Abstract
Here we report a two-step, hierarchical synthesis that assembles a trigonal prismatic organic cage into a more symmetric, higher-order tetrahedral cage, or ‘cage of cages’. Both the preformed [2+3] trigonal prismatic cage building blocks and the resultant tetrahedral [4[2+3]+6]cage molecule are constructed using ether bridges. This strategy affords the [4[2+3]+6]cage molecule excellent hydrolytic stability that is not a feature of more common dynamic cage linkers, such as imines. Despite its relatively high molar mass (3,001 g mol−1), [4[2+3]+6]cage exhibits good solubility and crystallizes into a porous superstructure with a surface area of 1,056 m2 g−1. By contrast, the [2+3] building block is not porous. The [4[2+3]+6]cage molecule shows high CO2 and SF6 uptakes due to its polar skeleton. The preference for the [4[2+3]+6]cage molecule over other cage products can be predicted by computational modelling, as can its porous crystal packing, suggesting a broader design strategy for the hierarchical assembly of organic cages with synthetically engineered functions.

Similar content being viewed by others
Main
The chemical synthesis of complex organic molecules is part of our toolkit to access materials with unique structures and functions1,2,3,4,5. Supramolecular self-assembly is a powerful strategy to synthesize molecules comprising a number of separate precursors6,7,8; these assemblies can also be nanometres in size9,10 or chemically interlocked11,12. However, obtaining the desired self-assembly outcomes for more complex molecules quickly becomes synthetically challenging, particularly when the bond-forming chemistry has low reversibility. This creates a dichotomy: the more successful supramolecular reactions often lead to labile, unstable products, and this can limit the scope for applications. This challenge can be tackled by careful tuning of precursor structure and functionality, such as molecular geometry, or by iterative optimization of the synthetic procedures, but the best reaction conditions are often not intuitively obvious.
Some of the earliest supramolecular systems were synthesized by condensing simple bidentate building blocks, such as ethylenediamine and triethylene glycol, to form cryptands and crown ethers, respectively13. These molecules inspired the synthesis of larger and more complex architectures. For example, Fujita and co-workers introduced the concept of emergent behaviour in the assembly of large self-assembled macrocyclic products using carefully designed precursors14. Such supramolecular design strategies have allowed us to synthesize more complex self-assembled structures and, hence, to unlock new applications2,15,16. However, high structural complexity is often accompanied by increased synthetic challenges and lower predictability because of sensitivity to parameters such as the precise bond angles in the precursors9,14,17.
Postsynthetic modifications have been used previously to enhance the porosity of organic cages18,19, such as by hooping parts of the cage together20. More recently, we and others have used hierarchical assembly strategies to form topologically complex hydrogen-bonded organic frameworks21,22 and covalently bonded materials, such as covalent organic frameworks23,24,25,26, using three-dimensional organic cages as the building blocks27. These studies have shown that cage-based building blocks can assemble into higher-order structures and increase the complexity of the resulting materials, for instance, by controlling network topology and interpenetration, while still offering a degree of structural predictability. In turn, this has afforded cage-based hydrogen-bonded organic frameworks and three-dimensional cage-based covalent organic frameworks with properties such as guest-responsive structural flexibility23 and self-healing behaviour28. However, this hierarchical structuring approach does not appear to have been extended to the preparation of porous organic cage molecules18,29: that is, to synthesize larger porous cages from smaller organic cage precursors.
The use of organic cages as precursors to synthesize higher-order porous structures is attractive because it embeds cage molecules, with their own chemical complexity, into larger, hierarchical cages with the potential to create new functions while retaining useful properties such as solution processability19,27,30. For example, this strategy might produce porous materials with more sophisticated hierarchical porosities. To tackle this goal, we considered three criteria: (1) geometry—the cage precursors need geometries that can be arranged into a higher-order structure in a useful yield; (2) chemical stability—the chemical bonding in the cages must not be too labile, both to impart stability for applications and also to avoid the dynamic scrambling that might occur, for example, in trying to construct an imine cage from another imine cage31; (3) rigidity—the precursors need sufficient rigidity to direct chemical reactivity to the desired product and to ensure that the resultant hierarchical cage is shape persistent and retains its porous structure after removal of solvent from the voids.
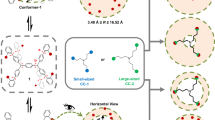
To meet these three criteria, we chose a trigonal prismatic [2+3] ether-bridged cage molecule, Cage-3-Cl, as the polyhedral building block to construct a hierarchical ‘cage of cages’ (Fig. 1). The preconfigured rigid geometry and excellent chemical stability of Cage-3-Cl allowed this [2+3] cage to assemble with tetrafluorohydroquinone (TFHQ) into the hierarchically structured organic ‘cage of cages’ compound, [4[2+3]+6]cage.
Results and discussion
Nucleophilic aromatic substitution (SNAr) reactions have been reported to undergo reversible covalent bond formation when using electron-poor aromatic compounds32,33,34, while still leading to stable molecular products. Reversible error-correction is important for the formation of complex molecules that must self-sort during the reaction from a variety of possible products. Although the SNAr reaction has been used in the synthesis of ether-bridged cages, most tend to be [2+3] or [2+4] cage products with small intrinsic cavities35,36,37, with the exception of a larger [4+6] ether-linked cage reported by Santos and co-workers32. One possible reason for the lack of larger cages synthesized via SNAr chemistry is the less predictable orientation of the ether bridges compared to the imines and boronate esters for which larger cages are more commonplace10,38,39,40,41,42.
Previous investigations by our group and others have demonstrated that Cage-3-Cl has a highly symmetric and rigid triangular prism geometry both in solution and in the solid state21,36. This geometry makes Cage-3-Cl an ideal building block for forming higher-order cage molecules, such as molecular barrels20. The three residual chlorine atoms exhibit high reactivity43,44, which is essential for forming ether bridges. We selected TFHQ as the linear bridge between Cage-3-Cl molecules because the fluorine atoms might afford extra barriers to restrict the rotation of the ether bridges, and might improve the solubility of the resulting cage–cage molecules36,45.
To explore the available bond angles and the relative flexibility of the ether bridges in possible hierarchical cage products, we performed molecular dynamics (MD) and density functional theory (DFT) calculations. Models were constructed with the supramolecular toolkit (stk) software46 to predict the most likely reaction products. As shown in Fig. 2, the [4[2+3]+6] stoichiometry is predicted to form a stable, shape-persistent cage structure that exhibits a much lower energy than alternative [2[2+3]+3] and [8[2+3]+12] topologies. The [2[2+3]+3] topology has by far the highest relative energy (660.8 kJ mol−1) due to its highly strained geometry. The [8[2+3]+12] topology has higher relative energy (24.04 kJ mol−1) than the [4[2+3]+6] cage, which suggests that the [4[2+3]+6] topology is the thermodynamically favoured product, although we stress that these calculations do not include any solvent effects. As such, the [8[2+3]+12] topology might also be accessible under other synthesis conditions, whereas we predict that the [2[2+3]+3] topology is not. The cis–trans configurations of the ether bridges in the hypothetical [8[2+3]+12]cage can result in various positional configurations; all of these structural conformers were predicted to have relative energies that were between 24.0 and 229.1 kJ mol−1 higher than the [4[2+3]+6]cage, indicating a strong preference for the [4[2+3]+6] product (Supplementary Information Section 1 and Supplementary Figs. 1–4).
x = number of Cage-3-Cl cages, y = number of TFHQ linkers. Atom colours: carbon, grey; nitrogen, blue; oxygen, red; fluorine, green. Hydrogen atoms are omitted for clarity. Note the break in the energy scale for the highly strained [2[2+3]+3]cage, which has by far the highest relative energy (660.8 kJ mol−1). The DFT energies indicate that the [4[2+3]+6] stoichiometry is predicted to form a stable, shape-persistent cage structure that has a lower relative energy (24.04 kJ mol−1) than the alternative [8[2+3]+12] topology.
These simulation results suggested that it might be possible to synthesize [4[2+3]+6]cage via the SNAr reaction between Cage-3-Cl and TFHQ (Fig. 1). We therefore attempted the reaction experimentally, and screened a range of conditions in which we varied the reagent concentration, solvent and base (Supplementary Table 1). From these experiments, we found that the reaction in acetone in the presence of the acid scavenger N,N-diisopropylethylamine (DIPEA) afforded a new product with the highest yield of 53% after purification. The 1H NMR spectrum for the purified reaction product from the acetone reaction with DIPEA showed two singlets at 7.09 and 6.85 ppm, which we assigned to the two aromatic protons in the [2+3] cage (Ha and Hb; Fig. 3a and Supplementary Fig. 5). The presence of two singlets indicates different environments, which we attribute to one of the protons being more shielded. However, apart from this splitting of the aromatic proton singlet in Cage-3-Cl, the NMR spectroscopy data indicated that the resulting product had high symmetry in solution. In the 13C NMR spectrum, we observed three signals in the 174.5–173.1 ppm range (Fig. 3b and Supplementary Fig. 6), which we assigned to the triazine ring carbon atoms. We attribute the characteristic splitting, observed at 142.5 and 140.0 ppm with a coupling constant of 250 MHz, to the coupling between the carbon and fluorine atoms in the TFHQ linker (Fig. 3b and Supplementary Fig. 6). We also confirmed the presence of these fluorinated aromatic rings by 19F NMR spectroscopy, observing a singlet at −155.62 ppm (Supplementary Fig. 7), indicating that the fluorine atoms were symmetrically equivalent in solution. We also used high-resolution matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry to analyse the reaction product. We found an ion with a mass-to-charge (m/z) ratio of 3,002.0756 (Fig. 3c and Supplementary Figs. 8 and 9), which matched well with the theoretical value of [[4[2+3]+6]cage + H]+ (3002.0871), indicating the formation of [4[2+3]+6]cage.
a, 1H NMR (400 MHz, acetone-d6) spectra of Cage-3-Cl (green, bottom) and [4[2+3]+6]cage (blue, top). b, 13C NMR (100 MHz, dioxane-d8) spectra: TFHQ (yellow, bottom), Cage-3-Cl (green, middle) and [4[2+3]+6]cage (blue, top). Insets: zoom-ins of the boxed regions. The NMR spectra highlight the splitting of peaks due to the formation of a hierarchical ‘cage of cages’ structure. c, High-resolution MALDI-TOF spectrum of [4[2+3]+6]cage, showing an ion with an m/z ratio of 3,002.0756 assigned to [[4[2+3]+6]cage + H]+. Two internal calibrants (Spherical) with m/z ratios of 2,979 and 3,423 that bracketed the ion of interest were used to limit the m/z error to ±5 ppm.
We next grew crystals for single-crystal X-ray diffraction analysis to confirm the structure of the [4[2+3]+6]cage molecule. Slow evaporation of a mixture of acetone/ethanol afforded single crystals suitable for X-ray analysis using synchrotron radiation (Supplementary Fig. 10 and Supplementary Table 2). The synchrotron single-crystal structure, which we refined in the monoclinic P21 space group, revealed that the [4[2+3]+6]cage molecule adopts a tetrahedral topology, where four Cage-3-Cl cage molecules serve as the vertices and six TFHQ molecules are located as the edges (Fig. 4a). The interior and the exterior aryl caps of the Cage-3-Cl cage molecules form a core–shell structure, defining an inner and outer truncated tetrahedron with edge lengths of 6.4 and 13.7 Å, respectively (Fig. 4b). We also calculated the electrostatic potentials for the [4[2+3]+6]cage molecule, which showed that the centre of the [4[2+3]+6]cage molecule is surrounded by aromatic rings, affording π–π interactions for any guest molecules within the cage (Fig. 4c and Supplementary Information Section 1).
a, Structure of an individual [4[2+3]+6]cage molecule. Atom colours: carbon, grey; hydrogen, white; nitrogen, blue; oxygen, red; fluorine, green. b, Representation of the [4[2+3]+6]cage molecule using two truncated tetrahedra on the inner and outer aryl caps of the [2+3] Cage-3-Cl cage molecules. For clarity, all atoms here are coloured grey. c, Electrostatic potential maps of the [4[2+3]+6]cage molecule. The red and blue surfaces represent negative and positive regions of potential, respectively. Colour bar, −31.4 to 94.1 kcal mol−1. d,e, Pore channels in the extended [4[2+3]+6]cage crystal structure as viewed along the a axis (d) and the b axis (e). For clarity, hydrogen atoms are omitted in b, e and f. The yellow surfaces in d and e represent the contact surface as measured using a 1.2 Å diameter probe. f, Scheme explaining the window splitting in the [4[2+3]+6]cage crystal structure along the a axis; the window of the lower blue cage is partially occluded by the aryl face of the upper yellow cage.
The interior of the cage core exhibits an electron-poor character because of the V-shaped electron-deficient clefts formed by the triazine rings of Cage-3-Cl and the fluorine-decorated aromatic rings. This environment might be useful for selective guest molecule separation47,48,49. In the extended crystal structure of this cage of cages, the asymmetric cell contains one [4[2+3]+6]cage molecule, which assembles into a porous supramolecular structure by interacting with 12 neighbouring [4[2+3]+6]cage molecules through van der Waals forces (Supplementary Fig. 11). Two of the windows in the [4[2+3]+6]cage molecule are narrowed into smaller channels by the Cage-3-Cl vertices from neighbouring cage molecules (Fig. 4d,f and Supplementary Fig. 12), yielding three-dimensional interconnected pore channels (Fig. 4d,e). Using Zeo++50, we calculated that the pore-limiting diameter of the [4[2+3]+6]cage crystal structure was 6.4 Å and the largest cavity diameter was 8.9 Å (Supplementary Table 3 and Supplementary Figs. 13–15), suggesting that the structure is microporous. From these calculations, we also determined that voids in the [4[2+3]+6]cage crystal structure that are accessible to a 1.65 Å CO2 probe occupy 32.0% of the unit cell volume (Supplementary Table 3).
There was strong agreement between the predicted structure for the [4[2+3]+6]cage molecule and the molecule observed in the crystal structure (Fig. 5). This validates the theoretical predictions, and the close match between the crystal structure prediction (CSP)-predicted structure and experimental crystal structure adds confidence in the crystal structure refinement (Supplementary Fig. 17). The root mean squared displacement (r.m.s.d.) was calculated as 0.5 Å with a maximum distance between atoms of 1.4 Å. However, the experimental displacement parameters are large due to disorder in the crystal structure (Supplementary Fig. 11a). Further attempts to synthesize the larger [8[2+3]+12] product by varying the reaction conditions were unsuccessful, based on MALDI-TOF analysis of the resulting products (Supplementary Table 1 and Supplementary Fig. 8), in line with the molecular stability predictions (Fig. 2).
a–c, The predicted structure (red) overlaid with the single-crystal X-ray diffraction structure (blue) is shown as viewed along the a (a), b (b) and c (c) crystallographic axes. The r.m.s.d. was calculated as 0.5 Å with a maximum distance between atoms of 1.4 Å, highlighting the close structural similarity between the predicted and experimental structures.
In principle, catenation of this cage is possible, given its large intrinsic voids (>10 Å diameter), as observed for considerably smaller imine cages11. However, we saw no evidence for catenated cage side-products, either by NMR or by MALDI-TOF characterization.
We next used CSP to explore the solid-state packing of these hierarchical cages. The lattice energy landscape was explored using quasi-random sampling of the crystal packing space with the Global Lattice Energy Explorer (GLEE)51. Initial trial structures were generated from rigid molecules and subjected to lattice energy minimization using an empirically parameterized potential with atomic multipole electrostatics52 (see Supplementary Information Section 4, Supplementary Tables 4 and 5, and Supplementary Figs. 16–25 for full details).
Surprisingly, the CSP landscape for [4[2+3]+6]cage (Fig. 6) showed catenated structures, along with the non-catenated cage that was observed experimentally, even though the discrete [4[2+3]+6]cage molecule was used for the CSP calculations. Three distinct catenations were identified in the predicted crystal structures: triply interlocked cage dimers (Fig. 6c), singly interlocked cage dimers (Fig. 6d) and singly interlocked one-dimensional (1D) cage chains12,53 (Fig. 6e). The details of the methods used for catenation detection are provided in Supplementary Information Section 4 and Supplementary Figs. 18‒20. All sampled structures within a 197 kJ mol−1 energy window from the global energy minimum were found to be catenanes (Supplementary Figs. 21 and 22), indicating a strong thermodynamic preference over the non-catenated cages observed by experiment. To verify the relative energies calculated using the rigid-molecule, force-field approach, a selection of catenated and non-catenated predicted structures were re-evaluated using periodic DFT, which confirmed this greater thermodynamic stability (see Supplementary Information Section 4 for full details).
a, Computational crystal energy landscape of [4[2+3]+6]cage with colour-coded categorization based on catenation type: discrete, non-catenated cages (uncoloured circles), triply interlocked cage dimers (green circles), singly interlocked cage dimers (blue) and singly interlocked 1D cage chains (orange). The yellow star and blue cross represent the predicted structures matching the experimentally observed [4[2+3]+6]cage crystal structure and [4[2+3]+6]cage·acetone solvated structure, respectively. b, Energy landscape after removal of the catenated structures, with colour coding based on the diameter of the largest sphere (Df) capable of freely moving within the crystal structure’s channel(s). Channels are found based on their ability to accommodate a CO2 molecule. Df = 0 corresponds to no channel being found. c–e, Atomic structures depicted for examples of a triply interlocked cage dimer (c), a singly interlocked cage dimer (d) and a singly interlocked 1D cage chain (e).
While the CSP study did not explicitly target catenated structures, the sampled catenated configurations suggest that triply interlocked catenanes (green points, Fig. 6a), in particular, might be much more thermodynamically stable in the solid state. This echoes previous findings for [4+6] imine cages, in which discrete cages were found to transform into triply interlocked catenanes upon exposure to acid, suggesting that the individual cages were the kinetic rather than the thermodynamic product11. The absence of catenanes in our experiments might be explained by the much lower reversibility of the ether bonding in the [4[2+3]+6]cage molecule, which is not accounted for in the CSP calculations. Prompted by these solid-state CSP results, we also explored the relative thermodynamic stability of catenanes at the molecular level. DFT calculations of catenane dimers showed that the energy difference between the molecular equivalent non-catenated [4[2+3]+6]cage dimer and trimer fragments retrieved from the global lowest-energy CSP, and the corresponding triply interlocked catenane molecular fragment was 373.7 kJ mol−1 and 324.7 kJ mol−1, respectively, reaffirming strong thermodynamic favour towards the catenane structures.
When we remove the catenated structures from the CSP plot (Fig. 6b and Supplementary Fig. 23), this reveals the observed experimental structure positioned at the bottom of a low-density ‘spike’ in the energy landscape, approximately 13.6 kJ mol−1 higher than the global energy minimum for non-catenated cages. The predicted crystal structure reproduces the geometry of the experimentally determined [4[2+3]+6]cage crystal structure accurately (Supplementary Fig. 17), confirming that the crystal structure determined by X-ray diffraction corresponds to a low-energy local minimum in lattice energy. The colour coding in this ‘non-catenated’ crystal structure landscape represents the diameter of the largest sphere capable of unrestricted movement within the crystal structure channels. Channel dimensions are determined based on their capacity to accommodate a CO2 molecule with a kinetic radius of 1.65 Å (Supplementary Figs. 24 and 25). In the landscape depicted in Fig. 6b, void analysis has been restricted to structures within 20 kJ mol−1 of the low-energy edge of the energy-density distribution of structures. Except for a very small number of predicted structures (purple points, Fig. 6b), all investigated structures, including the synthesized structure, show potential for CO2 uptake. That is, CSP suggests that [4[2+3]+6]cage has an intrinsic propensity to be porous in the majority of its potential crystalline packing modes.
Molecular crystals exhibiting permanent porosity in the solid state are attractive for applications such as gas capture, separation and catalysis18,54. One successful approach that we and others have developed is to form porous organic crystals by synthesizing cages with prefabricated shape-persistent cavities that are retained after solvents are removed during activation18,50,54. Our calculations revealed that the ether bridges in the [4[2+3]+6]cage skeleton appeared to be relatively rigid, suggesting shape persistence. We therefore investigated the porosity in the [4[2+3]+6]cage crystals using gas sorption analysis. We activated the [4[2+3]+6]cage crystals by first exchanging the ethanol and acetone crystallization solvents with diethyl ether or n-pentane, which we chose because of their low surface tensions. Then, we removed any residual solvent from the crystals under a dynamic vacuum at room temperature. Subsequent powder X-ray diffraction (PXRD) analysis revealed that the [4[2+3]+6]cage crystals retained some crystallinity after being activated using these conditions (Supplementary Fig. 26). The [4[2+3]+6]cage crystals activated via the diethyl ether solvent exchange route appeared more crystalline, and this sample was used for the subsequent gas sorption experiments described here.
Nitrogen sorption isotherms recorded at 77 K revealed that the crystalline [4[2+3]+6]cage exhibits a type I N2 sorption isotherm with a relatively high Brunauer–Emmett–Teller surface of 1,056 m2 g−1 (Fig. 6a and Supplementary Figs. 27‒29), consistent with a microporous solid and the pore size distribution plot calculated using Zeo++51 (Supplementary Table 3 and Supplementary Fig. 13). We found that crystalline [4[2+3]+6]cage has a CO2 uptake capacity of 3.98 mmol g−1 at 1 bar and 273 K (Fig. 7b and Supplementary Fig. 30). This CO2 uptake is high compared with other porous organic crystalline materials, such as covalent organic frameworks55, at comparable temperatures and pressures, and is one of the highest CO2 uptakes reported to date for a porous organic cage (Supplementary Table 6)56,57. The calculated isosteric heat of adsorption of CO2 on crystalline [4[2+3]+6]cage ranges between 21.1 and 23.2 kJ mol−1 (Supplementary Fig. 31), which indicates a strong affinity between the adsorbed CO2 gas and polar [4[2+3]+6]cage crystal pores, rationalizing this high uptake capacity. In addition, we found that crystalline [4[2+3]+6]cage has a high SF6 uptake capacity of 3.21 mmol g−1 at 1 bar and 273 K (Supplementary Fig. 32). The calculated isosteric heat of adsorption of SF6 on crystalline [4[2+3]+6]cage ranges between 29.2 and 29.5 kJ mol−1, which again indicates a strong affinity between adsorbed SF6 gas molecules and the [4[2+3]+6]cage crystal pores (Supplementary Fig. 33). Analysis of the [4[2+3]+6]cage powder after the gas sorption isotherms by PXRD analysis indicated that the material remained crystalline during these measurements (Supplementary Fig. 34).
a, N2 sorption isotherms recorded at 77 K showing hysteresis in the desorption isotherm. b, CO2 gas sorption isotherms recorded at 273 K (cyan) and 298 K (orange) showing an uptake capacity of 3.98 mmol g−1 at 1 bar and 273 K. Closed and open symbols represent the adsorption and desorption isotherms, respectively.
We also uncovered a second crystal structure of the [4[2+3]+6]cage molecule during this study, referred to as [4[2+3]+6]cage·acetone, which crystallized from slow evaporation of an acetone-d6 solution (Supplementary Fig. 35). [4[2+3]+6]cage·acetone crystallized in the cubic space group \(I\bar{4}3m\) (a = 23.2901(15) Å, V = 12633(2) Å3, Supplementary Table 7) with the ether-bridged cage adopting a perfect tetrahedral geometry in the structure (Supplementary Fig. 36). The [4[2+3]+6]cage·acetone lost crystallinity rapidly after being removed from the acetone-d6 solvent and cracked (Supplementary Fig. 35). We therefore performed single-crystal analysis by sealing a solvated crystal in a borosilicate capillary containing residual acetone-d6 solvent. However, due to the poorer crystal stability of 4[2+3]+6]cage·acetone, we did not investigate its solid-state properties further. The instability of this form was further investigated through computational geometry optimization of the crystal structure. Employing the same energy model as used in the CSP study, rigid-molecule geometry optimization of the structure after solvent removal resulted in considerable structural distortion from the original cubic lattice, adopting a monoclinic form, in keeping with the observed experimental instability. Details can be found in Supplementary Information Section 8. The relaxed structure, denoted by a blue cross in the landscape of Fig. 6a, is situated 103 kJ mol−1 above the global energy minimum on the landscape of non-catenated structures. This energy difference underscores the crucial role of solvent stabilization in the synthesis of this solvated structure, and can also help to rationalize why this tetrahedral molecular structure was not predicted using gas-phase (that is, solvent-free) DFT calculations (Fig. 5).
For practical applications, gas sorption capacity is not the only criterion. For example, most CO2 capture applications involve wet or humid gas streams, and hence water stability is important. Many porous organic cage materials, such as imine cages and (particularly) boronate ester cages, are unstable to water. We therefore explored the hydrolytic stability of the [4[2+3]+6]cage molecule by immersing the synthesized crystals in water for 12 days. Subsequent analysis of the sample by 1H NMR spectroscopy revealed that [4[2+3]+6]cage remained chemically intact under these conditions (Supplementary Fig. 38). PXRD analysis of the same sample also revealed that the [4[2+3]+6]cage crystals retained their crystallinity under these conditions (Supplementary Fig. 39). Hence, both the chemical and crystal structure of [4[2+3]+6]cage molecule appear to have good hydrolytic stability.
Conclusion
We report the assembly of a more complex type of porous organic cage—a ‘cage of cages’—that was synthesized using a two-step hierarchical self-assembly strategy. In this study, we demonstrate the strategy by assembling four trigonal cages into a larger tetrahedral cage. The resulting [4[2+3]+6]cage molecule exhibits excellent stability in water, and crystals of the [4[2+3]+6]cage show permanent porosity and a high surface area of 1,056 m2 g−1. The abundance of polar atoms in the cage cavity endows it with high CO2 and SF6 uptake capacity. The good solubility of [4[2+3]+6]cage in acetone indicates it has the potential to be used as a building block for even more complex structures, such as porous cage co-crystals. More broadly, this illustrates a strategy for hierarchical molecular assembly using computation as a guide to assess the most likely reaction products. For example, it might be possible in the future to design analogous systems where the [2+3] cages contribute discrete, prefabricated porosity into a higher-order, hierarchically porous crystal.
This study also showcases the use of computational design in supramolecular synthesis, both at the molecular level (Fig. 5) and in the solid state (Fig. 6). It is notable that triply interlocked cage catenane dimers emerged as the most stable predicted crystal packings (Fig. 6a). Such catenanes were not observed in experiments, most likely because they are kinetically disfavoured, but they are nonetheless synthetically plausible because analogous structures have been formed using more reversible [4+6] imine cage-forming reactions11. Less obviously, infinite 1D catenated cage chains are also produced in these simulations (Fig. 6e), and in some cases these structures are predicted to have similar lattice energies to the experimentally observed non-catenated cage (Fig. 6a). This highlights how a priori structure predictions have the power to suggest non-intuitive new materials, although it is unclear how one might design a kinetic pathway to these chain structures, even though analogous structures have been observed for less complex macrocycles53.
Methods
Molecular simulations
Both Cage-3-Cl and cage-of-cages models were constructed in Tri2Di3, Tri4Di6 and Tri8Di12 topologies using the stk software46. All cages were annealed with an MD simulation at 700 K for 50 ns with a time step of 0.5 fs after a 100 ps equilibration time with the OPLS4 force field as implemented in the Macromodel Suite58. Five hundred random configurations from the total MD duration were sampled and energy minimized, with the lowest energy configuration selected for DFT calculations. DFT calculations were performed with CP2K v.2023.1 (ref. 59) software using the generalized gradient approximation theory with the Perdew–Burke–Ernzerhof functional60 and def2-TZVP basis sets61. A planewave cut-off value of 400 Ry and a relative cut-off value of 100 Ry were parameterized to obtain converged energy levels and dispersion interactions were accounted for with Grimme’s DFT-D3 approach62.
The geometries of the [4[2+3]+6]cage were then fully optimized by means of the hybrid M06-2X functional in Gaussian16 (ref. 63). The def2-SVP basis set64,65 was applied for all atoms. No symmetry or geometry constraint was imposed during optimizations. The optimized geometries were verified as local minima on the potential energy surface by frequency computations at the same theoretical level63.
Synthesis of [4[2+3]+6]cage
To synthesize [4[2+3]+6]cage, DIPEA (61 µl, 0.35 mmol) was dissolved in acetone (25 ml) and purged with N2 for 10 min. To the acetone solution, a mixture of Cage-3-Cl (58.7 mg, 0.1 mmol) and TFHQ (27.3 mg, 0.15 mmol) in acetone (6 ml) was added dropwise over 3 h under a N2 atmosphere. After the addition was complete, the reaction was stirred at room temperature for 36 h. The solvent was then removed by rotary evaporation, and the crude product was purified by column chromatography using acetone/CH2Cl2 (10% vol/vol acetone) as eluent to afford [4[2+3]+6]cage as a white solid in 53% isolated yield: 40 mg (0.013 mmol). 1H NMR (400 MHz, acetone-d6): δ (ppm) 7.09 (s, 12H, Hb), 6.85 (s, 12H, Ha); 19F NMR (376 MHz, acetone-d6): δ (ppm) −155.62; 13C NMR (100 MHz, dioxane-d8): δ (ppm) 174.5, 173.5, 173.1, 153.2, 152.8, 142.5, 140.1, 140.0, 128.3, 115.2, 114.8. MALDI-TOF [M + H]+, [C120H24F24N36O36 + H]+: calculated, 3002.0871; found, 3002.0756.
CSP
CSP involves the following general steps: (1) molecular geometry optimization; (2) trial crystal structure generation; (3) local lattice energy minimization of trial structures; and (4) duplicate removal.
The geometry of the molecular cage was optimized at the B3LYP/6-311 G(d,p) level using Gaussian09 software66, and the resulting geometry was kept fixed throughout the subsequent steps. Trial crystal structures are generated using the Global Lattice Energy Explorer (GLEE) code51. Subsequently, these trial structures undergo lattice optimization while preserving the rigidity of the molecular cage. For this task, we employ an empirically parameterized intermolecular atom–atom exp-6 potential coupled with atomic multipole electrostatics. The force-field parameters are acquired from the FIT force field67,68. Atom-centred multipoles up to hexadecapole on each atom were derived from the electron density through DMA, and partial charges (used in early stages of optimization) were fitted to the molecular electrostatic potential generated by these multipoles69,70. The overall model is denoted as FIT + DMA.
The search for space groups involves sampling the ten most common space groups for organic crystals along with four trigonal space groups (143, 144, 145 and 146), each with one molecule in the asymmetric unit. A quasi-random method is used to search these selected space groups separately, and valid structures are lattice energy minimized using DMACRYS software52 in a two-stage protocol. The first stage involves FIT + DMA with partial charges, followed by the second stage with multipole electrostatics. More details can be found in Supplementary Information.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper, its Supplementary Information files, and the Cambridge Crystallographic Data Centre (deposition numbers 2303319 for [4[2+3]+6]cage and 2326368 for [4[2+3]+6]cage·acetone). The crystal structures and structure factor data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The CSP data are available at the University of Southampton Institutional Research Repository at https://doi.org/10.5258/SOTON/D2929 (ref. 71).
References
Lehn, J. M. Toward self-organization and complex matter. Science 295, 2400–2403 (2002).
McTernan, C. T., Davies, J. A. & Nitschke, J. R. Beyond platonic: how to build metal–organic polyhedra capable of binding low-symmetry, information-rich molecular cargoes. Chem. Rev. 122, 10393–10437 (2022).
Guillerm, V. & Eddaoudi, M. The importance of highly connected building units in reticular chemistry: thoughtful design of metal–organic frameworks. Acc. Chem. Res. 54, 3298–3312 (2021).
Montà-González, G., Sancenón, F., Martínez-Máñez, R. & Martí-Centelles, V. Purely covalent molecular cages and containers for guest encapsulation. Chem. Rev. 122, 13636–13708 (2022).
Lutz, J. F., Lehn, J. M., Meijer, E. W. & Matyjaszewski, K. From precision polymers to complex materials and systems. Nat. Rev. Mater. 1, 16024 (2016).
Acharyya, K. & Mukherjee, P. S. Organic imine cages: molecular marriage and applications. Angew. Chem. Int. Ed. 58, 8640–8653 (2019).
Deng, H. et al. Multiple functional groups of varying ratios in metal–organic frameworks. Science 327, 846–850 (2010).
Pullen, S. & Clever, G. H. Mixed-ligand metal–organic frameworks and heteroleptic coordination cages as multifunctional scaffolds—a comparison. Acc. Chem. Res. 51, 3052–3064 (2018).
Fujita, D. et al. Self-assembly of tetravalent Goldberg polyhedra from 144 small components. Nature 540, 563–566 (2016).
Koo, J. et al. Gigantic porphyrinic cages. Chem 6, 3374–3384 (2020).
Hasell, T. et al. Triply interlocked covalent organic cages. Nat. Chem. 2, 750–755 (2010).
Benke, B. P., Kirschbaum, T., Graf, J., Gross, J. H. & Mastalerz, M. Dimeric and trimeric catenation of giant chiral [8 + 12] imine cubes driven by weak supramolecular interactions. Nat. Chem. 15, 413–423 (2023).
Steed, J. W., Turner, D. R. & Wallace, K. J. Core Concepts in Supramolecular Chemistry and Nanochemistry (John Wiley, 2007).
Sun, Q. F. et al. Self-assembled M24L48 polyhedra and their sharp structural switch upon subtle liqand variation. Science 328, 1144–1147 (2010).
Fujita, D. et al. Protein stabilization and refolding in a gigantic self-assembled cage. Chem 7, 2672–2683 (2021).
Chakrabarty, R., Mukherjee, P. S. & Stang, P. J. Supramolecular coordination: self-assembly of finite two- and three-dimensional ensembles. Chem. Rev. 111, 6810–6918 (2011).
Santolini, V., Miklitz, M., Berardo, E. & Jelfs, K. E. Topological landscapes of porous organic cages. Nanoscale 9, 5280–5298 (2017).
Yang, X., Ullah, Z., Stoddart, J. F. & Yavuz, C. T. Porous organic cages. Chem. Rev. 123, 4602–4634 (2023).
Mastalerz, M. Porous shape-persistent organic cage compounds of different size, geometry, and function. Acc. Chem. Res. 51, 2411–2422 (2018).
Wang, Q. Q. et al. Molecular barrel by a hooping strategy: synthesis, structure, and selective CO2 adsorption facilitated by lone pair-π interactions. J. Am. Chem. Soc. 139, 635–638 (2017).
Zhu, Q. et al. Analogy powered by prediction and structural invariants: computationally led discovery of a mesoporous hydrogen-bonded organic cage crystal. J. Am. Chem. Soc. 144, 9893–9901 (2022).
Han, B. et al. Postsynthetic metalation of a robust hydrogen-bonded organic framework for heterogeneous catalysis. J. Am. Chem. Soc. 141, 8737–8740 (2019).
Zhu, Q. et al. 3D cage COFs: a dynamic three-dimensional covalent organic framework with high-connectivity organic cage nodes. J. Am. Chem. Soc. 142, 16842–16848 (2020).
Ji, C. et al. Tunable cage-based three-dimensional covalent organic frameworks. CCS Chem. 4, 3095–3105 (2022).
Ma, J.-X. et al. Cage based crystalline covalent organic frameworks. J. Am. Chem. Soc. 141, 3843–3848 (2019).
Kory, M. J. et al. Gram-scale synthesis of two-dimensional polymer crystals and their structure analysis by X-ray diffraction. Nat. Chem. 6, 779–784 (2014).
Giri, A., Sahoo, A., Dutta, T. K. & Patra, A. Cavitand and molecular cage-based porous organic polymers. ACS Omega 5, 28413–28424 (2020).
Zhu, Q. et al. Soft hydrogen-bonded organic frameworks constructed using a flexible organic cage hinge. J. Am. Chem. Soc. 145, 23352–23360 (2023).
Zhang, G. & Mastalerz, M. Organic cage compounds—from shape-persistency to function. Chem. Soc. Rev. 43, 1934–1947 (2014).
Das, S., Heasman, P., Ben, T. & Qiu, S. Porous organic materials: strategic design and structure-function correlation. Chem. Rev. 117, 1515–1563 (2017).
Jiang, S. et al. Porous organic molecular solids by dynamic covalent scrambling. Nat. Commun. 2, 207 (2011).
Santos, T. et al. Dynamic nucleophilic aromatic substitution of tetrazines. Angew. Chem. Int. Ed. 60, 18783–18791 (2021).
Terrier, F. Modern Nucleophilic Aromatic Substitution (Wiley, 2013); https://doi.org/10.1002/9783527656141
Katz, J. L., Geller, B. J. & Conry, R. R. Synthesis of oxacalixarenes incorporating nitrogen heterocycles: evidence for thermodynamic control. Org. Lett. 8, 2755–2758 (2006).
Katz, J. L. et al. Single-step synthesis of D3h-symmetric bicyclooxacalixarenes. Org. Lett. 7, 3505–3507 (2005).
Wang, D. X. et al. Versatile anion–π interactions between halides and a conformationally rigid bis(tetraoxacalix[2]arene[2]triazine) cage and their directing effect on molecular assembly. Chem. Eur. J. 16, 13053–13057 (2010).
Wang, Z. et al. Multicolor tunable polymeric nanoparticle from the tetraphenylethylene cage for temperature sensing in living cells. J. Am. Chem. Soc. 142, 512–519 (2020).
Zhang, G., Presly, O., White, F., Oppel, I. M. & Mastalerz, M. A permanent mesoporous organic cage with an exceptionally high surface area. Angew. Chem. Int. Ed. 53, 1516–1520 (2014).
Hong, S. et al. Porphyrin boxes: rationally designed porous organic cages. Angew. Chem. Int. Ed. 54, 13241–13244 (2015).
Ivanova, S. et al. Isoreticular crystallization of highly porous cubic covalent organic cage compounds. Angew. Chem. Int. Ed. 60, 17455–17463 (2021).
Hähsler, M. & Mastalerz, M. A giant [8 + 12] boronic ester cage with 48 terminal alkene units in the periphery for postsynthetic alkene metathesis. Chem. Eur. J. 27, 233–237 (2021).
Ono, K. et al. Self-assembly of nanometer-sized boroxine cages from diboronic acids. J. Am. Chem. Soc. 137, 7015–7018 (2015).
Kory, M. J., Bergeler, M., Reiher, M. & Schlüter, A. D. Facile synthesis and theoretical conformation analysis of a triazine-based double-decker rotor molecule with three anthracene blades. Chem. Eur. J. 20, 6934–6938 (2014).
Luo, N., Ao, Y. F., Wang, D. X. & Wang, Q. Q. Exploiting anion–π interactions for efficient and selective catalysis with chiral molecular cages. Angew. Chem. Int. Ed. 60, 20650–20655 (2021).
Mooibroek, T. J. & Gamez, P. The s-triazine ring, a remarkable unit to generate supramolecular interactions. Inorganica Chim. Acta 360, 381–404 (2007).
Turcani, L., Berardo, E. & Jelfs, K. E. stk: a Python toolkit for supramolecular assembly. J. Comput. Chem. 39, 1931–1942 (2018).
Itoh, Y. et al. Ultrafast water permeation through nanochannels with a densely fluorous interior surface. Science 376, 738–743 (2022).
Yoshizawa, M., Tamura, M. & Fujita, M. Diels–Alder in aqueous molecular hosts: unusual regioselectivity and efficient catalysis. Science 312, 251–254 (2006).
Yamashina, M. et al. An antiaromatic-walled nanospace. Nature 574, 511–515 (2019).
Willems, T. F., Rycroft, C. H., Kazi, M., Meza, J. C. & Haranczyk, M. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials. Microporous Mesoporous Mater. 149, 134–141 (2012).
Case, D. H., Campbell, J. E., Bygrave, P. J. & Day, G. M. Convergence properties of crystal structure prediction by quasi-random sampling. J. Chem. Theory Comput. 12, 910–924 (2016).
Price, S. L. et al. Modelling organic crystal structures using distributed multipole and polarizability-based model intermolecular potentials. Phys. Chem. Chem. Phys. 12, 8478–8490 (2010).
Loots, L. & Barbour, L. J. An infinite catenane self-assembled by π⋯π interactions. Chem. Commun. 49, 671–673 (2013).
Tozawa, T. et al. Porous organic cages. Nat. Mater. 8, 973–978 (2009).
Ozdemir, J. et al. Covalent organic frameworks for the capture, fixation, or reduction of CO2. Front. Energy Res. 7, 77 (2019).
Kunde, T., Pausch, T. & Schmidt, B. M. Porous organic compounds—small pores on the rise. Eur. J. Org. Chem. 2021, 5844–5856 (2021).
Kunde, T., Nieland, E., Schröder, H. V., Schalley, C. A. & Schmidt, B. M. A porous fluorinated organic [4+4] imine cage showing CO2 and H2 adsorption. Chem. Commun. 56, 4761–4764 (2020).
Lu, C. et al. OPLS4: improving force field accuracy on challenging regimes of chemical space. J. Chem. Theory Comput. 17, 4291–4300 (2021).
Kühne, T. D. et al. CP2K: an electronic structure and molecular dynamics software package—Quickstep: efficient and accurate electronic structure calculations. J. Chem. Phys. 152, 194103 (2020).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
VandeVondele, J. & Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 127, 114105 (2007).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Frisch, M. J. et al. Gaussian 16 revision a.03.2016 (Gaussian, 2016).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 8, 1057–1065 (2006).
Frisch, M. J. et al. Gaussian 09 revision D.01, C.01 (Gaussian, 2010).
Coombes, D. S., Price, S. L., Willock, D. J. & Leslie, M. Role of electrostatic interactions in determining the crystal structures of polar organic molecules. A distributed multipole study. J. Phys. Chem. 100, 7352–7360 (1996).
Beyer, T. & Price, S. L. Dimer or catemer? Low-energy crystal packings for small carboxylic acids. J. Phys. Chem. B 104, 2647–2655 (2000).
Stone, A. J. Distributed multipole analysis: stability for large basis sets. J. Chem. Theory Comput. 1, 1128–1132 (2005).
Ferenczy, G. G. Charges derived from distributed multipole series. J. Comput. Chem. 12, 913–917 (1991).
Hafizi, R. & Day, G. M. Supporting data for the journal article “Computationally guided synthesis of a hierarchical [4[2+3]+6] porous organic ‘cage of cages’”. University of Southampton Institutional Research Repository https://doi.org/10.5258/SOTON/D2929 (2024).
Acknowledgements
A.I.C. thanks the Royal Society for a Research Professorship (RSRP\S2\232003). C.Z. acknowledges the China Scholarship Council for financial support (202106745008). R.H. acknowledges the Iridis 5 High Performance Computing facility, and associated support services at the University of Southampton. Via our membership of the UK’s HEC Materials Chemistry Consortium, which is funded by the Engineering and Physical Sciences Research Council (EPSRC) (EP/R029431 and EP/X035859), this work used the Archer2 HPC facility. We acknowledge A. Hunter for performing the MALDI-TOF analysis at the National Mass Spectrometry Facility (NMSF) at Swansea University, and Diamond Light Source for access to beamlines I19 (CY30461). We received funding from the EPSRC (EP/V026887/1) and the Leverhulme Trust via the Leverhulme Research Centre for Functional Materials Design. This project has received funding from the European Research Council under the European Union’s Horizon 2020 Research and Innovation programme (grant CoMMaD number 758370).
Author information
Authors and Affiliations
Contributions
Q.Z. led the experimental work and the synthesis and characterization of the materials. A.I.C., K.E.J. and M.A.L. conceived the idea and modelling strategy with Q.Z. and supervised the project. H.Q. and M.A.L. conducted the single-crystal X-ray diffraction analysis and solved the structure. G.A., K.E.J. and C.Z. performed the molecular simulations. R.H. and G.M.D. performed the CSP, and G.M.D. supervised this part of the project. Q.Z., G.A., R.H., G.M.D., K.E.J., M.A.L. and A.I.C. analysed the data and prepared the paper. All authors discussed the results and contributed to the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Chenfeng Ke, Bernhard Schmidt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, Tables 1–8, Scheme 1, synthetic procedures and methods, molecular simulation, NMR, MALDI-TOF, powder X-ray diffraction, single-crystal X-ray diffraction, crystal structure prediction and gas sorption analysis.
Supplementary Data 1
Simulated structures of [2[2 + 3] + 3]cage.xyz, 4[2 + 3] + 6]cage.xyz, [8[2 + 3] + 12]cage.xyz, [8[2 + 3] + 12]cage_1.xyz, [8[2 + 3] + 12]cage_2.xyz, and [8[2 + 3] + 12]cage_3.xyz.
Supplementary Data 2
X-ray crystallographic data of [4[2 + 3] + 6]cage, CCDC 2303319.
Supplementary Data 3
Crystallographic data of [4[2 + 3] + 6]cage·acetone, CCDC 2326368.
Supplementary Video 1
Video showing the single crystal structure of [4[2 + 3] + 6]cage.
Supplementary Data 4
Tabulated source data used to prepare Supplementary Figs. 1–2, 8, 13, 14, 26–34 and 39.
Supplementary Data 5
Raw NMR spectroscopy data used to prepare Supplementary Figs. 5, 7 and 38.
Source data
Source Data Fig. 3
Raw NMR spectroscopy data and MALDI-TOF data.
Source Data Fig. 7
Source data for carbon dioxide gas sorption isotherms recorded at 273 and 298 K in Fig. 7.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, Q., Qu, H., Avci, G. et al. Computationally guided synthesis of a hierarchical [4[2+3]+6] porous organic ‘cage of cages’. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00531-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00531-7