Article
|
Open Access
Featured
-
-
Article
| Open Access Sequential appetite suppression by oral and visceral feedback to the brainstem
Sequential appetite suppression by oral and visceral feedback to the brainstem
Genetically distinct neural circuits in the caudal brainstem receive feedback from the mouth and gut to regulate feeding behaviour on short and long timescales.
- Truong Ly
- , Jun Y. Oh
- & Zachary A. Knight
-
Article
| Open Access An orexigenic subnetwork within the human hippocampus
An orexigenic subnetwork within the human hippocampus
An appetite-regulating subnetwork in humans involving the lateral hypothalamus and the dorsolateral hippocampus is implicated in obesity and related eating disorders.
- Daniel A. N. Barbosa
- , Sandra Gattas
- & Casey H. Halpern
-
Article
| Open Access Xiphoid nucleus of the midline thalamus controls cold-induced food seeking
Xiphoid nucleus of the midline thalamus controls cold-induced food seeking
Through leverage of whole-brain screening, in vivo calcium imaging and chemo- and optogenetic manipulations, it is demonstrated that the xiphoid nucleus serves as a key brain region in the promotion of cold-induced food-seeking behaviours.
- Neeraj K. Lal
- , Phuong Le
- & Li Ye
-
Article
| Open Access Mast cells link immune sensing to antigen-avoidance behaviour
Mast cells link immune sensing to antigen-avoidance behaviour
Mast cells are shown to function as sensor cells linking antigen recognition in type 2 immunity to antigen-specific avoidance behaviour, preventing immune activation and inflammation.
- Thomas Plum
- , Rebecca Binzberger
- & Hans-Reimer Rodewald
-
Article
| Open Access Brainstem ADCYAP1+ neurons control multiple aspects of sickness behaviour
Brainstem ADCYAP1+ neurons control multiple aspects of sickness behaviour
A studying using a set of unbiased methodologies shows that a specific subpopulation of neurons in the brainstem can regulate the diverse responses to a bacterial endotoxin that induces sickness behaviours.
- Anoj Ilanges
- , Rani Shiao
- & Jeffrey M. Friedman
-
Article |
 Sestrin mediates detection of and adaptation to low-leucine diets in Drosophila
Sestrin mediates detection of and adaptation to low-leucine diets in Drosophila
Fruitflies require Sestrin to regulate mTORC1 signalling in response to dietary leucine, survive a diet low in leucine, and control leucine-sensitive physiological characteristics, which establishes Sestrin as a physiologically relevant leucine sensor.
- Xin Gu
- , Patrick Jouandin
- & David M. Sabatini
-
Article
| Open Access Dopamine subsystems that track internal states
Dopamine subsystems that track internal states
Distinct dopaminergic neurons in the ventral tegmental area respond to physiological fluid balance and nutrient cues at specific stages of ingestion, driving learning about the physiological effects of ingestion.
- James C. R. Grove
- , Lindsay A. Gray
- & Zachary A. Knight
-
Article |
 The neuronal logic of how internal states control food choice
The neuronal logic of how internal states control food choice
High-resolution volumetric calcium imaging was used to create a functional atlas of the Drosophila melanogaster ventral brain and identify how and where metabolic and reproductive states alter processing of food-related sensory stimuli.
- Daniel Münch
- , Dennis Goldschmidt
- & Carlos Ribeiro
-
Article |
 A nutrient-specific gut hormone arbitrates between courtship and feeding
A nutrient-specific gut hormone arbitrates between courtship and feeding
Diuretic hormone 31 secreted by the gut in response to feeding on protein-rich food excites brain neurons that promote switching from feeding to mating behaviour in Drosophila.
- Hui-Hao Lin
- , Meihua Christina Kuang
- & Jing W. Wang
-
Article |
 Sensory representation and detection mechanisms of gut osmolality change
Sensory representation and detection mechanisms of gut osmolality change
Vagal afferents innervating the hepatic portal area respond to changes in gut osmolality and regulate thirst and drinking behaviour in mice.
- Takako Ichiki
- , Tongtong Wang
- & Yuki Oka
-
Article |
 Reverse-translational identification of a cerebellar satiation network
Reverse-translational identification of a cerebellar satiation network
Activity in anterior deep cerebellar nuclei reduces food consumption in mice without reducing metabolic rate, potentially identifying a therapeutic target for disorders involving excessive eating.
- Aloysius Y. T. Low
- , Nitsan Goldstein
- & J. Nicholas Betley
-
Article |
 Food cue regulation of AGRP hunger neurons guides learning
Food cue regulation of AGRP hunger neurons guides learning
In response to food cues, a hypothalamic circuit in the mouse brain transiently inhibits neurons expressing agouti-related peptide, and this promotes learning of cue-initiated food-seeking tasks.
- Janet Berrios
- , Chia Li
- & Bradford B. Lowell
-
Article |
 Response of the microbiome–gut–brain axis in Drosophila to amino acid deficit
Response of the microbiome–gut–brain axis in Drosophila to amino acid deficit
In Drosophila, an amino acid deficit triggers the expression of the neuropeptide CNMamide in gut enterocytes, which promotes a compensatory appetite for essential over non-essential amino acids, and this process is modulated by the microbiome.
- Boram Kim
- , Makoto I. Kanai
- & Won-Jae Lee
-
Article |
 Hunger enhances food-odour attraction through a neuropeptide Y spotlight
Hunger enhances food-odour attraction through a neuropeptide Y spotlight
Attraction to food odours is enhanced in mice that are experiencing hunger through a mechanism involving neuropeptide Y.
- Nao Horio
- & Stephen D. Liberles
-
Article |
 Enteric neurons increase maternal food intake during reproduction
Enteric neurons increase maternal food intake during reproduction
A multi-organ circuit is activated in female flies after mating, leading to changes in enteric neurons that increase food intake.
- Dafni Hadjieconomou
- , George King
- & Irene Miguel-Aliaga
-
Article |
 The cellular basis of distinct thirst modalities
The cellular basis of distinct thirst modalities
The authors uncover the diverse transcriptomic cell types of thirst-driving neurons in the lamina terminalis and show that unique combinations of neuron types respond to and mediate distinct thirst states.
- Allan-Hermann Pool
- , Tongtong Wang
- & Yuki Oka
-
Article |
 Neurons that regulate mouse torpor
Neurons that regulate mouse torpor
A specific neuronal population in the medial and lateral preoptic area of the hypothalamus regulates entry into torpor in mice.
- Sinisa Hrvatin
- , Senmiao Sun
- & Michael E. Greenberg
-
Article |
 A neural circuit mechanism for mechanosensory feedback control of ingestion
A neural circuit mechanism for mechanosensory feedback control of ingestion
A population of neurons in the parabrachial nucleus that expresses prodynorphin monitors ingestion using mechanosensory signals from the upper digestive tract, and mediates negative feedback control of intake when the digestive tract is distended.
- Dong-Yoon Kim
- , Gyuryang Heo
- & Sung-Yon Kim
-
Article |
 An intestinal zinc sensor regulates food intake and developmental growth
An intestinal zinc sensor regulates food intake and developmental growth
Hodor, an intestinal zinc-gated chloride channel, controls systemic growth in Drosophila by promoting food intake and by modulating Tor signalling and lysosomal homeostasis within enterocytes.
- Siamak Redhai
- , Clare Pilgrim
- & Irene Miguel-Aliaga
-
Article |
 A glucose-sensing neuron pair regulates insulin and glucagon in Drosophila
A glucose-sensing neuron pair regulates insulin and glucagon in Drosophila
A pair of glucose-sensing neurons identified in the brain of Drosophila melanogaster regulates secretion of adipokinetic hormone and Drosophila insulin-like peptide 2, suggesting that these neurons have key roles in maintenance of glucose homeostasis.
- Yangkyun Oh
- , Jason Sih-Yu Lai
- & Greg S. B. Suh
-
Letter |
 Chemosensory modulation of neural circuits for sodium appetite
Chemosensory modulation of neural circuits for sodium appetite
Sodium appetite in mice is driven by a neural circuit that is focused on neurons of the pre-locus coeruleus and integrates the sensory detection of sodium and internal signals.
- Sangjun Lee
- , Vineet Augustine
- & Yuki Oka
-
Letter |
 A gut-to-brain signal of fluid osmolarity controls thirst satiation
A gut-to-brain signal of fluid osmolarity controls thirst satiation
Drinking behaviour in mice is regulated by a signal derived from the water and salt content of the gastrointestinal tract that is transmitted to forebrain neurons that control thirst via the vagus nerve.
- Christopher A. Zimmerman
- , Erica L. Huey
- & Zachary A. Knight
-
Letter |
 Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour
Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour
Distinct but partially overlapping subsets of neurons in the orbitofrontal cortex of mice respond to feeding and/or social stimuli and, when optogenetically stimulated at single-cell resolution, specifically regulate reward-seeking behaviours.
- Joshua H. Jennings
- , Christina K. Kim
- & Karl Deisseroth
-
Letter |
 Genetic identification of leptin neural circuits in energy and glucose homeostases
Genetic identification of leptin neural circuits in energy and glucose homeostases
A subset of neurons in the hypothalamus is identified as the primary site of action for regulating energy balance and glucose homeostasis by leptin.
- Jie Xu
- , Christopher L. Bartolome
- & Dong Kong
-
Article |
 Encoding of danger by parabrachial CGRP neurons
Encoding of danger by parabrachial CGRP neurons
Single-cell recordings show that CGRP-expressing neurons in the parabrachial nucleus in mice respond to both noxious stimuli and signals of feeding satiety.
- Carlos A. Campos
- , Anna J. Bowen
- & Richard D. Palmiter
-
Outline |
 Fighting the fatty liver
Fighting the fatty liver
Increased levels of obesity are driving an epidemic of non-alcoholic fatty liver disease. Understanding, diagnosing and treating this progressive condition are now priorities.
- Liam Drew
-
Outline |
 Fatty liver disease: turning the tide
Fatty liver disease: turning the tide
A progressive and potentially life-threatening condition previously associated with alcoholism is becoming more common — even in non-drinkers.
- Liam Drew
-
Article |
 Homeostatic circuits selectively gate food cue responses in insular cortex
Homeostatic circuits selectively gate food cue responses in insular cortex
A combination of microprism-based cellular imaging to monitor insular cortex visual cue responses in behaving mice across hunger states with circuit mapping and manipulations reveals a neural basis for state-specific biased processing of motivationally relevant cues.
- Yoav Livneh
- , Rohan N. Ramesh
- & Mark L. Andermann
-
Article |
 MC4R-dependent suppression of appetite by bone-derived lipocalin 2
MC4R-dependent suppression of appetite by bone-derived lipocalin 2
Osteoblast-derived LCN2 activates the melanocortin 4 receptor in neurons of the paraventricular nucleus of the hypothalamus to suppress appetite, regulates insulin secretion and increases insulin sensitivity and glucose tolerance.
- Ioanna Mosialou
- , Steven Shikhel
- & Stavroula Kousteni
-
Letter |
 Gamma oscillations organize top-down signalling to hypothalamus and enable food seeking
Gamma oscillations organize top-down signalling to hypothalamus and enable food seeking
Coordinated gamma oscillations in the lateral hypothalamus, lateral septum and medial prefrontal cortex are shown to drive food-seeking behaviour in mice independently of nutritional need and to organize firing of feeding behaviour-related hypothalamic neurons.
- Marta Carus-Cadavieco
- , Maria Gorbati
- & Tatiana Korotkova
-
Letter |
 A cholinergic basal forebrain feeding circuit modulates appetite suppression
A cholinergic basal forebrain feeding circuit modulates appetite suppression
A mouse study reveals that acetylcholine signalling networks have a role in the regulation of body weight homeostasis, with increased activity of cholinergic neurons decreasing food consumption through downstream hypothalamic targets.
- Alexander M. Herman
- , Joshua Ortiz-Guzman
- & Benjamin R. Arenkiel
-
Letter |
 Thirst neurons anticipate the homeostatic consequences of eating and drinking
Thirst neurons anticipate the homeostatic consequences of eating and drinking
Feedback from the oral cavity to thirst-promoting neurons in the subfornical organ (SFO) during eating and drinking is integrated with information about blood composition, providing a prediction of how oral consumption will affect fluid balance and leading to changes in behaviour.
- Christopher A. Zimmerman
- , Yen-Chu Lin
- & Zachary A. Knight
-
Letter |
 Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism
Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism
Activation of glucose-sensing neurons in the ventromedial hypothalamic nucleus using radio waves or magnetic fields remotely and non-invasively in vivo increases plasma glucose and glucagon, and suppresses plasma insulin; conversely, remote inhibition of glucose-sensing neurons decreased blood glucose and increased plasma insulin.
- Sarah A. Stanley
- , Leah Kelly
- & Jeffrey M. Friedman
-
Article |
 Neurons for hunger and thirst transmit a negative-valence teaching signal
Neurons for hunger and thirst transmit a negative-valence teaching signal
Cell-type-specific electrical activity manipulations and deep-brain imaging in mice of neuronal populations associated with homeostasis of nutrient or fluid intake reveals that learning is conditioned by a negative-valence signal from the hunger-mediating AGRP neurons and also from the thirst-mediating neurons in the subfornical organ.
- J. Nicholas Betley
- , Shengjin Xu
- & Scott M. Sternson
-
Article |
 Hypothalamic POMC neurons promote cannabinoid-induced feeding
Hypothalamic POMC neurons promote cannabinoid-induced feeding
Cannabinoid-induced feeding signals are shown to enhance pro-opiomelanocortin (POMC) neuronal activity in mice, causing an enhancement of β-endorphin release, which is crucial in causing this cannabinoid-induced response; these results uncover an overlooked role of hypothalamic POMC neurons in the promotion of feeding by cannabinoids.
- Marco Koch
- , Luis Varela
- & Tamas L. Horvath
-
Letter |
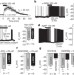 G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons
G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons
α-MSH and AgRP, two hypothalamus-derived peptides with opposing actions on the melanocortin-4 receptor (MC4R), modulate neurons driving feeding behaviour; although previous downstream mechanisms of cellular modulation by these peptides have been determined, here α-MSH and AgRP are shown to regulate neural activity by coupling MC4R to Kir7.1 potassium channels and closing or opening them, respectively.
- Masoud Ghamari-Langroudi
- , Gregory J. Digby
- & Roger D. Cone
-
-
Outlook |
 Perspective: Tricks of the trade
Perspective: Tricks of the trade
Processed foods that dilute protein content subvert our appetite control systems, say Stephen J. Simpson and David Raubenheimer.
- Stephen J. Simpson
- & David Raubenheimer
-
Outlook |
 Neuroscience: Dissecting appetite
Neuroscience: Dissecting appetite
A slew of new technologies are helping to map the neural circuits that control when, and how much, we eat.
- Bijal P. Trivedi
-
Letter |
 An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger
An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger
The AgRP-expressing neurons in the arcuate nucleus drive food-seeking behaviours during caloric restriction; a mouse study of monosynaptic retrograde rabies spread and optogenetic circuit mapping reveals that these neurons are activated by input from hypothalamic paraventricular nucleus cells and their activation or inhibition can modulate feeding behaviour.
- Michael J. Krashes
- , Bhavik P. Shah
- & Bradford B. Lowell
-
Letter |
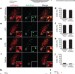 Genetic identification of a neural circuit that suppresses appetite
Genetic identification of a neural circuit that suppresses appetite
A neural circuit from the parabrachial nucleus to the central nucleus of the amygdala mediates appetite suppression.
- Matthew E. Carter
- , Marta E. Soden
- & Richard D. Palmiter
-
-
Outlook |
 Obesity: Heavy sleepers
Obesity: Heavy sleepers
A growing body of evidence shows that getting a good night's sleep plays an important role in regulating the body's metabolism.
- Brian Owens
-
Outlook |
 Obesity: Insensitive issue
Obesity: Insensitive issue
It is becoming clear that links between taste preferences and obesity go beyond simply having a sweet tooth.
- James Mitchell Crow
-
Research Highlights |
A pathway for feeding control
-
Editorial |
A whale of a story
A previously unknown sensory organ provides a lesson in coordination.
-
Letter |
 Discovery of a sensory organ that coordinates lunge feeding in rorqual whales
Discovery of a sensory organ that coordinates lunge feeding in rorqual whales
A newly discovered sensory organ in the jaws of rorqual whales is shown to have a crucial role during lunge feeding.
- Nicholas D. Pyenson
- , Jeremy A. Goldbogen
- & Robert E. Shadwick
-
Research Highlights |
 Jays plan meals in advance
Jays plan meals in advance
-
News & Views |
 Let them eat fat
Let them eat fat
A specialist neuron uses an intriguing process to help control the body's response to hunger. A lipid pathway involving the breakdown of cellular components regulates the expression of a neuropeptide that affects feeding and body weight.
- Scott M. Sternson

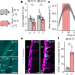 A brainstem–hypothalamus neuronal circuit reduces feeding upon heat exposure
A brainstem–hypothalamus neuronal circuit reduces feeding upon heat exposure Obesity
Obesity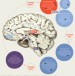 Structure: The anatomy of sleep
Structure: The anatomy of sleep