consistent with transcriptional patterns. We further support the finding of stochastic epigenetic aging by direct tissue and single-cell DNAm analyses and modeling of aging DNAm trajectories with a stochastic process akin to radiocarbon decay. Finally, we describe a single-cell algorithm for the identification of co-regulated and stochastic CpG clusters showing consistent transcriptomic coordination patterns. Together, our analyses increase our understanding of the basis of epigenetic clocks and highlight potential opportunities for targeting aging and evaluating longevity interventions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Code availability
The code used to produce the results and figures in the study is published on GitHub (https://github.com/TarkhovAndrei/scDNAm) and attached as a zip-archive in Supplementary Data 1.
References
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14, R115 (2013).
Hannum, G. et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 49, 359–367 (2013).
Johansson, Å., Enroth, S. & Gyllensten, U. Continuous aging of the human DNA methylome throughout the human lifespan. PLoS ONE 8, e67378 (2013).
Petkovich, D. A. et al. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metab. 25, 954–960.e6 (2017).
Thompson, M. J. et al. A multi-tissue full lifespan epigenetic clock for mice. Aging 10, 2832–2854 (2018).
Meer, M. V., Podolskiy, D. I., Tyshkovskiy, A. & Gladyshev, V. N. A whole lifespan mouse multi-tissue DNA methylation clock. eLife 7, e40675 (2018).
Levine, M. E. et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10, 573–591 (2018).
Lu, A. T. et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 11, 303–327 (2019).
Belsky, D. W. et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife 11, e73420 (2022).
Vershinina, O., Bacalini, M. G., Zaikin, A., Franceschi, C. & Ivanchenko, M. Disentangling age-dependent DNA methylation: deterministic, stochastic, and nonlinear. Sci. Rep. 11, 9201 (2021).
Seale, K., Horvath, S., Teschendorff, A., Eynon, N. & Voisin, S. Making sense of the ageing methylome. Nat. Rev. Genet. 23, 585–605 (2022).
Tarkhov, A. E., Denisov, K. A. & Fedichev, P. O. Aging clocks, entropy, and the limits of age-reversal. Preprint at bioRxiv https://doi.org/10.1101/2022.02.06.479300 (2022).
Levine, M. E., Higgins-Chen, A., Thrush, K., Minteer, C. & Niimi, P. Clock work: deconstructing the epigenetic clock signals in aging, disease, and reprogramming. Preprint at http://biorxiv.org/lookup/doi/10.1101/2022.02.13.480245 (2022).
Bell, C. G. et al. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 20, 249 (2019).
Jaffe, A. E. & Irizarry, R. A. Accounting for cellular heterogeneity is critical in epigenome-wide association studies. Genome Biol. 15, R31 (2014).
Marioni, R. E. et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 16, 25 (2015).
Tomusiak, A. et al. Development of a novel epigenetic clock resistant to changes in immune cell composition. Preprint at http://biorxiv.org/lookup/doi/10.1101/2023.03.01.530561 (2023).
Kim, J. Y., Tavaré, S. & Shibata, D. Counting human somatic cell replications: methylation mirrors endometrial stem cell divisions. Proc. Natl Acad. Sci. USA 102, 17739–17744 (2005).
Yang, Z. et al. Correlation of an epigenetic mitotic clock with cancer risk. Genome Biol. 17, 205 (2016).
Zhou, W. et al. DNA methylation loss in late-replicating domains is linked to mitotic cell division. Nat. Genet. 50, 591–602 (2018).
Teschendorff, A. E. A comparison of epigenetic mitotic-like clocks for cancer risk prediction. Genome Med. 12, 56 (2020).
Minteer, C. J. et al. More than bad luck: cancer and aging are linked to replication-driven changes to the epigenome. Sci. Adv. 9, eadf4163 (2023).
Trapp, A., Kerepesi, C. & Gladyshev, V. N. Profiling epigenetic age in single cells. Nat. Aging 1, 1189–1201 (2021).
Slieker, R. C. et al. Age-related accrual of methylomic variability is linked to fundamental ageing mechanisms. Genome Biol. 17, 191 (2016).
Teschendorff, A. E. et al. Age-dependent DNA methylation of genes that are suppressed in stem cells is a hallmark of cancer. Genome Res. 20, 440–446 (2010).
Rakyan, V. K. et al. Human aging-associated DNA hypermethylation occurs preferentially at bivalent chromatin domains. Genome Res. 20, 434–439 (2010).
Feil, R. & Fraga, M. F. Epigenetics and the environment: emerging patterns and implications. Nat. Rev. Genet. 13, 97–109 (2012).
Nejman, D. et al. Molecular rules governing de novo methylation in cancer. Cancer Res. 74, 1475–1483 (2014).
Levy, O. et al. Age-related loss of gene-to-gene transcriptional coordination among single cells. Nat. Metab. 2, 1305–1315 (2020).
Amit, G., Vaknin Ben Porath, D., Levy, O., Hamdi, O. & Bashan, A. Global coordination level in single-cell transcriptomic data. Sci. Rep. 12, 7547 (2022).
Horvath, S. & Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 19, 371–384 (2018).
Hayflick, L. Entropy explains aging, genetic determinism explains longevity, and undefined terminology explains misunderstanding both. PLoS Genet. 3, e220 (2007).
Lipsitz, L. A. Loss of ‘complexity’ and aging: potential applications of fractals and chaos theory to senescence. JAMA 267, 1806 (1992).
Gladyshev, V. N. et al. Molecular damage in aging. Nat. Aging 1, 1096–1106 (2021).
Yanai, S. & Endo, S. Functional aging in male C57BL/6J mice across the life-span: a systematic behavioral analysis of motor, emotional, and memory function to define an aging phenotype. Front. Aging Neurosci. 13, 697621 (2021).
Petr, M. A. et al. A cross-sectional study of functional and metabolic changes during aging through the lifespan in male mice. eLife 10, e62952 (2021).
Wang, S., Lai, X., Deng, Y. & Song, Y. Correlation between mouse age and human age in anti-tumor research: significance and method establishment. Life Sci. 242, 117242 (2020).
Thomas, J. et al. Running the full human developmental clock in interspecies chimeras using alternative human stem cells with expanded embryonic potential. NPJ Regen. Med. 6, 25 (2021).
Pyrkov, T. V. et al. Longitudinal analysis of blood markers reveals progressive loss of resilience and predicts human lifespan limit. Nat. Commun. 12, 2765 (2021).
Kerepesi, C., Zhang, B., Lee, S.-G., Trapp, A. & Gladyshev, V. N. Epigenetic clocks reveal a rejuvenation event during embryogenesis followed by aging. Sci. Adv. 7, eabg6082 (2021).
Sziráki, A., Tyshkovskiy, A. & Gladyshev, V. N. Global remodeling of the mouse DNA methylome during aging and in response to calorie restriction. Aging Cell 17, e12738 (2018).
Ying, K. et al. Causality-enriched epigenetic age uncouples damage and adaptation. Nat. Aging 4, 231–246 (2024).
Clemens, Z. et al. The biphasic and age-dependent impact of klotho on hallmarks of aging and skeletal muscle function. eLife 10, e61138 (2021).
Menichetti, G., Bianconi, G., Castellani, G., Giampieri, E. & Remondini, D. Multiscale characterization of ageing and cancer progression by a novel network entropy measure. Mol. Biosyst. 11, 1824–1831 (2015).
Sivakumar, S., LeFebre, R. W., Menichetti, G., Mugler, A. & Ambrosio, F. The fidelity of genetic information transfer with aging segregates according to biological processes. Preprint at http://biorxiv.org/lookup/doi/10.1101/2022.07.18.500243 (2022).
Gladyshev, V. N. Aging: progressive decline in fitness due to the rising deleteriome adjusted by genetic, environmental, and stochastic processes. Aging Cell 15, 594–602 (2016).
Argelaguet, R. et al. Multi-omics profiling of mouse gastrulation at single-cell resolution. Nature 576, 487–491 (2019).
Hernando-Herraez, I. et al. Ageing affects DNA methylation drift and transcriptional cell-to-cell variability in mouse muscle stem cells. Nat. Commun. 10, 4361 (2019).
Angermueller, C. et al. Parallel single-cell sequencing links transcriptional and epigenetic heterogeneity. Nat. Methods 13, 229–232 (2016).
Cooper, G. M. et al. Distribution and intensity of constraint in mammalian genomic sequence. Genome Res. 15, 901–913 (2005).
Siepel, A., Pollard, K. S. & Haussler, D. in Research in Computational Molecular Biology Vol. 3909 (eds Apostolico, A. et al.) 190–205 (Springer, 2006).
Yang, Z. Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: approximate methods. J. Mol. Evol. 39, 306–314 (1994).
Thomas, J. W. et al. Comparative analyses of multi-species sequences from targeted genomic regions. Nature 424, 788–793 (2003).
Siepel, A. & Haussler, D. Computational identification of evolutionarily conserved exons. In Proc. 8th Annual International Conference on Computational Molecular Biology—RECOMB ’04 (eds Gramada, A. & Bourne, P. E.) 177–186 (ACM, 2004); https://doi.org/10.1145/974614.974638
Siepel, A. et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 15, 1034–1050 (2005).
Felsenstein, J. & Churchill, G. A. A hidden Markov model approach to variation among sites in rate of evolution. Mol. Biol. Evol. 13, 93–104 (1996).
Lonsdale, J. et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Battram, T. et al. The EWAS Catalog: a database of epigenome-wide association studies. Wellcome Open Res. 7, 41 (2022).
Liao, Y., Wang, J., Jaehnig, E. J., Shi, Z. & Zhang, B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 47, W199–W205 (2019).
Cavalcante, R. G. & Sartor, M. A. annotatr: genomic regions in context. Bioinformatics 33, 2381–2383 (2017).
Fang, Y. et al. DNA methylation entropy is associated with DNA sequence features and developmental epigenetic divergence. Nucleic Acids Res. 51, 2046–2065 (2023).
Luo, C., Hajkova, P. & Ecker, J. R. Dynamic DNA methylation: in the right place at the right time. Science 361, 1336–1340 (2018).
Zhang, B. et al. Multi-omic rejuvenation and lifespan extension on exposure to youthful circulation. Nat. Aging 3, 948–964 (2023).
Olova, N., Simpson, D. J., Marioni, R. E. & Chandra, T. Partial reprogramming induces a steady decline in epigenetic age before loss of somatic identity. Aging Cell 18, e12877 (2019).
Gill, D. et al. Multi-omic rejuvenation of human cells by maturation phase transient reprogramming. eLife 11, e71624 (2022).
Lu, A. T. et al. Universal DNA methylation age across mammalian tissues. Nat. Aging 3, 1144–1166 (2023).
Zhang, B. et al. Epigenetic profiling and incidence of disrupted development point to gastrulation as aging ground zero in Xenopus laevis. Preprint at http://biorxiv.org/lookup/doi/10.1101/2022.08.02.502559 (2022).
Minteer, C. et al. Tick tock, tick tock: mouse culture and tissue aging captured by an epigenetic clock. Aging Cell 21, e13553 (2022).
Kabacik, S. et al. The relationship between epigenetic age and the hallmarks of ageing in human cells. Nat. Aging 2, 484–493 (2022).
Kerepesi, C. et al. Epigenetic aging of the demographically non-aging naked mole-rat. Nat. Commun. 13, 355 (2022).
Horvath, S. et al. DNA methylation clocks tick in naked mole rats but queens age more slowly than nonbreeders. Nat. Aging 2, 46–59 (2022).
Battich, N., Stoeger, T. & Pelkmans, L. Control of transcript variability in single mammalian cells. Cell 163, 1596–1610 (2015).
Raj, A. & Van Oudenaarden, A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135, 216–226 (2008).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Teschendorff, A. E., Breeze, C. E., Zheng, S. C. & Beck, S. A comparison of reference-based algorithms for correcting cell-type heterogeneity in epigenome-wide association studies. BMC Bioinformatics 18, 105 (2017).
Zheng, S. C. et al. A novel cell-type deconvolution algorithm reveals substantial contamination by immune cells in saliva, buccal and cervix. Epigenomics 10, 925–940 (2018).
Zheng, S. C. et al. EpiDISH web server: epigenetic dissection of intra-sample-heterogeneity with online GUI. Bioinformatics 36, 1950–1951 (2020).
Kent, W. J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Menees, K. B. et al. Sex- and age‐dependent alterations of splenic immune cell profile and NK cell phenotypes and function in C57BL/6J mice. Immun. Ageing 18, 3 (2021).
Sloan, C. A. et al. ENCODE data at the ENCODE portal. Nucleic Acids Res. 44, D726–D732 (2016).
Acknowledgements
We thank D. Santesmasses, J. Bang, W. Mitchell, A. Shindyapina and A. Trapp for discussion, and V. Ternovykh for his help with figure enhancements. The work was supported by National Institute on Aging grants, Impetus grant program, Hevolution and James Fickel and Michael Antonov Foundations (granted to V.N.G.).
Author information
Authors and Affiliations
Contributions
A.E.T. developed the concept of the work, designed and implemented the algorithm for identification of co-regulated and stochastic clusters in scDNAm data, and carried out most of the analyses reported in the paper. A.E.T. and T.L.-V. contributed to the simulations and numerical tests of the stochastic model. A.E.T., S.Z., K.Y., M.M., B.Z., A.T. and O.L. contributed to the computational analyses and biological annotation of the results. O.L. analyzed scRNA-seq data and contributed to the interpretation of the work. V.N.G. supervised the work and provided funding. The paper was written by A.E.T. and V.N.G. with input from other authors. All authors discussed the results and reviewed the paper.
Corresponding authors
Ethics declarations
Competing interests
Following the submission of this paper, A.E.T. underwent a change in employment status (Retro Biosciences). The major work on the paper was completed before this employment change. During the peer review process, A.E.T. was employed by and owned stocks of Retro Biosciences. The other authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Andrew Teschendorff and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Role of coverage depth on DNAm changes during aging.
The range of DNAm changes at CpGs sites in the dataset. Histograms are shown for all CpG sites and for the CpGs significantly changing with age. a. For CpG sites covered at the 30X coverage depth in no more than 50 mice out of 255 mice. b. For CpG sites covered at the 30X coverage depth in more than 200 mice out of 255 mice. c. Cell-type decomposition (EpiDISH) of tissue DNAm based on the CpG sites significantly changing with age after the Bonferroni correction for multiple testing (the Pearson correlation). Neutrophils (r = −0.6, p = 3.6·10−15), monocytes (r = −0.58, p = 4.9·10−14) and eosinophils (r = −0.6, p = 3.4·10−15) were inferred to be decreasing significantly with age. All values were normalized to the maximum for a corresponding cell type. d. Same as c for B (r = −0.09, p = 0.29), CD4T (r = 0.07, p = 0.4), CD8T (r = −0.05, p = 0.56) and NK (r = −0.28, p = 7.5·10−4) cells. Only NK cells changed significantly with age.
Extended Data Fig. 2 Biological annotation of co-regulated and stochastic clusters.
a. Number of co-regulated and stochastic sites as a function of the correlation threshold for 502 CpGs passing the coverage filter (15 cells of young mice, and 15 cells of old mice measured simultaneously). b. Number of co-regulated and stochastic sites as a function of the correlation threshold for 51,895 CpGs passing the coverage filter (5 cells of young mice, and 5 cells of old mice measured simultaneously). c. Evolutionary conservation score (phastCons) for co-regulated, stochastic and random sites as a function of the correlation threshold for 51,895 CpGs passing the coverage filter (5 cells of young mice, and 5 cells of old mice measured simultaneously). Data are presented as mean values ± SEM. d. phastCons evolutionary conservation score distributions for CpGs comprising stochastic (49,043 CpGs) and co-regulated (2,625 CpGs) clusters compared to random regions (51,895 CpGs) of the genome (left panel). Co-regulated clusters show significantly higher evolutionary conservation than the random regions, whereas stochastic clusters are significantly less conserved than both the random regions and co-regulated clusters. phastCons evolutionary conservation score distributions for CpGs comprising hypermethylated (24,514 CpGs) and hypomethylated (27,381 CpGs) clusters compared to random regions (51,895 CpGs) of the genome (right panel). Hypermethylated clusters show significantly lower evolutionary conservation scores than both the random regions and hypomethylated regions. The central box of the boxplot shows the IQR, the whiskers extend from the box to the furthest data point within 1.5 times the IQR. Two-sided Mann-Whitney-Wilcoxon test p-values: ns: \(0.05\, < \,p\,\le \,1\), *: \(0.01\, < \,p\,\le \,0.05\), **: \({10}^{-3}\, < \,p\,\le \,0.01\), ***: \({10}^{-4}\, < \,p\,\le \,{10}^{-3}\), ****: \(0.05\, < \,p\,\le \,{10}^{-4}\). e. Enrichment with transcription-factor (TF) binding sites and CpG islands for co-regulated and stochastic clusters, hypermethylated and hypomethylated regions vs. random regions of the genome. The level of statistical significance was chosen at \(0.05\) after the Bonferroni correction for multiple comparisons.
Extended Data Fig. 3 Co-regulation routine details for analyzing embryonic development and enrichment for alternative splicing events.
a. Number of CpGs in co-regulated and stochastic clusters for embryonic data as a function of the correlation threshold. b. Histogram of Pearson correlation coefficients for pairs of CpGs for embryonic development. The typical correlation coefficient is low, thus implying a high level of sequencing noise in the data. c. Enrichment analysis of age-associated alternative splicing events (Alt Spl) for the CpGs comprising co-regulated, stochastic, hypermethylated and hypomethylated clusters (left), and for the alternative splicing events within a 5 kb distance of the CpGs clusters in the genome (right). Co-regulated clusters showed a lower percentage of alternative splicing events than stochastic clusters, though the difference was not statistically significant (Fisher’s exact test). The correlation threshold is 0.4. d. Same as c for the correlation threshold 0.5.
Extended Data Fig. 4 Enrichment of co-regulated and stochastic clusters against EWAS hits.
Each horizontal bar represents an enriched term. The X-axis shows the -log10(P-value), signed by log2 (Odds ratio). Only the EWAS trait with significant enrichment (P < 0.05) are included and annotated. The results presented for correlation threshold: a. 0.4. b. 0.5. The statistical test details are described in the database methods58.
Extended Data Fig. 5 Tests of the algorithm for identification of co-regulated CpGs on simulated data.
Simulation assumes 40 old and 40 young cells, each having 200 CpGs forming 4 blocks of 50 CpGs. The first half of CpGs are stochastic, the second half is co-regulated. In each group of 100 CpGs, 50 CpGs originally have the DNAm level of 30%, and reaching 70% in older cells, another 50 CpGs start at 70% and decrease methylation to 30%. The percentage of missed CpGs and the percentage of errors in identification DNAm level are varied, as well as the correlation threshold. a. Accuracy and b. F1 score of identification of co-regulated sites as a function of correlation threshold, assuming 10% of errors in DNAm levels, and missing values varying from 0% to 90% (in legend). c. Simulated data for 0% missed values, and errors varied from 20%, 30% and 40% respectively, along with the predicted co-regulated/stochastic CpGs (red for stochastic, green for co-regulated). d. Same as c for 0% of errors, and 40%, 60% and 80% of missed values. e. Same as c for 20% of errors, and 40%, 60% and 80% of missed values.
Extended Data Fig. 6 Dynamics of CpG sites significantly associated with age in scDNAm data.
The stochastic CpG clusters are shown by green boxes, the co-regulated CpG clusters—by red boxes. Penalized regression models are biased towards stochastic CpG sites, since they penalize any kind of correlation across the CpG sites used in the model (Lasso and ElasticNet clocks). Color represents the value of the regression weights. Co-regulated clusters contain fewer non-zero weights in the regression models. Therefore, the penalized regression models lower the fraction of co-regulated sites used for building DNAm aging clocks.
Extended Data Fig. 7 Global coordination levels for the antisense DNA strand.
a. Global coordination level of gene expression for genes associated with the co-regulated and stochastic clusters of CpGs in young mice (\(2\) months old: Y4, Y5, Y7 and Y8) and old mice (\(24\) months old: O1 and O5). Blue distributions (‘Real’ in legend) represent true distributions of the GCL for the given gene sets, whereas ‘Surrogate’ distributions are for surrogate gene sets that were randomly selected from a subset of genes with similar expression levels as the gene-set genes. Each surrogate gene set preserved the size of the original gene set and mimicked its expression profile, but did not represent any known KEGG pathway. b. For normalization of results presented in a, we use Z-scores of the GCL relative to the corresponding surrogate gene sets. Co-regulated genes show a significantly higher level of transcriptomic coordination than the stochastic ones for all mice. The results in a and b are shown for the antisense DNA strand. In boxplots of a and b, the central box of the boxplot shows the IQR, the whiskers extend from the box to the furthest data point within 1.5 times the IQR. Two-sided Mann-Whitney-Wilcoxon test p-values: ns: \(0.05\, < \,p\,\le \,1\), *: \(0.01\, < \,p\,\le \,0.05\), **: \({10}^{-3}\, < \,p\,\le \,0.01\), ***: \({10}^{-4}\, < \,p\,\le \,{10}^{-3}\), ****: \(0.05\, < \,p\,\le \,{10}^{-4}\). For each group we used 20 randomly sampled surrogate sets.
Extended Data Fig. 8 Heatmap of scDNAm (left) and scRNAseq (right) for co-regulated CpG clusters and the corresponding gene lists for young and old cells.
Side-by-side comparison of the dynamics of the co-regulated CpG clusters (left panel) with the dynamics of gene expression for the genes lying in close proximity to these CpGs (right panel) in 2-month-old and 24-month-old mice. Gene names and their extent are shown in the bottom panel.
Extended Data Fig. 9 Heatmap of scDNAm (left) and scRNAseq (right) for stochastic CpG clusters and the corresponding gene lists for young and old cells.
Side-by-side comparison of the dynamics of the stochastic CpG clusters (left panel) with the dynamics of gene expression for the genes lying in close proximity to these CpGs (right panel) in 2-month-old and 24-month-old mice. Gene names and their extent are shown in the bottom panel.
Extended Data Fig. 10 Heatmap of scDNAm (left) and scRNAseq (right) for random CpG clusters and corresponding gene lists for young and old cells.
Side-by-side comparison of the dynamics of the random CpG clusters (left panel) with the dynamics of gene expression for the genes lying in close proximity to these CpGs (right panel) in 2-month-old and 24-month-old mice. Gene names and their extent are shown in the bottom panel.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4.
Supplementary Code 1
Source code used to conduct analyses in the paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tarkhov, A.E., Lindstrom-Vautrin, T., Zhang, S. et al. Nature of epigenetic aging from a single-cell perspective. Nat Aging (2024). https://doi.org/10.1038/s43587-024-00616-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43587-024-00616-0
This article is cited by
-
Quantifying stochasticity in the aging DNA methylome
Nature Aging (2024)