Abstract
Introduction:
The underlying causes of longitudinally extensive transverse myelitis (LETM) are broad and include inflammatory processes, compression and spinal dural arteriovenous fistula (SDAVF). Presenting symptoms of SDAVF are nonspecific and often go misdiagnosed. Acute clinical deterioration from SDAVF has been described following exertion or valsalva. However, deterioration has been recently recognized following steroid administration and may contribute to increased morbidity.
Case Presentation:
We describe a 63-year-old woman with a 2-year history of intermittent lower extremity numbness and back pain, lumbar stenosis, who presented with subacute worsening of symptoms following a course of oral steroids for an upper respiratory infection. Initial whole-spine imaging was concerning for LETM and lumbar puncture was concerning for an inflammatory process. The patient was treated with intravenous (IV) methylprednisolone, after which she developed acute onset bilateral lower extremity paraparesis with a sensory level. Angiogram confirmed the diagnosis of SDAVF and the patient was treated surgically. Post-operative course was complicated and subsequent clinical improvement has been slow with incomplete recovery to date.
Discussion:
This case illustrates the nonspecific presentation of SDAVF and the difficulty of differentiating it from other causes of LETM. It demonstrates acute clinical deterioration of SDAVF following steroid administration, a recently recognized clinical entity. The most likely mechanism is hydrostatic steroid effect coupled with iatrogenic fluid co-administration causing increased venous congestion. Previous cases have demonstrated this effect to be transient and resolves after discontinuation of steroids. This case highlights a recent association of increased morbidity following steroid administration despite definitive treatment.
Similar content being viewed by others
Introduction
Longitudinally extensive transverse myelitis (LETM) is defined as spinal cord signal change on magnetic resonance imaging (MRI) involving three or more successive vertebral levels.1 The etiologies of LETM includes infectious and parainfectious causes, autoimmune and rheumatological conditions including neuromyelitis optica, demyelinating diseases, paraneoplastic conditions, chronic compressive myelopathy, cord infarction and spinal dural arteriovenous fistulas (SDAVF).1 Neuromyelitis optica has received increasing attention as an etiology of LETM due in part to the availability of testing for aquaporin-4 antibodies, a relationship to other autoimmune entities such as lupus and Sjogren’s disease, and availability of treatment options including immunomodulatory agents and plasma exchange.1,2
SDAVF is the most common form of spinal vascular malformation but SDAVF is a rare cause of LETM and can go misdiagnosed for years.1,3,4 SDAVF can sometimes be differentiated from other causes of LETM by imaging characteristics, including the presence of venous flow voids on T2 and cord signal change involving the conus.1 Acute clinical deterioration from SDAVF has been described following exertion or valsalva and improvement with rest. However, transient deterioration has recently been recognized following steroid administration, and may contribute to increased morbidity.5,6, 7,8, 9,10,11
We present a case of LETM complicated by multiple premorbid conditions. Following treatment with intravenous (IV) steroids, the patient developed acute onset paraparesis and sensory loss. The patient was subsequently diagnosed with SDAVF and treated surgically with only minimal improvement.
Case presentation
We present a 63-year-old woman with a past medical history of obesity, deep venous thrombosis and pulmonary embolism on therapeutic anticoagulation, and a 2-year history of intermittent lateral thigh numbness and chronic lower back pain that reportedly worsened with activity and improved with rest. She was previously diagnosed with lumbar radiculopathy from multilevel lumbar stenosis. She had previously underwent treatment with spinal injections but reported only minimal improvement. She presented with 2-month history of progressive worsening of symptoms, including hyperesthesia of right L3-5 dermatomes and lower back pain. She also complained of new bladder retention with incontinence and gait difficulty without weakness. This presentation followed a viral upper respiratory infection for which she received a course of oral steroids. She denied personal history of optic neuritis. Her family history was positive for a sister with lupus.
Her initial examination revealed intact strength and brisk deep tendon reflexes of the bilateral lower extremities. The left lower extremity revealed clonus and mild distal decreased vibration and proprioception. The remainder of the examination was unremarkable.
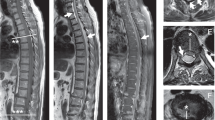
Complete spinal MRI confirmed severe multilevel lumbar foraminal stenosis. MRI also revealed abnormal T2 signal hyperintensity from T3 to the conus and contrast enhancement from T7-8 to T10-11, concerning for transverse myelitis (Figures 1a and b).
MRI spine imaging, L spine sag T2 (a) showing abnormal signal to the conus, T spine T1 post gadolinium (b) demonstrating enhancement T7-8 to T10-11, T spine sag T2 (c) showing dilated flow voids (arrow), spinal angiogram, pre-treatment subtracted Rt L2 segment run AP view (d), lateral view (e) demonstrating dural arteriovenous fistula (thick) and arterialized vein (thin), post-treatment angiogram of Rt L2 segment AP view (f) showing resolution of fistula.
Lumbar puncture was performed on hospital day 3 and demonstrated 651 red blood cells per ul (normal 0), 48 white blood cells per ul (normal 0–10), glucose 46 mg dl−1 (normal 50–75), protein 132 mg dl−1 (normal 15–45), elevated central nervous system IgG synthesis of 13.2 mg dl−1 (normal 0.6–4.2), but was otherwise unremarkable. Serological testing was performed and included workup for infectious, rheumatological and neuropathic etiologies. This testing was unremarkable except for a positive anti-nuclear antibody screen by indirect immunofluorescence assay and enzyme immunoassay and a positive anti-SSA/Ro antibody. An autoimmune or demyelinating process was suspected as the underlying etiology. The patient started treatment on hospital day 5 with IV methylprednisolone at a dose of 1 g daily, planned for 5 days duration. Confirmatory testing for aquaporin-4 antibody returned negative in both serum and cerebrospinal fluid.
Approximately 4 h after receiving the second scheduled dose of IV methylprednisolone and 28 h after initiation of therapy, the patient reported bilateral lower extremity paraparesis that was worse on the right and a sensory level to T10. Prothrombin time and International Normalized Ratio remained within the normal range. Brain MRI was unremarkable. Repeat spine MRI demonstrated similar cord signal abnormality to the prior study, with improvement of the contrast enhancement at T11 but a new focus at T6-7, and no evidence of interval hematoma development. Upon further review of imaging, flow voids were noted (Figure 1c). SDAVF was suspected and the patient underwent diagnostic spinal angiogram which demonstrated a type 1 SDAVF with feeding vessel of the right L2 segmental artery. She was treated surgically with an L2 and partial L1 laminectomy. She had successful ligation of the SDAVF, which was confirmed on post-operative spinal angiogram (Figures 1d–f). Post-operative course was complicated by acute bilateral femoral deep venous thrombosis and initiation of anticoagulation led to development of a concurrent spinal hematoma. The patient was eventually discharged to acute rehabilitation.
At 2 months follow-up, the patient had some mild subjective improvement in strength with physical therapy, primarily of the left lower extremity greater than right, and some sensory improvement.
Discussion
SDAVF are a rare cause of LETM; however, they remain the most common form of spinal vascular malformation. In SDAVF, the fistula develops between a branch of the radicular artery and vein, typically at the level of a nerve root within the dural root sleeve.3,4 Presenting symptoms are nonspecific and include weakness, sensory deficits of the lower extremities, pain, urinary incontinence or retention and bowel issues including constipation.1,3,4 The mechanism is believed to be due to relatively high-pressure venous drainage at the level of the cord surface leading to venous congestion.4 SDAVF can be misdiagnosed for years, typically as a polyneuropathy, polyradiculopathy or myelopathy.1,3,4 SDAVFs most often occur in middle-aged to older patients, are male predominant, and are most commonly thoracolumbar in location.3 In the case of cerebral dural fistulas, there is a strong association with cerebral venous thrombosis and thrombophilia. However, no known association between SDAVF and thrombophilia has been demonstrated.1,12,13
Spinal imaging can be helpful in diagnosis. MRI findings can include T2 flow voids representing dilated veins, cord T2/FLAIR signal hyperintensity with involvement of the conus, and cord contrast enhancement.1 The gold standard for diagnosis of an SDAVF is a spinal angiogram. Treatment of SDAVF typically involves endovascular embolization, laminectomy and ligation, or both.
Lumbar puncture is often performed to aid in the diagnosis and routine analysis of cerebrospinal fluid, including protein, glucose and cell counts, and can provide supportive information for differentiating inflammatory from infectious processes involving the central nervous system. In SDAVF, lumbar puncture results can be misleading and can range from unremarkable,5,7,14,15 to isolated elevated protein levels,8,16 to greatly elevated white blood cell and protein levels mimicking an infectious or inflammatory process.6,15,17
Acute clinical deterioration in patients with SDAVF can present as progression of motor weakness to paraparesis or paraplegia, acute loss of sensory modalities and/or progression of bowel or bladder dysfunction. Previously, acute clinical deterioration was considered transient and typically followed exertion or valsalva and patients saw clinical improvement with rest.1,3,12 However, this phenomenon has been recently described following an empiric steroid administration for myelopathic symptoms,5,6,7,8,9,10,11 and clinical deterioration occurs within hours to days. In early case reports, clinical deterioration was transient, with some clinical improvement or even complete return to prior baseline after cessation of steroid treatment.5,7,8 In more recent case reports of this phenomenon, it is becoming increasingly clear that the effects of steroids may not always be transient.6,9,10 Two recent retrospective case series have reported an incidence of this phenomenon in greater than 50% in SDAVF patients that were treated with steroids.10,11 Also in most previously described cases, patients experienced meaningful improvement of symptoms following definitive treatment of the fistula (Table 1).
The pathophysiology of steroid-induced acute clinical deterioration in SDAVF is hypothesized to be a relative or localized increase of venous blood flow leading to worsening venous congestion and cord ischemia via two hypothesized mechanisms. The first is a direct hypervolemia from iatrogenic saline administration. The second is an indirect mechanism from hydrostatic fluid retention due to then steroid effect on the kidney and increased vasodilatation.5,6,7,8,9,10,11
Our patient is currently the third reported patient in the literature of SDAVF with clinical deterioration following oral steroid administration and the 24th reported patient following IV steroid administration.5,6,7,8,9,10,11This patient is also unique because her clinical deterioration following IV steroids was not transient and did not improve rapidly following the cessation of treatment. She also suffered a complicated post-operative course due to premorbid hypercoagulability and anticoagulation management. To date, she has had only mild and slow clinical improvement following definitive surgical treatment without a return to her premorbid baseline.
Conclusions
This case illustrates the difficulty of diagnosis of the underlying cause for LETM as SDAVF in a patient who presented with nonspecific signs and symptoms and multiple comorbid conditions.1,3 Acute clinical deterioration of the myelopathic symptoms following steroid administration is a recently recognized sign of SDAVF. However, it is becoming increasingly apparent that clinical deterioration following steroids in SDAVF may not be a transient finding and can result in increased morbidity.6,9,10 Clinicians need to be knowledgeable of such associations and potential outcomes.
References
Tobin WO, Weinshenker BG, Lucchinetti CF . Longitudinally extensive transverse myelitis. Curr Opin Neurol 2014; 27: 279–289.
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189.
Jellema K, Tijssen CC, van Gijn J . Spinal dural arteriovenous fistulas: a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain 2006; 129: 3150–3164.
Schick U, Hassler W . Treatment and outcome of spinal dural arteriovenous fistulas. Eur Spine J 2003; 12: 350–355.
Söderlund ME, Benisty S, Gaston A, Djindjian M, Cesaro P, Créange A . [Can myelopathies secondary to arterio-venous dural fistulae be aggravated by intravenous corticosteroid therapy?]. Rev Neurol (Paris) 2007; 163: 235–237.
Strowd RE, Geer C, Powers A, Abou-Zeid N . A unique presentation of a spinal dural arteriovenous fistula exacerbated by steroids. J Clin Neurosci 2012; 19: 466–468.
Cabrera M, Paradas C, Márquez C, González A . Acute paraparesis following intravenous steroid therapy in a case of dural spinal arteriovenous fistula. J Neurol 2008; 255: 1432–1433.
O’Keeffe DT, Mikhail MA, Lanzino G, Kallmes DF, Weinshenker BG . Corticosteroid-induced paraplegia—a diagnostic clue for spinal dural arterial venous fistula. JAMA Neurol 2015; 72: 833–834.
McKeon A, Lindell EP, Atkinson JLD, Weinshenker BG, Piepgras DG, Pittock SJ . Pearls & oy-sters: clues for spinal dural arteriovenous fistulae. Neurology 2011; 76: e10–e12.
Nasr DM, Brinjikji W, Rabinstein AA, Lanzino G . Clinical outcomes following corticosteroid administration in patients with delayed diagnosis of spinal arteriovenous fistulas. J Neurointerv Surg 2016 (e-pub ahead of print 3 June 2017; doi:10.1136/neurintsurg-2016-012430).
Lee C-S, Pyun HW, Chae EY, Kim K-K, Rhim SC, Suh DC . Reversible aggravation of neurological deficits after steroid medication in patients with venous congestive myelopathy caused by spinal arteriovenous malformation. Interv Neuroradiol 2009; 15: 325–329.
Koch C . Spinal dural arteriovenous fistula. Curr Opin Neurol 2006; 19: 69–75.
Jellema K, Tijssen CC, Fijnheer R, De Groot PG, Koudstaal PJ, Van Gijn J . Spinal dural arteriovenous fistulas are not associated with prothrombotic factors. Stroke 2004; 35: 2069–2071.
Awad IA, Barnett GH . Neurological deterioration in a patient with a spinal arteriovenous malformation following lumbar puncture. Case report. J Neurosurg 1990; 72: 650–653.
Rastogi S, Liebeskind DS, Zager EL, Volpe NJ, Weigele JB, Hurst RW . Rapid cognitive decline following lumbar puncture in a patient with a dural arteriovenous fistula. Surg Neurol 2004; 62: 341–345.
Foote AM, Bower SPC, Danks RA, Chong W . Multiple spinal dural arteriovenous fistulae and deterioration post lumbar puncture. J Clin Neurosci 2010; 17: 137–138.
García-Cabo C, Morís G . Sudden paraplegia after lumbar puncture as a clue in the diagnosis of a patient with spinal dural arteriovenous fistula. Eur Spine J (e-pub ahead of print 1 February 2017; doi:10.1007/s00586-017-4946-5).
Acknowledgements
We thank Drs Thomas Masaryk and Mark Bain for their assistance in the care of this patient.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
DiSano, M., Cerejo, R. & Mays, M. Acute paraparesis and sensory loss following intravenous corticosteroid administration in a case of longitudinally extensive transverse myelitis caused by spinal dural arteriovenous fistula: case report and review of literature. Spinal Cord Ser Cases 3, 17025 (2017). https://doi.org/10.1038/scsandc.2017.25
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2017.25