Abstract
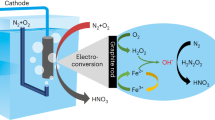
Renewable electricity-driven electrochemical nitrogen oxidation is a promising alternative to traditional Haber–Bosch and Ostwald processes to directly synthesize nitrate from nitrogen. However, its efficiency is hindered by strong competition from the oxygen evolution reaction in aqueous environments, along with a deficiency in standardized testing protocols. Here we present an efficient approach for nitrogen oxidation, substituting the oxygen evolution reaction with hydroxyl radicals (·OH) generated through hydrogen peroxide decomposition to serve as an active oxygen source. Electrochemical tests demonstrate that the nitrogen oxidation, facilitated by ·OH, can achieve a Faradaic efficiency of 25.6% and a nitrate yield of 8.3 nmol s−1 cm−2. Furthermore, we employed in situ electrochemical mass spectrometry, gas-phase infrared and electron paramagnetic resonance spectroscopy to establish a comprehensive set of benchmarks to confirm the authenticity of nitrogen activation and to examine the reaction mechanism mediated by ·OH. Techno-economic analysis underscores the promising feasibility and sustainable economic value of the presented method.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the finding of the study are available in the paper and its Supplementary Information. Source data are provided with this paper and in the Mendeley Data repository at https://doi.org/10.17632/kcwgbv68y6.1 (ref. 50).
References
Markit, I. Nitric Acid-Chemical Economics Handbook (IHS Markit, 2015).
Chen, J. G. et al. Beyond fossil fuel–driven nitrogen transformations. Science 360, eaar6611 (2018).
Canfield, D. E., Glazer, A. N. & Falkowski, P. G. The evolution and future of Earth’s nitrogen cycle. Science 330, 192–196 (2010).
Galloway, J. N. et al. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320, 889–892 (2008).
Li, L. et al. Efficient nitrogen fixation to ammonia through integration of plasma oxidation with electrocatalytic reduction. Angew. Chem. Int. Ed. 60, 14131–14137 (2021).
Wang, Y., Li, T., Yu, Y. & Zhang, B. Electrochemical synthesis of nitric acid from nitrogen oxidation. Angew. Chem. Int. Ed. 61, e202115409 (2022).
Iriawan, H. et al. Methods for nitrogen activation by reduction and oxidation. Nat. Rev. Methods Primers 1, 56 (2021).
Nie, Z. et al. Catalytic kinetics regulation for enhanced electrochemical nitrogen oxidation by Ru-nanoclusters-coupled Mn3O4 catalysts decorated with atomically dispersed Ru atoms. Adv. Mater. 34, 2108180 (2022).
Kuang, M. et al. Efficient nitrate synthesis via ambient nitrogen oxidation with Ru-doped TiO2/RuO2 electrocatalysts. Adv. Mater. 32, 2002189 (2020).
Armstrong, D. A. et al. Standard electrode potentials involving radicals in aqueous solution: inorganic radicals (IUPAC Technical Report). Pure Appl. Chem. 87, 1139–1150 (2015).
Jin, H. et al. Dynamic rhenium dopant boosts ruthenium oxide for durable oxygen evolution. Nat. Commun. 14, 354 (2023).
Fei, H. et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 1, 63–72 (2018).
Grdeń, M., Łukaszewski, M., Jerkiewicz, G. & Czerwiński, A. Electrochemical behaviour of palladium electrode: oxidation, electrodissolution and ionic adsorption. Electrochim. Acta 53, 7583–7598 (2008).
Plauck, A., Stangland, E. E., Dumesic, J. A. & Mavrikakis, M. Active sites and mechanisms for H2O2 decomposition over Pd catalysts. Proc. Natl Acad. Sci. USA 113, E1973–E1982 (2016).
Li, J., Staykov, A., Ishihara, T. & Yoshizawa, K. Theoretical study of the decomposition and hydrogenation of H2O2 on Pd and Au@Pd surfaces: understanding toward high selectivity of H2O2 synthesis. J. Phys. Chem. C 115, 7392–7398 (2011).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Ignarro, L. J., Fukuto, J. M., Griscavage, J. M., Rogers, N. E. & Byrns, R. E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from l-arginine. Proc. Natl Acad. Sci. USA 90, 8103–8107 (1993).
Anand, M., Abraham, C. S. & Nørskov, J. K. Electrochemical oxidation of molecular nitrogen to nitric acid-towards a molecular level understanding of the challenges. Chem. Sci. 12, 6442–6448 (2021).
Wan, H., Bagger, A. & Rossmeisl, J. Limitations of electrochemical nitrogen oxidation toward nitrate. J. Phys. Chem. Lett. 13, 8928–8934 (2022).
Guo, Y. et al. Electrochemical nitrate production via nitrogen oxidation with atomically dispersed Fe on N-doped carbon nanosheets. ACS Nano 16, 655–663 (2022).
A checklist for reproducibility in electrochemical nitrogen fixation. Nat. Commun. 13, 4642 (2022).
Choi, J. et al. Identification and elimination of false positives in electrochemical nitrogen reduction studies. Nat. Commun. 11, 5546 (2020).
Lee, S. A., Lee, M. G. & Jang, H. W. Catalysts for electrochemical ammonia oxidation: trend, challenge, and promise. Sci. China Mater. 65, 3334–3352 (2022).
Liu, H. Y. et al. Electrochemical ammonia oxidation with molecular catalysts. ACS Catal. 13, 4675–4682 (2023).
Xia, Y. & Zweier, J. L. Direct measurement of nitric oxide generation from nitric oxide synthase. Proc. Natl Acad. Sci. USA 94, 12705–12710 (1997).
Bordignon, E. EPR spectroscopy of nitroxide spin probes. eMagRes 6, 235–254 (2017).
Li, Y. et al. Ternary PtIrNi catalysts for efficient electrochemical ammonia oxidation. ACS Catal. 10, 3945–3957 (2020).
Dong, K. et al. Plasma-induced defective TiO2-x with oxygen vacancies: a high-active and robust bifunctional catalyst toward H2O2 electrosynthesis. Chem Catal. 1, 1437–1448 (2021).
Zhang, X. et al. Photothermal-assisted photocatalytic nitrogen oxidation to nitric acid on palladium-decorated titanium oxide. Adv. Energy Mater. 12, 2103740 (2022).
Hu, X. et al. Simultaneous generation of H2O2 and formate by co-electrolysis of water and CO2 over bifunctional Zn/SnO2 nanodots. Angew. Chem. Int. Ed. 62, e202304050 (2023).
Yu, M. et al. Self-supported Mo-doped TiO2 electrode for ambient electrocatalytic nitrogen oxidation. Electrochim. Acta 435, 141333 (2022).
MacFarlane, D. R. et al. A roadmap to the ammonia economy. Joule 4, 1186–1205 (2020).
Lim, J., Fernández, C. A., Lee, S. W. & Hatzell, M. C. Ammonia and nitric acid demands for fertilizer use in 2050. ACS Energy Lett. 6, 3676–3685 (2021).
Dong, K. et al. Epoxidation of olefins enabled by an electro-organic system. Green Chem. 24, 8264–8269 (2022).
Dong, K. et al. Noble-metal-free electrocatalysts toward H2O2 production. J. Mater. Chem. A 8, 23123–23141 (2020).
Chen, W., He, F. & Chen, Y. X. in Encyclopedia of Solid–Liquid Interfaces, 497–513 (Elsevier, 2023).
Abdiaziz, K., Salvadori, E., Sokol, K. P., Reisner, E. & Roessler, M. M. Protein film electrochemical EPR spectroscopy as a technique to investigate redox reactions in biomolecules. Chem. Commun. 55, 8840–8843 (2019).
Chen, L. et al. Accurate identification of radicals by in-situ electron paramagnetic resonance in ultraviolet-based homogenous advanced oxidation processes. Water Res. 221, 118747 (2022).
Dong, K. et al. Conductive two-dimensional magnesium metal–organic frameworks for high-efficiency O2 electroreduction to H2O2. ACS Catal. 12, 6092–6099 (2022).
Dong, K. et al. Honeycomb carbon nanofibers: a superhydrophilic O2-entrapping electrocatalyst enables ultrahigh mass activity for the two-electron oxygen reduction reaction. Angew. Chem. Int. Ed. 60, 10583–10587 (2021).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. Climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Monkhorst, H. J. & Pack, J. D. Special points for Brillonin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
NIST Standard Reference Database Number 69. National Institute of Standards and Technology http://webbook.nist.gov/chemistry/ (2023).
Wang, V., Xu, N., Liu, J. C., Tang, G. & Geng, W. T. VASPKIT: a user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033 (2021).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. A. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Dong, K. H2O2-mediated electrosynthesis of nitrate from air. Mendeley Data https://doi.org/10.17632/kcwgbv68y6.1 (2024).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos. 22072015 and 21927811), the Free Exploration Project of Frontier Technology for Laoshan Laboratory (no. 16-02), the Program for Science & Technology Innovation Talents in Universities of Henan Province (no. 20HASTIT028) and China Postdoctoral Science Foundation (2023M731175 and GZB20230232). We thank S. Li from Nanjing University of Aeronautics and Astronautics and J. Nie from Jilin University for their invaluable contributions in the initial phases of the experiment. Furthermore, we appreciate X. Wang from Gaossunion Corporation for designing the in situ electrochemical electrolytic cell.
Author information
Authors and Affiliations
Contributions
K.D. performed the catalyst preparation, characterizations and catalytic tests. D.M. and Haobo Li conceived and conducted the theoretical investigation of the nitrogen oxidation mechanism. K.D., Y.Y., Huangjingwei Li, S.S., Y.W., Y.L., D.Z. and Qian Liu contributed to the structure characterizations and data analysis. K.D., Quan Li, D.M., X.S. and B.T. designed this study and wrote the paper. All authors contributed and reviewed the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–39, Discussion, Technical economic analysis and Tables 1–4.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, K., Yao, Y., Li, H. et al. H2O2-mediated electrosynthesis of nitrate from air. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00522-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00522-8