Abstract
Osteoarthritis (OA) is the most prevalent chronic degenerative joint disease with few treatment options. The pathogenesis of OA is characterized by sustained inflammation, oxidative stress and chondrocyte apoptosis that eventually lead to cartilage degradation and joint dysfunction. In the present study, we identified a synthetic triterpenoid CDDO-Im(1-[2-cyano-3,12-dioxooleana-1,9(11)–dien-28-oyl] imidazole) as an activator of Nrf2 (nuclear factor erythroid 2-related factor 2) that displayed strong anti-OA effects. We showed that CDDO-Im (20 nM) significantly alleviated TNF-α-induced apoptosis of primary human chondrocytes and extracellular matrix degradation. In a mouse OA model incurred by DMM (destabilization of medial meniscus), administration of CDDO-Im (2.5 mg/kg, ip, every other day for 8 weeks) effectively reduced knee joint cartilage erosion and serum levels of inflammatory cytokines IL-1β and IL-6. We revealed that CDDO-Im (20 nM) significantly enhanced autophagy activities in chondrocytes, whereas the autophagy inhibition by chloroquine (CQ, 50 μM) or 3-methyladenine (3-MA, 5 mM) abrogated the anti-apoptosis and chondroprotective effects of CDDO-Im in TNF-α-treated chondrocytes. Moreover, we confirmed that CDDO-Im (1–20 nM) dose-dependently activated Nrf2 pathway in TNF-α-treated chondrocytes, and its chondroprotective and autophagy-enhancing effects were significantly diminished when Nrf2 signaling was blocked by Nrf2 inhibitor ML385 (20 μM) or siRNA-mediated Nrf2 knockdown. Together, our results demonstrate that CDDO-Im exhibits prominent chondroprotective and anti-OA activities owing to its Nrf2 activation and autophagy-enhancing properties, which might provide new insights into the strategies of OA clinical prevention and treatment.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is a common and chronic degenerative joint disease that affects more than 250 million people worldwide, and imposes massive health and social burdens [1]. For patients with mild and early OA, some anti-OA drugs such as diacerein, glucosamine and nonsteroidal anti-inflammatory drugs (NSAIDs), are only palliative [2,3,4,5,6], and for patients with advanced OA, surgical replacement of the joint is the treatment of last resort [7]. Owing to the complex pathogenesis of OA, effective treatments to stop or reverse OA progression are lacking.
OA pathogenesis is characterized by sustained inflammation and oxidative stress, resulting in chondrocyte apoptosis and cartilage degeneration [8,9,10]. Recent studies have shown that the development of OA is significantly affected by dysfunction of autophagy, an essential cellular function that protects cells from apoptosis by degrading waste or damaged proteins and organelles [11, 12]. During OA progression, the autophagic activity of chondrocytes is defective, leading to increased cellular apoptosis that exacerbates OA pathologies [13]. In contrast, restoration of chondrocyte autophagy by various pharmacological agents has been shown to protect chondrocytes from apoptosis in cell and animal studies of OA [14].
It has been established that there is a close connection between oxidative stress and autophagy [15,16,17]. Oxidative stress, which results from an imbalance between cellular oxidant species production and antioxidant capability, is a pathological factor for OA [8]. Oxidative stress modifies and causes the accumulation of proteins, lipids, carbohydrates, and nucleic acids that need to be properly removed by autophagy to maintain cellular homeostasis. However, sustained oxidative stress might also adversely affect autophagy, which exacerbates oxidative damage. Nrf2 (nuclear factor erythroid-derived 2-related factor-2), as a transcription factor, essential for cellular redox and chondrocyte homeostasis, exerts its anti-oxidative stress functions mainly by binding to the antioxidant-response element on target gene promoters, thereby transactivating multiple antioxidant and detoxification enzymes [18, 19]. Nrf2 has been recognized as an effective target for OA intervention and effective strategies targeting Nrf2 might improve chondrocyte autophagy, which contributes to its anti-OA activities [10].
In this study, we explored the anti-OA effects of CDDO-Im, a synthetic triterpenoid (saponin compound) and an Nrf2 activator known to have anti-inflammatory and anti-oxidative stress properties [20], in cultured primary human chondrocytes and a mouse model of OA induced by DMM (destabilization of medial meniscus). We found that CDDO-Im possesses strong anti-OA activities, a considerable portion of which are attributed to its anti-apoptotic and autophagy-enhancing properties. These results have important clinical therapeutic implications.
Materials and methods
Isolation and culture of human primary chondrocytes
Human primary chondrocytes were isolated using a previously reported protocol [21]. Briefly, the human cartilage tissues from the relatively normal area collected from total knee arthroplasty patients were cut into pieces and digested in type II collagenase (0.2%) in a 37 °C shaker for 8 h. After filtration with a 100 μm cell strainer (Corning, USA), the suspension was centrifuged, and the isolated chondrocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 1 g/L glucose (Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco BRL, Grand Island, NY, USA) and 1% penicillin–streptomycin (Invitrogen, Carlsbad, CA, USA) at a 37 °C, 5% CO2 incubator. Second or third passage chondrocytes were used in cell experiments, in which, CDDO-Im (MCE, MedChem Express, New Jersey, USA) and ML385 (20 μM, MCE-MedChemExpress, New Jersey, USA) were added 2 h before TNF-α treatment (50 ng/mL, Peprotech, Rocky Hill, NJ, USA). For the autophagic flux assay, chondrocytes were pretreated with chloroquine (CQ, 50 μM, MCE-MedChemExpress, New Jersey, USA) or 3-methyladenine (3-MA, 5 mM, MCE-MedChemExpress, New Jersey, USA). All experiments were performed in triplicate and repeated three times.
Animals and model
Wild-type C57BL/6 male mice aged 12 weeks were obtained from the Model Animal Research Center of Nanjing University, and maintained in SPF (specific pathogen-free) environment with freely available water and standard mouse chow. The experimental mice were randomly divided into the following three groups: (1) sham operation (sham); (2) destabilization of medial meniscus (DMM); and (3) DMM with CDDO-Im treatment (2.5 mg/kg in DMSO-PBS, intraperitoneal injection, every other day, 8 weeks). DMM surgery was performed according to a previously established procedure [10]. The use of animals and the animal protocols were approved by the Ethics Committee and the Institutional Animal Care and Use Committee of Drum Tower Hospital, Nanjing University Medical School.
Western blot analysis
A whole Cell Lysis Assay Kit (KeyGEN, Nanjing, China) was used to isolate total proteins according to the manufacturer’s instructions. Protein concentrations were quantified using a BCA Protein Assay Kit (Beyotime, Nanjing, China). After electrophoresis on SDS-polyacrylamide gels, proteins were transferred to PVDF membranes and incubated with the following primary antibodies: anti-Collagen II (Boster, Wuhan, China); anti-Aggrecan, anti-P62/SQSTM1, anti-LC3 and anti-Nrf2 (Proteintech, Wuhan, China); anti-matrix metalloproteinase-13 (MMP13), anti-Adamts5, anti-Bax, anti-Bcl-2 and anti-ATG7 (Abcam, Cambridge, UK); anti-cleaved caspase 3, anti-Beclin1, anti-HO-1 and anti-NQO1 (Cell Signaling Technology, Boston, MA, USA); anti-β-actin (Abclonal, Wuhan, China) as the internal control. The membranes were further incubated with HRP-conjugated secondary antibodies (Proteintech, Wuhan, China), and an ECL Western Blotting Substrate Kit (Tanon, China) was then used to visualize signals. Finally, band densities were analyzed by ImageJ software [22].
Histological analysis
Eight weeks after the operation, the right knee joints of mice were surgically removed and fixed with 4% paraformaldehyde, decalcified with EDTA and embedded in paraffin. The joints were sliced into 5 μm sections and stained with safranin O/fast green (Sigma-Aldrich, St. Louis, USA), and the Osteoarthritis Research Society International (OARSI) scoring system was used to evaluate the degradation of joint cartilage [21].
Immunohistochemical (IHC) staining
The standard IHC protocol was used to stain the knee joint sections [23]. Briefly, the sections were incubated with primary antibodies against Collagen II and MMP13 at 4 °C overnight. The next day, the sections were incubated with HRP-conjugated secondary antibody, processed with 3,3’-diaminobenzidine (DAB) horseradish peroxidase color development kit (Beyotime, Nanjing, China) and counterstained with hematoxylin.
Immunofluorescence (IF) staining
Immunofluorescence staining was performed as previously described [24]. Briefly, chondrocytes were seeded on glass slides with various treatments, fixed with 4% paraformaldehyde for 15 min and permeated with 0.2% Triton-100 for 20 min. After washing, the slices were blocked with 10% goat serum (Boster, Wuhan, China) for 2 h and then incubated with primary antibodies against Collagen II (Boster, Wuhan, China); caspase 3, LC3 (CST, Boston, MA, USA); MMP13 (Abcam, Cambridge, UK) or Nrf2 (Proteintech, Wuhan, China) overnight at 4 °C. The next day, the slices were incubated with a fluorescein-conjugated secondary antibody (Proteintech, Wuhan, China) at room temperature for 1.5 h and nuclei were stained with DAPI (Santa Cruz, USA). A laser confocal microscope (Olympus, Tokyo, Japan) was used to acquire the images.
TdT-mediated dUTP Nick-End Labeling (TUNEL)
The DeadEndTM Fluorometric TUNEL System (Promega, Madison, Wisconsin, USA) was used to evaluate chondrocyte apoptosis. The cells on slides were fixed and permeabilized, and then incubated with reagents from the cell death detection kit according to the manufacturer’s protocol. Nuclei were stained with aqueous mounting medium containing DAPI (Santa Cruz, USA). Finally, an inverted fluorescence microscope (Olympus, Tokyo, Japan) was used to acquire images [23].
Cell viability assay
Cell Counting Kit-8 (CCK-8, MCE, New Jersey, USA) and trypan blue exclusion assays were used to evaluate cell growth and viability. For the CCK-8 assay, a total of 5000 human primary chondrocytes were seeded into 96-well plates overnight and treated with various concentrations of CDDO-Im (0, 1, 2, 5, 10, 20, 40 nM) for 24 h, followed by the addition of 10 μL of CCK8 solution and culture at 37 °C for 1.5 h. The OD values at 450 nm were measured with a microplate reader (MolecularDevices, California, USA). For the trypan blue exclusion assay, chondrocytes treated with various concentrations of CDDO-Im (0, 1, 2, 5, 10, 20, 40 nM) for 24 h were treated with Trypan blue dyes according to the manufacturer’s recommendation (Beyotime, Nanjing, China). The results are presented as the percentage of live cells to total cells [25].
Measurement of Nitric Oxide (NO) Production
NO (nitric oxide) production was detected with an NO Detection Assay kit (Beyotime, Nanjing, China) according to the manufacturer’s protocol. In short, 50 μL of Griess Reagent I was mixed with an equal amount of fresh medium and added to cells, followed by the addition of 50 μL Griess Reagent II. After mixing, the absorbance was measured at 540 nm using a microplate reader (Molecular Devices, California, USA).
Measurement of Reactive Oxygen Species (ROS)
A ROS Assay Kit (Beyotime, Nanjing, China) was used to measure cellular ROS levels according to the manufacturer’s protocol [26]. Briefly, chondrocytes subjected to various treatments were incubated with the DCFH-DA probe (10 μM) for 20 min and washed. ROS fluorescence signals were imaged with a confocal laser microscope (Olympus, Tokyo, Japan) at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Enzyme-linked Immunosorbent Assay (ELISA)
Mouse blood was collected and centrifuged at 3000 × g for 15 min. The upper plasma was aspirated and tested for IL-1β and IL-6 levels using respective mouse IL-1β or IL-6 ELISA kits (KeyGEN, Nanjing, China) according to the manufacturer’s instructions.
Small interfering RNA-mediated Nrf2 knockdown
Nrf2 knockdown in chondrocytes was achieved by small interfering RNA (siRNA). Initially, three siRNAs targeting the different regions of Nrf2 mRNA were tested and the one showing highest knockdown efficacy (siN2, 5′-GCCCAUUGAUGUUUCUGAUTT-3′/5′-AUCAGAAACAUCAAUGGGCCC-3′), and the control containing scrambled sequences were transfected into cells with FuGENE® HD Transfection Reagent (Promega, Madison, Wisconsin, USA) according to a standard protocol [24]. The siRNAs were synthesized by Hippobio (Huzhou, China) and the knockdown efficiency was verified by Western blot analysis.
Transmission electron microscopy
Chondrocytes were fixed with electron microscope fixation solution for 30 min, fixed with 2% osmium tetroxide for 2 h, and then stained with 0.5% uranyl acetate for 12 h. After dehydration, samples on slices were observed and images were acquired using a transmission electron microscope (Hitachi TEM system, Japan) [24].
Statistical analysis
Data are presented as the mean ± standard deviation (SD) of at least three independent experiments for cell assays or the mean ± SEM values for animal studies. Statistical analyses were performed with GraphPad Prism 7 software (La Jolla, CA, USA). Differences between group means were assessed with Student’s test/one-way ANOVA or two-way ANOVA. P < 0.05 was considered statistically significant.
Results
CDDO-Im attenuates TNF-α-induced chondrocyte apoptosis and ECM degradation
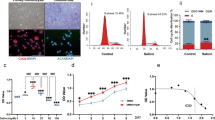
To determine the chondroprotective effects of CDDO-Im, we first treated primary human chondrocytes from the third passage with increasing concentrations of CDDO-Im (0, 1, 2, 5, 10, 20, 40 nM) for 24 h, and then performed cell growth and viability analyses. As shown in Fig. 1a, CDDO-Im treatment neither inhibited cell growth nor reduced cell viability at concentrations of up to 20 nM. Next, we performed Western blotting and found that the addition of TNF-α, a proinflammatory cytokine involved in OA pathogenesis [27,28,29,30], to chondrocytes increased the expression of the major metalloproteinases Adamts5 and MMP13, and decreased the levels of the cartilage matrix proteins Collagen II and Aggrecan; these changes were significantly reveresd by CDDO-Im treatment (Fig. 1b and c). To determine whether CDDO-Im affected chondrocyte apoptosis, we found that TNF-α treatment significantly increased the expression of the apoptotic proteins Bax and cleaved caspase 3 and reduced the expression of the antiapoptotic protein Bcl-2, and these levels were normalized by CDDO-Im treatment (Fig. 1d and e). In addition, immunofluorescence staining confirmed that CDDO-Im blocked the TNF-α-induced Collagen II reduction and induction of Caspase 3 (Fig. 1f–h). These data suggest that CDDO-Im reduces arthritic chondrocyte apoptosis and the associated ECM degradation.
a Cell viability assay. Primary chondrocytes were treated with various doses of CDDO-Im for 24 h. Cell viability was assayed by a cell counting kit (CCK-8, upper panel) and the trypan blue exclusion test (lower panel). Western blot analysis of protein expression and quantification of Collagen II, Aggrecan, MMP13 and Adamts5 (b and c) and cleaved caspase 3, Bax and Bcl-2 (d and e) in chondrocytes treated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) for 24 h. Representative images of immunofluorescence staining of Collagen II (f) or Caspase 3 (g). Chondrocytes were treated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) for 24 h. h Quantitative analysis of the data in Fig. 1f and g. The values are presented as the means ± SDs. *P < 0.05 versus control, #P < 0.05 versus TNF-α-treated chondrocytes.
CDDO-Im ameliorates DMM-induced cartilage erosion in mouse knee joints, chondrocyte apoptosis and OA pathologies
To further explore the therapeutic effects of CDDO-Im in vivo, we adopted a DMM animal model of OA and divided the experimental mice into the sham, DMM and CDDO-Im-treated DMM groups. We performed safranin O/fast green staining of mouse knee joint sections and found that DMM caused marked cartilage erosion and loss and increased OARSI scores, which were significantly reduced by CDDO-Im treatment (Fig. 2a). Moreover, CDDO-Im treatment significantly reduced the serum levels of IL-1β and IL-6 in DMM mice (Fig. 2b). IHC staining of the joint sections revealed reduced Collagen II and increased MMP13 in DMM mouse cartilage, which were alleviated by CDDO-Im treatment (Fig. 2c, d and f). Furthermore, CDDO-Im treatment significantly reduced the number of TUNEL-positive cells in DMM mouse cartilage (Fig. 2e and f). In addition, we monitored the changes in mouse body weight; histomorphology of the heart, liver, lung, spleen and kidney; and several parameters of liver (ALT, alanine aminotransferase; and AST, aspartate aminotransferase) and kidney (BUN, blood urea nitrogen; and Scr, serum creatinine) function and found no adverse alterations (Fig. 2g–i). Taken together, these data reveal that CDDO-Im inhibits DMM-induced cartilage erosion and chondrocyte apoptosis in vivo with very low, if any, cytotoxicity in major organs.
Mice were divided into Sham, DMM, and CDDO-Im-treated DMM groups (n = 6 per group, 8 weeks). a Representative images of safranin-O/fast green stained knee joint sections from sham, DMM and CDDO-Im-treated DMM mice. The arrows indicated the damaged cartilage areas. OARSI scores were on the right side. b The serum levels of IL-1β and IL-6 were measured by ELISA. c Representative immunohistochemical (IHC) staining of Collagen II and d MMP13. The arrows indicate damaged cartilage areas (c) or positively- stained chondrocytes (d). e Representative images of TUNEL staining of the sections. f Quantitative analysis of the data in Fig. 2c, d and e. g Representative H&E staining images of heart, liver, lung, spleen and kidney of sham, DMM and CDDO-Im-treated DMM mice. h Body weight changes of sham, DMM and CDDO-Im-treated DMM mice during the 8-week of experimental period. i Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN) and serum creatinine (Scr). The values are presented as means ± SEMs. *P < 0.05 versus sham mice, #P < 0.05 versus DMM mice.
CDDO-Im autophagy-sensitively regulates TNF-α-induced arthritic phenotypes of chondrocytes
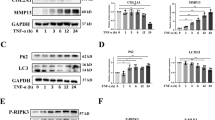
It is well known that OA pathogenesis is associated with impaired autophagic activity that might exacerbate chondrocyte apoptosis [31,32,33]. Therefore, we investigated whether CDDO-Im regulates autophagy in this process. We found that CDDO-Im treatment enhanced the expression of the autophagy-related proteins Beclin1, ATG7 and LC3 II and inhibited P62 by Western blotting (Fig. 3a and b) and by immunofluorescent staining of LC3 (Fig. 3c and d). Transmission electron microscopy (TEM) confirmed that CDDO-Im treatment increased the number of autophagy-associated vesicles (Fig. 3e). These results suggest that the antiapoptotic activity of CDDO-Im might be related to its beneficial regulation of autophagy.
a, b Western blot analysis of protein expression and quantification of ATG7, P62, Beclin1 and LC3 I/II in chondrocytes treated with control vehicle, CDDO-Im (20 nM), TNF-α (50 ng/mL) or CDDO-Im plus TNF-α for 24 h. The relative levels of LC3 are expressed as the ratio of LC3II/LC3I. c Representative images of Immunofluorescence-stained LC3 in chondrocytes treated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) for 6 h. d Quantitative analysis of the data in Fig. 3c. e Representative TEM images of chondrocytes treated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) for 6 h. The arrows point to autophagic vesicles. The values are presented as the means ± SDs. *P < 0.05 versus control, #P < 0.05 versus TNF-α-treated chondrocytes.
To confirm this idea, we repeated chondrocyte assays in the absence and presence of two autophagy inhibitors 3-MA and CQ, and found that CDDO-Im increased the expression levels of Beclin1 and ATG7 and the ratio of LC3II/LC3I and inhibited P62 expression (Fig. 4a and b); in addition, its ability to correct the levels of Collagen II, Aggrecan, MMP13 and Adamts5 (Fig. 4c and d) as well as those of cleaved caspase 3, Bax and Bcl-2 (Fig. 4e and f) were significantly reduced in the presence of the two autophagy inhibitors. These results indicate that CDDO-Im enhancement of autophagy contributes significantly to its chondroprotective activities.
a Representative Western blot of ATG7, P62, Beclin1 and LC3 in chondrocytes pretreated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) in the presence or absence of CQ (50 μM) or 3-MA (5 mM) for 24 h. b Quantitative analysis of the protein levels in Fig. 4a. c Representative Western blot of Collagen II, Aggrecan, MMP13 and Adamts5 in chondrocytes pretreated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) in the presence or absence of CQ (50 μM) or 3-MA (5 mM) for 24 h. d Quantitative analyses of the protein levels in Fig. 4c. e Representative Western blot of cleaved caspase 3, Bax and Bcl-2 in chondrocytes pretreated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) in the presence or absence of CQ (50 μM) or 3-MA (5 mM) for 24 h. f Quantitative analysis of the protein levels in Fig. 4e. The values are presented as the means ± SDs. *P < 0.05 versus control, #P < 0.05 versus TNF-α-treated chondrocytes, †P < 0.05 versus TNF-α and CDDO-Im treated chondrocytes.
CDDO-Im exerts chondroprotective effects by activating Nrf2
Since CDDO-Im is an Nrf2 activator and increases in Nrf2 or its downstream signaling by various pharmacological strategies are chondroprotective and exhibit anti-OA activities [10, 20, 34,35,36,37], we examined CDDO-Im-mediated regulation of Nrf2 and its relevance to chondroprotective functions. We proved that CDDO-Im significantly increased the Nrf2 protein abundance in chondrocytes (Fig. 5a and b), accompanied by increases in the levels of its downstream targets HO-1 and NQO1 in a concentration-dependent manner (Fig. 5c and d). Furthermore, immunofluorescence staining confirmed that CDDO-Im increased the nuclear translocation of Nrf2 (Fig. 5e and f), an indicator of Nrf2 activation.
a, b Western blot analysis of protein expression and quantification of Nrf2 in chondrocytes treated with control vehicle, CDDO-Im (20 nM), TNF-α (50 ng/mL) or CDDO-Im plus TNF-α for 24 h. c, d Western blot of protein expression and quantification of HO-1 and NQO1 in chondrocytes treated with various concentrations of CDDO-Im for 24 h. e Representative images of immunofluorescence-stained Nrf2 in chondrocytes treated with or without CDDO-Im. f Quantitative analysis of the data in Fig. 5e. The values are presented as the means ± SDs. *P < 0.05 versus control, #P < 0.05 versus TNF-α-treated chondrocytes.
Next, we used both pharmacological and gene-knockdown strategies to test whether CDDO-Im exerts its chondroprotective effects via its activation of Nrf2. ML385 is a potent and widely used pharmacological inhibitor of Nrf2. We found that ML385 significantly reduced the Nrf2 level in chondrocytes and partially blocked the chondroprotective effects of CDDO-Im on the expression of MMP13, Adamts5, Collagen II, Aggrecan, cleaved caspase 3, Bcl-2 and Bax (Fig. 6a and b). To further prove this, we tried three Nrf2 siRNAs and found that one siRNA (siN2) showed the highest Nrf2 knockdown efficacy (Fig. 6c and d). Transfection of siN2 into chondrocytes exhibited regulatory effects on CDDO-Im similar to those of ML385 (Fig. 6e and f).
a Representative Western blot of Nrf2, Collagen II, Aggrecan, MMP13, Adamts5, cleaved caspase 3, Bax and Bcl-2 in chondrocytes pretreated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) in the presence or absence of ML385 (20 μM) for 24 h. b Quantitative analysis of the protein levels in Fig. 6a. c Western blot analysis of Nrf2 protein expression in chondrocytes treated with three siRNAs. d Quantitative analysis of the protein levels in Fig. 6c. e Representative Western blotting of Collagen II, Aggrecan, MMP13, Adamts5, cleaved caspase 3, Bax and Bcl-2 in chondrocytes pretreated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) in the presence or absence of siN2 for 24 h. f Quantitative analysis of the protein levels in Fig. 6e. The values are presented as the means ± SDs. *P < 0.05 versus control, #P < 0.05 versus TNF-α-treated chondrocytes, †P < 0.05 versus TNF-α- and CDDO-Im-treated chondrocytes.
Furthermore, in chondrocytes, TNF-α-induced accumulation of NO (Fig. 7a), TUNEL positive cells (Fig. 7b), MMP13 (Fig. 7c and e) and cellular ROS (Fig. 7d and e) were significantly inhibited by CDDO-Im treatment, but ML385 blocked this inhibition (Fig. 7a–e). These data indicated that CDDO-Im-mediated activation of Nrf2 is critical for its chondroprotective effects.
a NO levels in chondrocyte culture medium pretreated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) in the presence or absence of ML385 (20 μM) for 24 h. b Representative images and quantification analyses of TUNEL staining in chondrocytes pretreated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) in the presence or absence of ML385 (20 μM) for 24 h. c Representative images of immunofluorescence staining of MMP13 in chondrocytes pretreated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) in the presence or absence of ML385 (20 μM) for 24 h. d Representative images of ROS in chondrocytes pretreated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) in the presence or absence of ML385 (20 μM) for 2 h. e Quantitative analysis of the data in Fig. 7c and d. The values are presented as the means ± SDs. *P < 0.05 versus control, #P < 0.05 versus TNF-α-treated chondrocytes, †P < 0.05 versus TNF-α and CDDO-Im treated chondrocytes.
CDDO-Im regulates chondrocyte autophagy by activating Nrf2
Finally, to determine whether the beneficial regulation of chondrocyte autophagy by CDDO-Im is due to its activation of Nrf2, we examined the effects of pharmacological Nrf2 inhibition and Nrf2 knockdown on CDDO-Im-mediated regulation of autophagy protein expression. The results showed that both Nrf2 inhibition by ML385 (Fig. 8a and b) and siRNA-mediated Nrf2 knockdown (Fig. 8c and d) significantly reduced the beneficial regulatory effects on the expression of the autophagy proteins ATG7 and Beclin1 and the LC3II/LC3I ratio. These results indicate that CDDO-Im-mediated activation of Nrf2 is essential for its regulation of autophagy in chondrocytes.
Western blotting and quantifications. Chondrocytes were treated with control, TNF-α (50 ng/mL), or CDDO-Im (20 nM) plus TNF-α in the presence or absence of ML385 (20 μM) for 24 h, and then the protein expression (a) and quantification (b) of ATG7, P62, Beclin1 and LC3 I/II were assessed. c Representative Western blot of Nrf2, ATG7, P62, Beclin1 and LC3 I/II in chondrocytes pretreated with CDDO-Im (20 nM) with or without TNF-α (50 ng/mL) in the presence of siControl (siCtrl) or siNrf2 for 24 h. d Quantitative analysis of the protein levels in Fig. 8c. The values are presented as the means ± SDs. *P < 0.05 versus control, #P < 0.05 versus TNF-α-treated chondrocytes, †P < 0.05 versus TNF-α- and CDDO-Im-treated chondrocytes.
Discussion
In this study, our explorations of the anti-OA functions of CDDO-Im led to several novel and important discoveries: (a) In the in vitro cell assay, CDDO-Im significantly reduced the apoptosis of arthritic chondrocytes and ECM degradation induced by TNF-α. (b) In the in vivo animal study, CDDO-Im alleviated articular cartilage damage caused by DMM and maintained the homeostasis of the cartilage matrix. (c) CDDO-Im effectively activates Nrf2 and its downstream signaling, which increases the antioxidative stress potential of chondrocytes. (d) CDDO-Im-mediated activation in Nrf2 enhances autophagy of chondrocytes, which contributes significantly to its antiapoptotic and anti-OA functions.
Nrf2 is a key regulator of cellular redox responses and an essential factor of OA, as Nrf2 knockout in mice exacerbates OA pathologies [10]. Nrf2 is ubiquitously expressed in almost all types of cells. Under normal conditions, Nrf2 is continuously expressed and degraded mediated by its inhibitor Keap1 via the ubiquitination pathway, but under stress conditions of various natures, Nrf2 is released from Keap1, enters the cell nucleus and transactivates a large spectrum of antioxidant and detoxifying molecules/enzymes [10, 34, 38]. In addition, Nrf2 beneficially regulates multiple signaling pathways and cellular processes that are critically involved in OA pathogenesis, such as chondrocyte proliferation, inflammation, autophagy and apoptosis. Activation of Nrf2 and its downstream signaling are protective against OA in many cell and animal disease models [18, 39, 40]. CDDO-Im-induced activation of Nrf2 beneficially regulates the undesirable expression of the presentative antioxidant enzymes HO-1 and NQO1; autophagy proteins ATG7, Beclin1 and LC3; apoptotic proteins Bcl-2 and Bax; ECM proteins Collagen II and Aggrecan, and catabolic enzymes MMP13 and Adamts5. CDDO-Im also inhibits chondrocyte apoptosis, reduces ROS and increases autophagosome numbers. In particular, we demonstrated that these protective functions occur in a Nrf2-dependent manner. These findings provide strong evidence suggesting that Nrf2 activation by CDDO-Im and possibly other similar chemicals or natural components may exert sufficient anti-OA activities.
One important observation of our study is that the antiapoptostic function of CDDO-Im in chondrocytes is related to its regulation of autophagy. Autophagy, especially the well-studied macroautophagy, is a conserved cellular function that protects cells from apoptosis by removing extra damaged cellular components and organelles [33, 41]. The initiation of autophagy starts with the formation of a double-layer membrane structue called phagophore, which wraps unwanted materials to form an autophagosome, which then fuses with a lysosome to form an autolysosome, ultimately leading to the degradation or reuse of intra-autophagosomal substances [42]. During autophagy, the cytosolic form of LC3 (LC3-I) is conjugated to phosphatidylethanolamine to form an LC3-phosphatidylethanolamine conjugate (LC3-II), which is recruited to autophagosomal membranes as an indication of autophagy activation [43]. In addition, a variety of autophagy-related proteins (ATGs) participate in the autophagy process. For example, P62 is a cargo-carrier that recognizes unwanted cellular waste products, ATG5 (autophagy-related 5) and ATG7 coordinate the expansion and extension of phagophores, and Beclin1 is essential for the induction of autophagosomes [12, 33]. Dysregulated and deficient autophagic activity has been observed during OA development [13, 14, 32, 44, 45]. Our results demonstrated that CDDO-Im enhances autophagic activity and corrects the altered expression of autophagy proteins, accompanied by a marked reduction in chondrocyte apoptosis, both in vitro and in vivo. However, the protective effects are blocked in the presence of the autophagy inhibitors CQ or 3-MA. These data support the hypothesis that the beneficial regulation of autophagy by CDDO-Im contributes significantly to its antiapoptotic and anti-OA functions.
CDDO-Im is a synthetic Nrf2 activator with a clearly-defined mechanism of action and very low, if any, toxicity even at high doses [46]. CDDO-Im can directly modify thiols of cysteine on the Nrf2 inhibitor Keap1, promoting its release from Keap1 and subsequent nuclear translocation/activation. In addition, CDDO-Im has a second reactive site (imidazolide) and can covalently bind to arginine and serine residues on other target proteins, such as glutathione S-transferase pi (GSTP) [47] and likely PPARγ (peroxisome proliferator-activated receptor-gamma) [48]. CDDO-Im’s therapeutic effects on lung injury, inflammatory diseases, chronic kidney disease and neurodegenerative disorders [46, 49,50,51,52] are mainly due to its upregulation of signaling by the master antioxidant transcription factor Nrf2 and subsequent activation of Nrf2-controlled antioxidant genes. Its beneficial regulation of PPARγ signaling might also contribute to its additive chondroprotective effects. Notably, clinical trials for cancer and cardiovascular disease have not shown equally positive results [46, 53]. Although our data support the hypothesis that CDDO-Im is chondroprotective and causes no noticeable adverse histomorphological alterations in other major organs, its derivatives, such as CDDO-Me, have shown the ability to increase adverse cardiovascular events [54]. Further studies are needed to clarify the safety profile of CDDO-Im.
Conclusion
In conclusion, we demonstrate for the first time that CDDO-Im has marked chondroprotective and anti-OA effects in vitro and in vivo. These effects are mainly achieved via its activation of Nrf2 and beneficial regulation of autophagic flux. Many OA patients on long-term treatment with NSAIDs might experience side effects such as liver and kidney injury [2]. CDDO-Im or its related derivatives may provide an alternative and effective strategy to treat OA and related joint diseases.
References
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–59.
Pelletier JP, Raynauld JP, Dorais M, Bessette L, Dokoupilova E, Morin F, et al. An international, multicentre, double-blind, randomized study (DISSCO): effect of diacerein vs celecoxib on symptoms in knee osteoarthritis. Rheumatology. 2020;59:3858–68.
Jain A, Mishra SK, Vuddanda PR, Singh SK, Singh R, Singh S. Targeting of diacerein loaded lipid nanoparticles to intra-articular cartilage using chondroitin sulfate as homing carrier for treatment of osteoarthritis in rats. Nanomedicine. 2014;10:1031–40.
Aghazadeh-Habashi A, Jamali F. The glucosamine controversy; a pharmacokinetic issue. J Pharm Pharm Sci. 2011;14:264–73.
Lin Y, Wu C, Wang X, Liu S, Zhao K, Kemper T, et al. Glucosamine promotes hepatitis B virus replication through its dual effects in suppressing autophagic degradation and inhibiting MTORC1 signaling. Autophagy. 2020;16:548–61.
Zeng C, Zhang W, Doherty M, Persson MSM, Mallen C, Swain S, et al. Initial analgesic prescriptions for osteoarthritis in the United Kingdom, 2000–2016. Rheumatology. 2021;60:147–59.
Hamilton DF, Beard DJ, Barker KL, Macfarlane GJ, Tuck CE, Stoddart A, et al. Targeting rehabilitation to improve outcomes after total knee arthroplasty in patients at risk of poor outcomes: randomised controlled trial. BMJ. 2020;371:m3576.
Ansari MY, Ahmad N, Haqqi TM. Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed Pharmacother. 2020;129:110452.
Marchev AS, Dimitrova PA, Burns AJ, Kostov RV, Dinkova-Kostova AT, Georgiev MI. Oxidative stress and chronic inflammation in osteoarthritis: can NRF2 counteract these partners in crime? Ann NY Acad Sci. 2017;1401:114–35.
Cai D, Yin S, Yang J, Jiang Q, Cao W. Histone deacetylase inhibition activates Nrf2 and protects against osteoarthritis. Arthritis Res Ther. 2015;17:269.
Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383:1564–76.
Yang H, Wen Y, Zhang M, Liu Q, Zhang H, Zhang J, et al. MTORC1 coordinates the autophagy and apoptosis signaling in articular chondrocytes in osteoarthritic temporomandibular joint. Autophagy. 2020;16:271–88.
Duan R, Xie H, Liu ZZ. The role of autophagy in osteoarthritis. Front Cell Dev Biol. 2020;8:608388.
Matsuzaki T, Alvarez-Garcia O, Mokuda S, Nagira K, Olmer M, Gamini R, et al. FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci Transl Med. 2018;10:428.
Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–88.
Gao Q. Oxidative stress and autophagy. Adv Exp Med Biol. 2019;1206:179–98.
Tang Z, Hu B, Zang F, Wang J, Zhang X, Chen H. Nrf2 drives oxidative stress-induced autophagy in nucleus pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect intervertebral disc from degeneration. Cell Death Dis. 2019;10:510.
Gao X, Jiang S, Du Z, Ke A, Liang Q, Li X. KLF2 protects against osteoarthritis by repressing oxidative response through activation of Nrf2/ARE signaling in vitro and in vivo. Oxid Med Cell Longev. 2019;2019:8564681.
Hou M, Zhang Y, Zhou X, Liu T, Yang H, Chen X, et al. Kartogenin prevents cartilage degradation and alleviates osteoarthritis progression in mice via the miR-146a/NRF2 axis. Cell Death Dis. 2021;12:483.
Xu D, Chen L, Chen X, Wen Y, Yu C, Yao J, et al. The triterpenoid CDDO-imidazolide ameliorates mouse liver ischemia-reperfusion injury through activating the Nrf2/HO-1 pathway enhanced autophagy. Cell Death Dis. 2017;8:e2983.
Zhu X, Chen F, Lu K, Wei A, Jiang Q, Cao W. PPARgamma preservation via promoter demethylation alleviates osteoarthritis in mice. Ann Rheum Dis. 2019;78:1420–9.
Lin J, Yu J, Zhao J, Zhang K, Zheng J, Wang J, et al. Fucoxanthin, a marine carotenoid, attenuates beta-amyloid oligomer-induced neurotoxicity possibly via regulating the PI3K/Akt and the ERK pathways in SH-SY5Y cells. Oxid Med Cell Longev. 2017;2017:6792543.
Liao Z, Luo R, Li G, Song Y, Zhan S, Zhao K, et al. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics. 2019;9:4084–100.
Li G, Song Y, Liao Z, Wang K, Luo R, Lu S, et al. Bone-derived mesenchymal stem cells alleviate compression-induced apoptosis of nucleus pulposus cells by N6 methyladenosine of autophagy. Cell Death Dis. 2020;11:103.
Qin T, Du R, Huang F, Yin S, Yang J, Qin S, et al. Sinomenine activation of Nrf2 signaling prevents hyperactive inflammation and kidney injury in a mouse model of obstructive nephropathy. Free Radic Biol Med. 2016;92:90–9.
Bayliak MM, Lushchak VI. Pleiotropic effects of alpha-ketoglutarate as a potential anti-ageing agent. Ageing Res Rev. 2021;66:101237.
Deng Y, Lu J, Li W, Wu A, Zhang X, Tong W, et al. Reciprocal inhibition of YAP/TAZ and NF-kappaB regulates osteoarthritic cartilage degradation. Nat Commun. 2018;9:4564.
Hosseinzadeh A, Kamrava SK, Joghataei MT, Darabi R, Shakeri-Zadeh A, Shahriari M, et al. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J Pineal Res. 2016;61:411–25.
Son YO, Park S, Kwak JS, Won Y, Choi WS, Rhee J, et al. Estrogen-related receptor gamma causes osteoarthritis by upregulating extracellular matrix-degrading enzymes. Nat Commun. 2017;8:2133.
Hui W, Litherland GJ, Elias MS, Kitson GI, Cawston TE, Rowan AD, et al. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann Rheum Dis. 2012;71:455–62.
Li YS, Zhang FJ, Zeng C, Luo W, Xiao WF, Gao SG, et al. Autophagy in osteoarthritis. Jt Bone Spine. 2016;83:143–8.
Duarte JH. Osteoarthritis: autophagy prevents age-related OA. Nat Rev Rheumatol. 2015;11:683.
Carames B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012;71:575–81.
Cuadrado A, Manda G, Hassan A, Alcaraz MJ, Barbas C, Daiber A, et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev. 2018;70:348–83.
Zhang F, Wang S, Zhang M, Weng Z, Li P, Gan Y, et al. Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke. 2012;43:1390–7.
Liu M, Reddy NM, Higbee EM, Potteti HR, Noel S, Racusen L, et al. The Nrf2 triterpenoid activator, CDDO-imidazolide, protects kidneys from ischemia-reperfusion injury in mice. Kidney Int. 2014;85:134–41.
Chen J, Li L, Zhou Y, Zhang J, Chen L. Gambogic acid ameliorates high glucose- and palmitic acid-induced inflammatory response in ARPE-19 cells via activating Nrf2 signaling pathway: ex vivo. Cell Stress Chaperones. 2021;26:367–75.
Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98:1169–203.
Yan Z, Qi W, Zhan J, Lin Z, Lin J, Xue X, et al. Activating Nrf2 signalling alleviates osteoarthritis development by inhibiting inflammasome activation. J Cell Mol Med. 2020;24:13046–57.
Khan NM, Haseeb A, Ansari MY, Devarapalli P, Haynie S, Haqqi TM. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human osteoarthritis chondrocytes. Free Radic Biol Med. 2017;106:288–301.
D’Adamo S, Cetrullo S, Guidotti S, Silvestri Y, Minguzzi M, Santi S, et al. Spermidine rescues the deregulated autophagic response to oxidative stress of osteoarthritic chondrocytes. Free Radic Biol Med. 2020;153:159–72.
Bao J, Chen Z, Xu L, Wu L, Xiong Y. Rapamycin protects chondrocytes against IL-18-induced apoptosis and ameliorates rat osteoarthritis. Aging. 2020;12:5152–67.
Nakatogawa H. Mechanisms governing autophagosome biogenesis. Nat Rev Mol Cell Biol. 2020;21:439–58.
Lotz MK, Carames B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat Rev Rheumatol. 2011;7:579–87.
Sacitharan PK, Bou-Gharios G, Edwards JR. SIRT1 directly activates autophagy in human chondrocytes. Cell Death Discov. 2020;6:41.
Mathis BJ, Cui T. CDDO and its role in chronic diseases. Adv Exp Med Biol. 2016;929:291–314.
Meng X, Waddington JC, Tailor A, Lister A, Hamlett J, Berry N, et al. CDDO-imidazolide targets multiple amino acid residues on the Nrf2 Adaptor, Keap1. J Med Chem. 2020;63:9965–76.
Chintharlapalli S, Papineni S, Konopleva M, Andreef M, Samudio I, Safe S. 2-Cyano-3,12-dioxoolean-1,9-dien-28-oic acid and related compounds inhibit growth of colon cancer cells through peroxisome proliferator-activated receptor gamma-dependent and -independent pathways. Mol Pharmacol. 2005;68:119–28.
Reddy NM, Suryanaraya V, Yates MS, Kleeberger SR, Hassoun PM, Yamamoto M, et al. The triterpenoid CDDO-imidazolide confers potent protection against hyperoxic acute lung injury in mice. Am J Respir Crit Care Med. 2009;180:867–74.
Lin X, Tawch S, Wong HT, Roy S, Gaudino S, Castillo P, et al. Nrf2 through aryl hydrocarbon receptor regulates IL-22 response in CD4+ T cells. J Immunol. 2021;206:1540–8.
Rush BM, Bondi CD, Stocker SD, Barry KM, Small SA, Ong J, et al. Genetic or pharmacologic Nrf2 activation increases proteinuria in chronic kidney disease in mice. Kidney Int. 2021;99:102–16.
Hou X, Liu H, Ping Y, Zhang F, Zhi L, Jiang X, et al. CDDO-Im exerts antidepressant-like effects via the Nrf2/ARE pathway in a rat model of post-stroke depression. Brain Res Bull. 2021;173:74–81.
Shanmugam MK, Dai X, Kumar AP, Tan BK, Sethi G, Bishayee A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: preclinical and clinical evidence. Cancer Lett. 2014;346:206–16.
de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–503.
Acknowledgements
This work was supported by the Key Program of NSFC (81730067), Major Project of NSFC (81991514), Jiangsu Provincial Key Medical Center Foundation, Jiangsu Provincial Medical Outstanding Talent Foundation, Jiangsu Provincial Medical Youth Talent Foundation and Jiangsu Provincial Key Medical Talent Foundation. The Fundamental Research Funds for the Central Universities (14380493, 14380494). Key project in Medical Science and Technology Development of Nanjing (ZKS18020). China Postdoctoral Science Foundation (Grant No 2019M661806). Thanks to Ph.D. Xiao-bo Zhu for his sincere guidance on experimental technology.
Author information
Authors and Affiliations
Contributions
JD (Jian Dong) and QJ designed the experimental plan. JD (Jian Dong) and GCL performed the surgical operations and wrote the manuscript. KJZ performed some cell experiments. XRC, JWL and JJL analyzed the experimental data. ZYL and ZZD collected cartilage tissue specimens. QJ and JD (Jin Dai) performed some surgical operations. WC designed the revision plan, rearranged the results and revised the writing of the manuscript. The final manuscript was approved by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Dong, J., Zhang, Kj., Li, Gc. et al. CDDO-Im ameliorates osteoarthritis and inhibits chondrocyte apoptosis in mice via enhancing Nrf2-dependent autophagy. Acta Pharmacol Sin 43, 1793–1802 (2022). https://doi.org/10.1038/s41401-021-00782-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-021-00782-6
Keywords
This article is cited by
-
Isoginkgetin-loaded reactive oxygen species scavenging nanoparticles ameliorate intervertebral disc degeneration via enhancing autophagy in nucleus pulposus cells
Journal of Nanobiotechnology (2023)
-
Mitochondrial proteotoxicity: implications and ubiquitin-dependent quality control mechanisms
Cellular and Molecular Life Sciences (2022)