Article
|
Open Access
Featured
-
-
Article |
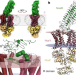 Molecular interplay of an assembly machinery for nitrous oxide reductase
Molecular interplay of an assembly machinery for nitrous oxide reductase
Cryo-electron microscopy structures of the bacterial protein machinery that is involved in the production and function of nitrous oxide provide insight into the assembly pathway of this enzyme and the mechanisms of copper transport.
- Christoph Müller
- , Lin Zhang
- & Oliver Einsle
-
Article
| Open Access Discovery, structure and mechanism of a tetraether lipid synthase
Discovery, structure and mechanism of a tetraether lipid synthase
In Methanocaldococcus jannaschii, a radical S-adenosylmethionine enzyme catalyses the formation of the biphytanyl chain.
- Cody T. Lloyd
- , David F. Iwig
- & Squire J. Booker
-
Article |
 Overcoming universal restrictions on metal selectivity by protein design
Overcoming universal restrictions on metal selectivity by protein design
An alternative approach to metalloprotein design shows that it is possible to overcome the restrictions of the Irving–Williams series and achieve both flexibility and specificity in the binding of metal ions.
- Tae Su Choi
- & F. Akif Tezcan
-
Article |
 Structure of a B12-dependent radical SAM enzyme in carbapenem biosynthesis
Structure of a B12-dependent radical SAM enzyme in carbapenem biosynthesis
X-ray crystal structures of TokK, a cobalamin- or B12-dependent radical SAM methylase, provide insight into how these enzymes use sequential radical-mediated methylations to assemble the C6 side chain of carbapenem antibiotics.
- Hayley L. Knox
- , Erica K. Sinner
- & Squire J. Booker
-
Article |
 Structural basis for tRNA methylthiolation by the radical SAM enzyme MiaB
Structural basis for tRNA methylthiolation by the radical SAM enzyme MiaB
Crystal structures reveal the catalytic mechanism through which the radical S-adenosylmethionine enzyme MiaB adds a methylthio group onto tRNA.
- Olga A. Esakova
- , Tyler L. Grove
- & Squire J. Booker
-
Article |
 Constructing protein polyhedra via orthogonal chemical interactions
Constructing protein polyhedra via orthogonal chemical interactions
An inorganic chemical approach to biomolecular design is used to generate ‘cages’ that can simultaneously promote symmetry and multiple modes of protein interactions.
- Eyal Golub
- , Rohit H. Subramanian
- & F. Akif Tezcan
-
Letter |
 A naturally occurring antiviral ribonucleotide encoded by the human genome
A naturally occurring antiviral ribonucleotide encoded by the human genome
Viperin inhibits the replication of various viruses by catalysing the conversion of CTP to ddhCTP, which is a unique nucleotide that functions as replication-chain terminator of RNA-dependent RNA polymerases.
- Anthony S. Gizzi
- , Tyler L. Grove
- & Steven C. Almo
-
Article |
 A B12-dependent radical SAM enzyme involved in oxetanocin A biosynthesis
A B12-dependent radical SAM enzyme involved in oxetanocin A biosynthesis
The biosynthesis of oxetanocin A involves OxsB, a B12-dependent S-adenosylmethionine radical enzyme, which catalyses an unusual ring contraction of a 2′-deoxyadenosine phosphate.
- Jennifer Bridwell-Rabb
- , Aoshu Zhong
- & Hung-wen Liu
-
Article |
 In-crystal reaction cycle of a toluene-bound diiron hydroxylase
In-crystal reaction cycle of a toluene-bound diiron hydroxylase
Crystal structures and DFT calculations suggest a possible mechanism for diiron enzyme arene hydroxylation.
- Justin F. Acheson
- , Lucas J. Bailey
- & Brian G. Fox
-
Letter |
 Charge-density analysis of an iron–sulfur protein at an ultra-high resolution of 0.48 Å
Charge-density analysis of an iron–sulfur protein at an ultra-high resolution of 0.48 Å
The ultra-high-resolution structure of the high-potential iron–sulfur protein at 0.48 Å, the highest-resolution X-ray crystal structure of a protein reported so far.
- Yu Hirano
- , Kazuki Takeda
- & Kunio Miki
-
Letter |
 The inner workings of the hydrazine synthase multiprotein complex
The inner workings of the hydrazine synthase multiprotein complex
Hydrazine is an intermediate in the process of anaerobic ammonium oxidation which has a major role in the Earth’s nitrogen cycle; the crystal structure of a hydrazine synthase enzyme provides insights into the mechanism of hydrazine synthesis.
- Andreas Dietl
- , Christina Ferousi
- & Thomas R. M. Barends
-
Letter |
 A four-helix bundle stores copper for methane oxidation
A four-helix bundle stores copper for methane oxidation
Csp1, a novel copper-binding protein that is exported from the cytosol of the methanotroph Methylosinus trichosporium OB3b and stores copper ions for particulate methane monooxygenase, is identified and characterized.
- Nicolas Vita
- , Semeli Platsaki
- & Christopher Dennison
-
Letter |
 The octahaem MccA is a haem c–copper sulfite reductase
The octahaem MccA is a haem c–copper sulfite reductase
Sulfite-reducing microbes couple the reduction of sulfite to the generation of a proton motive force that sustains organismic growth; here, two X-ray crystal structures are solved of MccA, a c-type cytochrome enzyme with eight haem groups that catalyses the six-electron reduction of sulfite to sulfide at a novel haem–copper active site.
- Bianca Hermann
- , Melanie Kern
- & Oliver Einsle
-
Letter |
 Hydrogens detected by subatomic resolution protein crystallography in a [NiFe] hydrogenase
Hydrogens detected by subatomic resolution protein crystallography in a [NiFe] hydrogenase
A sub-ångström-resolution X-ray crystal structure of [NiFe] hydrogenase, with direct detection of the products of the heterolytic splitting of dihydrogen into a hydride bridging the Ni and Fe and a proton attached to the sulphur of a cysteine ligand.
- Hideaki Ogata
- , Koji Nishikawa
- & Wolfgang Lubitz
-
Letter |
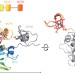 Structural insight into magnetochrome-mediated magnetite biomineralization
Structural insight into magnetochrome-mediated magnetite biomineralization
The magnetosome-associated protein mamP is an iron oxidase that reveals a unique arrangement of a self-plugged PDZ domain fused to two magnetochrome domains, defining a new class of c-type cytochrome exclusively found in magnetotactic bacteria.
- Marina I. Siponen
- , Pierre Legrand
- & David Pignol
-
Letter |
 PAAR-repeat proteins sharpen and diversify the type VI secretion system spike
PAAR-repeat proteins sharpen and diversify the type VI secretion system spike
An X-ray structure of bacterial type VI secretion system components reveals that PAAR family proteins bind at the tip of the VgrG spike, providing new insights into the mechanisms of type VI secretion; experiments using bacteria confirmed the importance of PAAR proteins.
- Mikhail M. Shneider
- , Sergey A. Buth
- & Petr G. Leiman
-
Letter |
 Elucidation of the Fe(iv)=O intermediate in the catalytic cycle of the halogenase SyrB2
Elucidation of the Fe(iv)=O intermediate in the catalytic cycle of the halogenase SyrB2
Synchrotron-based nuclear resonance vibrational spectroscopy is used to characterize the reactive Fe(iv)=O intermediate of the halogenase SyrB2; the substrate directs the orientation of this intermediate, presenting specific frontier molecular orbitals that can activate the selective halogenation.
- Shaun D. Wong
- , Martin Srnec
- & Edward I. Solomon
-
Letter |
 Biomimetic assembly and activation of [FeFe]-hydrogenases
Biomimetic assembly and activation of [FeFe]-hydrogenases
Three synthetic mimics of the di-iron centre in [FeFe]-hydrogenases are loaded onto the HydF protein and then transferred to apo-HydA1; full activation of HydA1 was achieved only with the HydF hybrid protein that contained the mimic with an azadithiolate bridge, confirming the presence of this ligand in the active site of native [FeFe]-hydrogenases.
- G. Berggren
- , A. Adamska
- & M. Fontecave
-
Letter |
 Control of substrate access to the active site in methane monooxygenase
Control of substrate access to the active site in methane monooxygenase
The crystal structure of the complex between the hydroxylase and regulatory component of soluble methane monooxygenase is presented, revealing how the latter component controls substrate access to the hydroxylase active site.
- Seung Jae Lee
- , Michael S. McCormick
- & Uhn-Soo Cho
-
Letter |
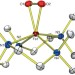 Structure and reactivity of a mononuclear non-haem iron(III)–peroxo complex
Structure and reactivity of a mononuclear non-haem iron(III)–peroxo complex
- Jaeheung Cho
- , Sujin Jeon
- & Wonwoo Nam
-
Letter |
 Microbial metalloproteomes are largely uncharacterized
Microbial metalloproteomes are largely uncharacterized
Metalloproteins are important in many biological processes, including respiration, photosynthesis and drug metabolism. Using genome sequences to predict the numbers and types of metal an organism uses is currently very challenging. These authors used a proteomics approach to identify and characterize a large number of a microorganism's metalloproteins on a genome-wide scale, and successfully separated and identified its cytoplasmic metalloproteins.
- Aleksandar Cvetkovic
- , Angeli Lal Menon
- & Michael W. W. Adams

 Enhanced rare-earth separation with a metal-sensitive lanmodulin dimer
Enhanced rare-earth separation with a metal-sensitive lanmodulin dimer