Abstract
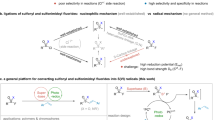
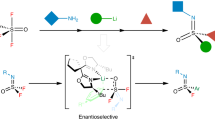
Sulfur(VI) fluoride exchange reactions have been applied to the linkage of a diverse range of molecules. However, the connectivity of fluorosulfuryls with alkynes remains a formidable challenge owing to the high reactivity of π systems. Here, we report a divergent sulfur(VI) fluoride exchange reaction between sulfonimidoyl fluorides and alkynes to afford vinylic and acetylenic sulfoximines. Experimental and computational mechanistic studies elucidated key BF3-bridged six-membered transition states that enabled the synchronous activation of silicon-capped alkynes and sulfonimidoyl fluorides via the interactions of F···Si and B···F. Mechanistic studies also revealed that N-benzyl sulfonimidoyls undergo a 1,5-hydrogen migration from the benzylic position to the acetylenic position, which generates the observed vinylic sulfoximines. A range of synthetic transformations, which include azide–alkyne cycloadditions were demonstrated on the vinylic and acetylenic sulfoximine products.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The X-ray crystallographic coordinates for the structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2094839 (4y) and CCDC 2094841 (5f). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. All other data supporting the findings of this study are available within the paper and Supplementary Information.
References
Dong, J., Krasnova, L., Finn, M. G. & Sharpless, K. B. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem. Int. Ed. 53, 9430–9448 (2014).
Fattah, T. A., Saeed, A. & Albericio, F. Recent advances towards sulfur(VI) fluoride exchange (SuFEx) click chemistry. J. Fluorine Chem. 213, 87–112 (2018).
Revathi, L., Ravindar, L., Leng, J., Rakesh, K. P. & Qin, H.-L. Synthesis and chemical transformations of fluorosulfates. Asian J. Org. Chem. 7, 662–682 (2018).
Ball, N. D. in Emerging Fluorinated Motifs: Synthesis, Properties, and Applications Vol. 2 (eds Ma, J.-A. & Cahard, D.) 621–674 (Wiley-VCH, 2020).
Lee, C. et al. The emerging applications of sulfur(VI) fluorides in catalysis. ACS Catal. 11, 6578–6589 (2021).
Barrow, A. S. et al. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 48, 4731–4758 (2019).
Guo, T. et al. A new portal to SuFEx click chemistry: a stable fluorosulfuryl imidazolium salt emerging as an ‘F–SO2+’ donor of unprecedented reactivity, selectivity, and scope. Angew. Chem. Int. Ed. 57, 2605–2610 (2018).
Smedley, C. J. et al. Bifluoride ion mediated SuFEx trifluoromethylation of sulfonyl fluorides and iminosulfur oxydifluorides. Angew. Chem. Int. Ed. 58, 4552–4556 (2019).
Smedley, C. J. et al. Diversity oriented clicking (DOC): divergent synthesis of SuFExable pharmacophores from 2-substituted-alkynyl-1-sulfonyl fluoride (SASF) hubs. Angew. Chem. Int. Ed. 59, 12460–12469 (2020).
Zheng, Q. et al. Sulfur [18F]fluoride exchange click chemistry enabled ultrafast late-stage radiosynthesis. J. Am. Chem. Soc. 143, 3753–3763 (2021).
Kende, A. S. & Mendoza, J. S. A one-pot synthesis of α-ester sulfones. J. Org. Chem. 55, 1125–1126 (1990).
Chen, W. T. et al. Synthesis of sulfotyrosine-containing peptides by incorporating fluorosulfated tyrosine using an Fmoc-based solid-phase strategy. Angew. Chem. Int. Ed. 55, 1835–1838 (2016).
Li, S. H., Wu, P., Moses, J. E. & Sharpless, K. B. Multidimensional SuFEx click chemistry: sequential sulfur(VI) fluoride exchange connections of diverse modules launched from an SOF4 hub. Angew. Chem. Int. Ed. 56, 2903–2908 (2017).
Gao, B., Li, S., Wu, P., Moses, J. E. & Sharpless, K. B. SuFEx chemistry of thionyl tetrafluoride (SOF4) with organolithium nucleophiles: synthesis of sulfonimidoyl fluorides, sulfoximines, sulfonimidamides, and sulfonimidates. Angew. Chem. Int. Ed. 57, 1939–1943 (2018).
Greed, S. et al. Synthesis of highly enantioenriched sulfonimidoyl fluorides and sulfonimidamides by stereospecific sulfur–fluorine exchange(SuFEx) reaction. Chem. Eur. J. 26, 12533–12538 (2020).
Liang, D.-D. et al. Silicon-free SuFEx reactions of sulfonimidoyl fluorides: scope, enantioselectivity, and mechanism. Angew. Chem. Int. Ed. 59, 7494–7500 (2020).
Liu, C. et al. A general approach to O-sulfation by a sulfur(VI) fluoride exchange reaction. Angew. Chem. Int. Ed. 59, 18435–18441 (2020).
Wei, M. et al. A broad-spectrum catalytic amidation of sulfonyl fluorides and fluorosulfates. Angew. Chem. Int. Ed. 60, 7397–7404 (2021).
Narayanan, A. & Jones, L. H. Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. 6, 2650–2659 (2015).
Jones, L. H. Emerging utility of fluorosulfate chemical probes. ACS Med. Chem. Lett. 9, 584–586 (2018).
Chen, W. et al. Arylfluorosulfates inactivate intracellular lipid binding protein(s) through chemoselective SuFEx reaction with a binding site Tyr residue. J. Am. Chem. Soc. 138, 7353–7364 (2016).
Warshel, A. & Bora, R. P. Perspective: defining and quantifying the role of dynamics in enzyme catalysis. J. Chem. Phys. 144, 180901–180904 (2016).
Liu, F. et al. Biocompatible SuFEx click chemistry: thionyl tetrafluoride (SOF4)-derived connective hubs for bioconjugation to DNA and proteins. Angew. Chem. Int. Ed. 58, 8029–8033 (2019).
Fadeyi, O. O. et al. Covalent enzyme inhibition through fluorosulfate modification of a noncatalytic serine residue. ACS Chem. Biol. 12, 2015–2020 (2017).
Mortenson, D. E. et al. ‘Inverse drug discovery’ strategy to identify proteins that are targeted by latent electrophiles as exemplified by aryl fluorosulfates. J. Am. Chem. Soc. 140, 200–210 (2018).
Liu, Z. et al. SuFEx click chemistry enabled late-stage drug functionalization. J. Am. Chem. Soc. 140, 2919–2925 (2018).
Zhenga, Q. et al. SuFEx-enabled, agnostic discovery of covalent inhibitors of human neutrophil elastase. Proc. Natl Acad. Sci. USA 116, 18808–18814 (2019).
Brighty, G. J. et al. Using sulfuramidimidoyl fluorides that undergo sulfur(VI) fluoride exchange for inverse drug discovery. Nat. Chem. 12, 906–913 (2020).
Kitamura, S. et al. Sulfur(VI) fluoride exchange (SuFEx)-enabled high-throughput medicinal chemistry. J. Am. Chem. Soc. 142, 10899–10904 (2020).
Meng, G. et al. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature 574, 86–89 (2019).
Dong, J., Sharpless, K. B., Kwisnek, L., Oakdale, J. S. & Fokin, V. V. SuFEx-based synthesis of polysulfates. Angew. Chem. Int. Ed. 53, 9466–9470 (2014).
Yatvin, J., Brooks, K. & Locklin, J. SuFEx on the surface: a flexible platform for postpolymerization modification of polymer brushes. Angew. Chem. Int. Ed. 54, 13370–13373 (2015).
Oakdale, J. S., Kwisnek, L. & Fokin, V. V. Selective and orthogonal post-polymerization modification using sulfur(VI) fluoride exchange (SuFEx) and copper-catalyzed azide–alkyne cycloaddition (CuAAC) reactions. Macromolecules 49, 4473–4479 (2016).
Gao, B. et al. Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates. Nat. Chem. 9, 1083–1088 (2017).
Wang, H. et al. SuFEx-based polysulfonate formation from ethenesulfonyl fluoride–amine adducts. Angew. Chem. Int. Ed. 56, 11203–11208 (2017).
Yang, C., Flynn, J. P. & Niu, J. Facile synthesis of sequence-regulated synthetic polymers using orthogonal SuFEx and CuAAC click reactions. Angew. Chem. Int. Ed. 57, 16194–16199 (2018).
Kolomeitsev, A. A., Movchun, V. N., Kondratenko, N. V. & Yagupolski, Y. L. A convenient route to aryl trifluoromethyl sulfones by fluoride-catalyzed cross-coupling of arenesulfonyl fluorides with (trifluoromethyl)trimethylsilane and (trifluoromethyl)trimethylstannane. Synthesis 12, 1151–1152 (1990).
Tang, S. et al. Metallomicelle catalyzed aerobic tandem desilylation/Glaser reaction in water. Green Chem. 21, 2899–2904 (2019).
Nishimura, T., Takiguchi, Y. & Hayashi, T. Effect of chiral diene ligands in rhodium-catalyzed asymmetric addition of arylboronic acids to α,β-unsaturated sulfonyl compounds. J. Am. Chem. Soc. 134, 9086–9089 (2012).
Mukherjee, P. et al. Sulfonamide synthesis via calcium triflimide activation of sulfonyl fluorides. Org. Lett. 20, 3943–3947 (2018).
Mahapatra, S. et al. SuFEx activation with Ca(NTf2)2: a unified strategy to access sulfamides, sulfamates, and sulfonamides from S(VI) fluorides. Org. Lett. 22, 4389–4394 (2020).
Wada, M., Sakurai, Y. & Akiba, K. Addition of alkynyl anions to aldimines containing α-hydrogens: a novel synthesis of β-aminoacetylenes. Tetrahedron Lett. 25, 1083–1084 (1984).
Wilkins, L. C. et al. Contrasting frustrated Lewis pair reactivity with selenium- and boron-based Lewis acids. Angew. Chem. Int. Ed. 55, 11292–11295 (2016).
Soltani, Y., Wilkins, L. C. & Melen, R. L. Stoichiometric and catalytic C–C and C–H bond formation with B(C6F5)3 via cationic intermediates. Angew. Chem. Int. Ed. 56, 11995–111999 (2017).
Lu, T. & Chen, Q. Shermo: a general code for calculating molecular thermochemistry properties. Comput. Theor. Chem. 1200, 113249 (2021).
Acknowledgements
We are grateful for financial support provided by NSFC (22125103, 22071057 and 21871089), STCSM (20XD1421500 and 20JC1416800), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
X.J. and M.W. conceived the idea and supervised the project. M.W. and D.Z. designed and carried out the experiments. Y.M. conducted the DFT calculations. X.J., M.W. and W.-P.D. discussed the results, contributed to the writing of the manuscript and commented on the manuscript. All the authors approved the final version of the manuscript for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Han Zuilhof and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6 and Tables 1–7.
Supplementary Data 1
Crystallographic data of 4y CCDC 2094839.
Supplementary Data 2
Crystallographic data of 5f CCDC 2094841.
Rights and permissions
About this article
Cite this article
Zeng, D., Ma, Y., Deng, WP. et al. Divergent sulfur(VI) fluoride exchange linkage of sulfonimidoyl fluorides and alkynes. Nat. Synth 1, 455–463 (2022). https://doi.org/10.1038/s44160-022-00060-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00060-1
This article is cited by
-
C-SuFEx linkage of sulfonimidoyl fluorides and organotrifluoroborates
Nature Communications (2024)
-
Enantioselective sulfur(VI) fluoride exchange reaction of iminosulfur oxydifluorides
Nature Chemistry (2024)
-
Strain-promoted S-arylation and alkenylation of sulfinamides using arynes and cyclic alkynes
Science China Chemistry (2024)
-
Bioinspired zinc-mediated umpolung thiolation of alkyl electrophiles: reaction development, scope and mechanism
Science China Chemistry (2024)
-
Turning sulfonyl and sulfonimidoyl fluoride electrophiles into sulfur(VI) radicals for alkene ligation
Nature Communications (2023)