Abstract
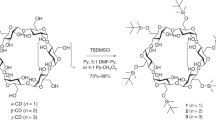
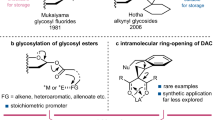
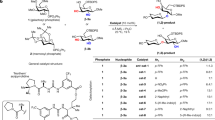
For over a century, naturally occurring cyclodextrins (CDs) have been investigated intensively and extensively. CDs possess inherently stable chiralities, which render them versatile players in diverse arenas of technology. Although naturally occurring CDs can be mass-produced by the enzymatic modification of amylose, their mirror-images have, however, remained inaccessible. Here we report the syntheses of three mirror-image CDs—namely, α-, β- and γ-l-CDs, which are composed of six, seven and eight α-1,4-linked l-glucopyranosyl residues, respectively. Hallmarks of their syntheses include the highly diastereoselective installations of multiple contiguous 1,2-cis l-glucopyranosidic linkages, the rapid assembly of linear oligosaccharides employing one-pot glycosylation strategies and three efficient diastereoselective cyclizations. The structures and inherent chiralities of all three synthetic l-CDs have been established unambiguously by single-crystal X-ray diffraction and induced electronic circular dichroism spectroscopy. The availability of l-CDs has enabled the elucidation of an unprecedented chiral self-sorting of a racemic modification of β-CDs in the solid state and an investigation of the chiral recognition of enantiomeric fenchone by α-l-CD. This research identifies a missing piece of the cyclodextrin jigsaw and sets the stage for scientists to explore the mirror-image world of naturally occurring CDs.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data that support the findings of this study are available in the main text and the Supplementary Information. X-Ray crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 2246203 (α-l-CD), 2246204 (β-l-CD), 2246205 (γ-l-CD) and 2266813 (β-d- and β-l-CD cocrystal). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Villiers, A. Sur la fermentation de la fécule par l’action du ferment butyrique. C. R. Hebd. Acad. Sci. Paris 112, 536–538 (1891).
Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1753 (1998).
Crini, G. A history of cyclodextrins. Chem. Rev. 114, 10940–10975 (2014).
Rekharsky, M. V. & Inoue, Y. Complexation thermodynamics of cyclodextrins. Chem. Rev. 98, 1875–1918 (1998).
Liu, Z., Nalluri, S. K. M. & Stoddart, J. F. Surveying macrocyclic chemistry: from flexible crown ethers to rigid cyclophanes. Chem. Soc. Rev. 46, 2459–2478 (2017).
Cram, D. J. The design of molecular hosts, guests, and their complexes (Nobel lecture). Angew. Chem. Int. Ed. Engl. 27, 1009–1020 (1988).
Lehn, J. -M. Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices (Nobel lecture). Angew. Chem. Int. Ed. Engl. 27, 89–112 (1988).
Chen, Y. & Liu, Y. Cyclodextrin-based bioactive supramolecular assemblies. Chem. Soc. Rev. 39, 495–505 (2010).
Chen, G. & Jiang, M. Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem. Soc. Rev. 40, 2254–2266 (2011).
Davis, M. E. & Brewster, M. E. Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 3, 1023–1035 (2004).
Nepogodiev, S. A. & Stoddart, J. F. Cyclodextrin-based catenanes and rotaxanes. Chem. Rev. 98, 1959–1976 (1998).
Harada, A., Takashima, Y. & Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 38, 875–882 (2009).
Harada, A. Cyclodextrin-based molecular machines. Acc. Chem. Res. 34, 456–464 (2001).
Saenger, W. et al. Structures of the common cyclodextrins and their larger analogues beyond the doughnut. Chem. Rev. 98, 1787–1802 (1998).
Easton, C. J. & Lincoln, S. F. Chiral discrimination by modified cyclodextrins. Chem. Soc. Rev. 25, 163–170 (1996).
Tang, W. & Ng, S.-C. Facile synthesis of mono-6-amino-6-deoxy-α-, β-, γ-cyclodextrin hydrochlorides for molecular recognition, chiral separation and drug delivery. Nat. Protoc. 3, 691–697 (2008).
Chen, L., Chen, Y., Zhang, Y. & Liu, Y. Photo‐controllable catalysis and chiral monosaccharide recognition induced by cyclodextrin derivatives. Angew. Chem. Int. Ed. 60, 7654–7658 (2021).
Armstrong, D. W., Ward, T. J., Armstrong, R. D. & Beesley, T. E. Separation of drug stereoisomers by the formation of β-cyclodextrin inclusion complexes. Science 232, 1132–1135 (1986).
Armstrong, D. W., Li, W. & Pitha, J. Reversing enantioselectivity in capillary gas chromatography with polar and nonpolar cyclodextrin derivative phases. Anal. Chem. 62, 214–217 (1990).
Tang, W. & Ng, S.-C. Synthesis of cationic single-isomer cyclodextrins for the chiral separation of amino acids and anionic pharmaceuticals. Nat. Protoc. 2, 3195–3200 (2007).
Breslow, R. & Dong, S. D. Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev. 98, 1997–2012 (1998).
Takahashi, K. Organic reactions mediated by cyclodextrins. Chem. Rev. 98, 2013–2034 (1998).
Guitet, M. et al. NHC‐capped cyclodextrins (ICyDs): insulated metal complexes, commutable multicoordination sphere, and cavity‐dependent catalysis. Angew. Chem. Int. Ed. 52, 7213–7218 (2013).
Xu, G. et al. Capturing the monomeric (L)CuH in NHC-capped cyclodextrin: cavity-controlled chemoselective hydrosilylation of α,β-unsaturated ketones. Angew. Chem. Int. Ed. 59, 7591–7597 (2020).
Tugny, C. et al. β-Cyclodextrin–NHC–gold (I) complex (β-ICyD) AuCl: a chiral nanoreactor for enantioselective and substrate-selective alkoxycyclization reactions. ACS Catal. 10, 5964–5972 (2020).
Huang, J., Zhang, X. & Armstrong, D. W. Highly efficient asymmetric direct stoichiometric aldol reactions on/in water. Angew. Chem. Int. Ed. 46, 9073–9077 (2007).
Ke, C. et al. Catalytic enantiodifferentiating photocyclodimerization of 2‐anthracenecarboxylic acid mediated by a non‐sensitizing chiral metallosupramolecular host. Angew. Chem. Int. Ed. 48, 6675–6677 (2009).
Wei, X. et al. Supramolecular photochirogenesis driven by higher-order complexation: enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate to slipped cyclodimers via a 2:2 complex with β-cyclodextrin. J. Am. Chem. Soc. 140, 3959–3974 (2018).
Ji, J. et al. An ultimate stereocontrol in supramolecular photochirogenesis: photocyclodimerization of 2-anthracenecarboxylate mediated by sulfur-linked β-cyclodextrin dimers. J. Am. Chem. Soc. 141, 9225–9238 (2019).
Wei, X. et al. Synthesis of cyclodextrin derivatives for enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate. Nat. Protoc. 17, 2494–2516 (2022).
Chen, X.-Y. et al. Selective photodimerization in a cyclodextrin metal–organic framework. J. Am. Chem. Soc. 143, 9129–9139 (2021).
Chen, A. X.-Y. et al. Site-selective C–H functionalization in a cyclodextrin metal–organic framework. Chem 10, 234–249 (2024).
Smaldone, R. A. et al. Metal–organic frameworks from edible natural products. Angew. Chem. Int. Ed. 49, 8630–8634 (2010).
Roy, I. & Stoddart, J. F. Cyclodextrin metal–organic frameworks and their applications. Acc. Chem. Res. 54, 1440–1453 (2021).
Shigemitsu, H. et al. Cyclodextrins with multiple pyrenyl groups: an approach to organic molecules exhibiting bright excimer circularly polarized luminescence. Angew. Chem. Int. Ed. 61, e202114700 (2022).
Tu, C. et al. Host–guest complexation‐induced aggregation based on pyrene‐modified cyclodextrins for improved electronic circular dichroism and circularly polarized luminescence. Angew. Chem. Int. Ed. 61, e202203541 (2022).
Song, X., Zhu, X., Qiu, S., Tian, W. & Liu, M. Self‐assembly of adaptive chiral [1]rotaxane for thermo‐rulable circularly polarized luminescence. Angew. Chem. Int. Ed. 61, e202208574 (2022).
Hu, L., Zhu, X., Yang, C. & Liu, M. Two‐dimensional chiral polyrotaxane monolayer with emergent and steerable circularly polarized luminescence. Angew. Chem. Int. Ed. 61, e202114759 (2022).
Yang, S. et al. Dynamic enzymatic synthesis of γ-cyclodextrin using a photoremovable hydrazone template. Chem 7, 2190–2200 (2021).
Erichsen, A., Peters, G. H. J. & Beeren, S. R. Templated enzymatic synthesis of δ-cyclodextrin. J. Am. Chem. Soc. 145, 4882–4891 (2023).
Ogawa, T. & Takahashi, Y. Total synthesis of α-cyclodextrin. Carbohydr. Res. 138, C5–C9 (1985).
Takahashi, Y. & Ogawa, T. Total synthesis of cyclomaltooctaose and an isomer of cyclomaltohexaose, cyclo{→6)-[α-D-Glcp-(1→4)]5-α-D-Glcp-(1-}.Carbohydr. Res. 169, 127–149 (1987).
Nicolaou, K. C., Vourloumis, D., Winssinger, N. & Baran, P. S. The art and science of total synthesis at the dawn of the twenty‐first century. Angew. Chem. Int. Ed. 39, 44–122 (2000).
Stoddart, J. F. Unnatural product synthesis. Nature 334, 10–11 (1988).
Ikuta, D. et al. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science 364, 674–677 (2019).
Stoddart, J. F. From carbohydrates to enzyme analogues. Chem. Soc. Rev. 8, 85–142 (1979).
Gattuso, G., Nepogodiev, S. A. & Stoddart, J. F. Synthetic cyclic oligosaccharides. Chem. Rev. 98, 1919–1958 (1998).
Bodine, K. D., Gin, D. Y. & Gin, M. S. Synthesis of readily modifiable cyclodextrin analogues via cyclodimerization of an alkynyl−azido trisaccharide. J. Am. Chem. Soc. 126, 1638–1639 (2004).
Li, X. et al. Promoter-controlled synthesis and conformational analysis of cyclic mannosides up to a 32-mer. Angew. Chem. Int. Ed. 62, e202307851 (2023).
Boltje, T. J., Kim, J.-H., Park, J. & Boons, G.-J. Chiral-auxiliary-mediated 1,2-cis-glycosylations for the solid-supported synthesis of a biologically important branched α-glucan. Nat. Chem. 2, 552–557 (2010).
Yasomanee, J. P. & Demchenko, A. V. Hydrogen bond mediated aglycone delivery: synthesis of linear and branched α-glucans. Angew. Chem. Int. Ed. 53, 10453–10456 (2014).
Wang, L., Overkleeft, H. S., van der Marel, G. A. & Codée, J. D. C. Reagent controlled stereoselective synthesis of α-glucans. J. Am. Chem. Soc. 140, 4632–4638 (2018).
Zhang, Y. et al. A new method for α-specific glucosylation and its application to the one-pot synthesis of a branched α-glucan. Org. Chem. Front. 6, 762–772 (2019).
Zhang, Y. et al. Merging reagent modulation and remote anchimeric assistance for glycosylation: highly stereoselective synthesis of α-glycans up to a 30-mer. Angew. Chem. Int. Ed. 60, 12597–12606 (2021).
Zhu, Y., Delbianco, M. & Seeberger, P. H. Automated assembly of starch and glycogen polysaccharides. J. Am. Chem. Soc. 143, 9758–9768 (2021).
Zhang, C. et al. Halogen-bond-assisted radical activation of glycosyl donors enables mild and stereoconvergent 1,2-cis-glycosylation. Nat. Chem. 14, 686–694 (2022).
Hettikankanamalage, A. A., Lassfolk, R., Ekholm, F. S., Leino, R. & Crich, D. Mechanisms of stereodirecting participation and ester migration from near and far in glycosylation and related reactions. Chem. Rev. 120, 7104–7151 (2020).
Huang, X., Huang, L., Wang, H. & Ye, X.-S. Iterative one‐pot synthesis of oligosaccharides. Angew. Chem. Int. Ed. 43, 5221–5224 (2004).
Wu, Y., Xiong, D.-C., Chen, S.-C., Wang, Y.-S. & Ye, X.-S. Total synthesis of mycobacterial arabinogalactan containing 92 monosaccharide units. Nat. Commun. 8, 14851 (2017).
Saouane, S. & Fabbiani, F. P. A. Structural elucidation of α-cyclodextrin-succinic acid pseudo dodecahydrate: expanding the packing types of α-cyclodextrin inclusion complexes. Crystals 6, 2 (2016).
Aree, T. & Chaichit, N. Crystal form III of β-cyclodextrin–ethanol inclusion complex: layer-type structure with dimeric motif. Carbohydr. Res. 343, 2285–2291 (2008).
Steiner, T. & Saenger, W. Channel-type crystal packing in the very rare space group P4212 with Z’ = 3/4: crystal structure of the complex γ-cyclodextrin–methanol–n-hydrate. Acta Crystallogr. B 54, 450–455 (1998).
Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 40, 1305–1323 (2011).
Nowakowski, M. & Ejchart, A. Complex formation of fenchone with α-cyclodextrin: NMR titrations. J. Incl. Phenom. Macrocycl. Chem. 79, 337–342 (2014).
Vidal, S. (ed) Protecting Groups: Strategies and Applications in Carbohydrate Chemistry (Wiley, 2019).
Khan, A. R., Forgo, P., Stine, K. J. & D’Souza, V. T. Methods for selective modifications of cyclodextrins. Chem. Rev. 98, 1977–1996 (1998).
Liu, J. et al. Programmed synthesis of hepta‐differentiated β‐cyclodextrin: 1 out of 117,655 arrangements. Angew. Chem. Int. Ed. 60, 12090–12096 (2021).
Chen, J., Chen, M. & Zhu, T. F. Directed evolution and selection of biostable l-DNA aptamers with a mirror-image DNA polymerase. Nat. Biotechnol. 40, 1601–1609 (2022).
Hahm, H. S. et al. Automated glycan assembly using the Glyconeer 2.1 synthesizer. Proc. Natl Acad. Sci. USA 114, E3385–E3389 (2017).
Yao, W. et al. Automated solution-phase multiplicative synthesis of complex glycans up to a 1,080-mer. Nat. Synth. 1, 854–863 (2022).
Cheng, H. & Wang, P. G. Machine assembly of carbohydrates with more than 1,000 sugar units. Nature 610, 266–267 (2022).
Shishiuchi, R. et al. Discovery of α-l-glucosidase raises the possibility of α-l-glucosides in nature. ACS Omega 7, 47411–47423 (2022).
Acknowledgements
Northwestern University (NU, J.F.S.), the University of Hong Kong (J.F.S.) and the Robert A. Welch Foundation (Y-0026 to D.W.A.) are acknowledged for financial support. This work made use of the Integrated Molecular Structure Education and Research Center (IMSERC) at NU, which receives support from the NIH (1S10OD012016-01/1S10RR019071-01A1), Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205), the State of Illinois and the International Institute for Nanotechnology (IIN). Use of resources of the Keck Biophysics Facility was supported in part by National Cancer Institute award CCSG-P30-CA060553 to the Robert H. Lurie Comprehensive Cancer Center at NU.
Author information
Authors and Affiliations
Contributions
Y.W. and J.F.S. conceived the study and designed experiments. Y.W. performed research under J.F.S.’s guidance. S.A., G.W. and D.W.A. contributed to the chiral recognition experiments. H.H. conducted density functional theory calculations. C.T. contributed to the IECD spectroscopy and X-ray crystallographic analysis. C.L.S. collected and solved the X-ray diffraction data. X.L., H.W., Q.-H.G., Y.Q., A.X.-Y.C., Y.J., R.Z. and A.H.G.D. commented on the data and all authors contributed to data analysis. Y.W. and J.F.S. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
Y.W. and J.F.S. have filed a patent application with Northwestern University (Innovation and New Ventures Office Reference No. NU 2024-020) based on this research. The other authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Sophie Beeren, William Harrison, Chang-Chun Ling and Peng George Wang for their contribution to the peer review of this work. Primary handling editor: Peter Seavill.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s note The editorial team of Nature Synthesis declare that Alison Stoddart has had no involvement in the editorial handling of this article.
Supplementary information
Supplementary Information
Experimental Details, Supplementary Figs. 1–48 and Schemes 1–17.
Supplementary Data 1
X-ray crystallographic data for α-l-CD, CCDC 2246203.
Supplementary Data 2
X-ray crystallographic data for β-l-CD, CCDC 2246204.
Supplementary Data 3
X-ray crystallographic data for γ-l-CD, CCDC 2246205.
Supplementary Data 4
X-ray crystallographic data for β-d- and β-l-CD cocrystal, CCDC 2266813.
Source data
Source Data Fig. 5
Source data for Fig. 5.
Source Data Fig. 6
Source data for Fig. 6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Y., Aslani, S., Han, H. et al. Mirror-image cyclodextrins. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00495-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00495-8