Abstract
Four red polyketide pigments, uroleuconaphins A1 (1) and B1 (2) and their glucosides 3 and 4, were isolated from the red goldenrod aphid Uroleucon nigrotuberculatum. Although these red pigments exist only as glucosides 3 and 4 in the intact insect body, 3 and 4 convert instantly to aglycones 1 and 2 at death. Pigments 1 and 2 inhibited the growth of Lecanicillium sp. (Ascomycota: Cordycipitaceae) and 1, 2, and 3 were active against Conidiobolus obscurus (Entomophthoromycota; Entomophthorales); these fungal species are pathogenic. We therefore regard aphid pigments 1–4 as chemopreventive agents that aid in the resistance of infection by entomopathogenic fungi at the level of the individual aphid and/or at the species level.
Similar content being viewed by others
Introduction
Aphids have novel polyketide pigments [1,2,3,4,5,6,7,8,9], such as furanaphin [1, 2] uroleuconaphins [3,4,5,6], and viridaphin A1 glucoside [7, 8], which affect body color and predator–prey interactions [10]. Furthermore, since aphid pigments possess interesting biological activities such as cytotoxicity [1, 3, 6, 7] and antibacterial activities [7], we hypothesized that aphid pigments may protect the aphids from pathogenic species such as viruses, bacteria, and fungi [7]. As part of our continuing efforts toward testing this hypothesis and elucidating the biological functions of aphid pigments, we focused on two entomopathogenic fungi: Lecanicillium sp. (Ascomycota: Cordycipitaceae) and Conidiobolus obscurus (Entomophthoromycota: Entomophthorales). Although C. obscurus is a known pathogen of several aphids [11], no field observations of the infection of the red goldenrod aphid Uroleucon nigrotuberculatum, which has red-pigment uroleuconaphins [3], with this fungus have been reported. In contrast, infection of this aphid with Lecanicillium sp. is frequently observed; sometimes the fungus causes outbreaks of epizootic disease in populations of this aphid. We therefore evaluated the growth-inhibition activity of the red pigments of this aphid against both fungal species.
Results and discussion
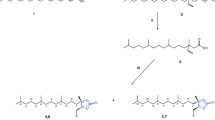
Red pigments 1 and 2 (Fig. 1a, c) were obtained from an ether extract of live U. nigrotuberculatum (Fig. 1d), as reported previously [3]. Surprisingly, we were unable to isolate 1 and 2 from an ethanol extract of the live aphids; instead, we obtained very polar red pigments whose structures were determined to be the β-D-glucosides of 1 and 2 (compounds 3 and 4, respectively; Fig. 1b, c). We established the structures of 3 and 4 as follows. The aphids (24.8 g) were crushed with a pestle and washed repeatedly with ethanol. The combined ethanol extract was concentrated and subjected to silica gel column chromatography (CHCl3: ethanol = 20: 1, 10: 1, 6: 1) and high performance liquid chromatography to yield molecules 3 (88 mg) and 4 (13 mg) as red solids. Since the density of the aphid’s body is ~1.06 g/mL (Experimental Section), the molar concentrations of 3 and 4 in the aphid body were approximately 5.2 and 0.8 mM, respectively.
The molecular formula of 3 was established as C36H38O16 by FABHRMS (m/z 749.2058, calculated for C36H38O16Na). The IR spectrum of 3 indicated the presence of hydroxy groups (3396 cm−1, br) and a conjugated carbonyl moiety (1668 cm−1). The 1H and 13C NMR data in MeOH-d4 are shown in Table 1. The presence of a β-glucopyranosyl linkage was suggested by the 1H NMR (methanol-d4) signals at δ 4.76 (J = 7.6 Hz); by the 13C NMR (methanol-d4) signals at δ 62.5, 71.2, 74.8, 77.2, 78.6, and 104.5. The HMBC correlation between the anomeric proton (δ H 4.76) of the glucose and C-9 (δ C 160.6) of the aglycone moiety revealed the position of the sugar linkage (Fig. 2). Furthermore, detailed NMR studies (1H NMR, 13C NMR, HMBC, HMQC) of 3 comparing with that of 1 [3] suggested that 3 was the monoglucoside of 1. This conclusion was confirmed by hydrolysis of the glycosyl linkages of 3, which was carried out under acidic conditions (0.5 M H2SO4 in dioxane-H2O) to yield D-glucose and under enzymatic conditions (naringinase) [12] to yield aglycone 1.
The molecular formula of 4 was established as C36H38O17 by FABHRMS (m/z 765.2007, calculated for C36H38O17Na). The structure of 4 was determined by detailed experiments in the same as 3 (Table 1 and Fig. 2).
Compounds 3 and 4 could not be obtained from the ethanol extracts of aphids that died due to infection with Lecanicillium sp. or that starved to death; only aglycones 1 and 2 were obtained. These findings suggest that 1) red pigments exist as glucosides 3 and 4 in the live aphid; 2) the aphid must contain a glucosidase that can quickly hydrolyze 3 and 4 to yield aglycones 1 and 2; 3) the ethanol used for pigment extraction must deactivate the glucosidase instantly to prevent the hydrolysis of glucosides 3 and 4 to aglycones 1 and 2; 4) the ether used for pigment extraction must be unable to deactivate the glucosidase to yield 1 and 2; and 5) although the glucosidase is inactive when the aphid is alive, it must become active instantly to produce aglycones 1 and 2 when death begins. Actually, we could confirm the generation of aglycone 1 using TLC, when a separately prepared pure sample of glucoside 3 was mixed with a residue of crushed aphid’s body, which was obtained after pigment extraction with ether (Fig. 3). Furthermore, the existence of some kind of glucosidase was suggested by earlier studies of pigments in the aphid Eriosoma lanigerum [13]. With these observations and conjectures in mind, we evaluated the growth inhibition activities of compounds 1–4 against the insect pathogens Lecanicillium sp. and C. obscurus, in order to elucidate the biological functions of these aphid pigments.
TLC using CHCl3:methanol (10:1) as solvent. a Glucoside 3. b Ether extract of a mixture of 3 and the aphid residue, which was prepared as follows. A few red aphids were crushed in ether, and the ether fraction was removed. Insoluble material in the ether (aphid residue) was washed with ether until the red color (compound 1 and 2) disappeared. A separately prepared, pure sample of glucoside 3 was added to the aphid residue in ether and mixed vigorously. TLC of this solution indicated that nearly all glucoside 3 was converted to aglycone 1. c Ether extract of the live aphid
The activities of each pigment were evaluated by measuring the inhibition of growth of fungal colonies on medium mixed with each pigment. Although glucoside 3 caused concentration-dependent growth inhibition of C. obscurus (Fig. 4a), glucoside 4 did not (Fig. 4b). As the molar concentration of 3 in the aphid body was quite high (5.2 mM as mentioned above), the aphid may be resistant to C. obscurus due to the presence of 3. Thus, aphid pigment 3 may be a chemopreventive agent that aids in the resistance of infection at the level of the individual aphid. Moreover, aglycones 1 and 2 also inhibited fungal growth in a concentration-dependent fashion (Fig. 4c, d) and the activities of them were more potent than that of 3. Although, the biological functions of these aglycones for the living aphid are unclear, they may function as a secondary system for resistance against the fungi. Further experiments are necessary.
On the other hand, although glucosides 3 and 4 were not active against Lecanicillium sp. (Fig. 5a, b), aglycones 1 and 2 inhibited growth at concentrations above 0.06 mM (Fig. 5c, d). These results indicate that this aphid is susceptible to infection by Lecanicillium sp. due to the inactivity of 3 and 4 against this fungus. However, when death by infection begins, aglycones 1 and 2 are produced and inhibit the growth of this ascomycete. Hence, we hypothesize that death of the individual aphid retards ascomycete growth on the aphid cadaver, lowering the rate of transmission to other individual aphids in the same colony and transmission to other aphid colonies. Although future investigation of the role of aglycones 1 and 2 in reducing horizontal transmission are necessary, we predict that the colony (at the species level) may be protected to some extent from infection with Lecanicillium sp. based on self-sacrifice of individual aphid.
Thus, aphid pigments 1–4 may be chemopreventive agents that aid in the resistance against infection by some entomopathogenic fungi. Hence, the aphid polyketide pigments assume the host defense system of individual level and/or species level of the aphid to increase the aphid’s inclusive fitness.
Several recent studies have shown that bacterial endosymbionts protect host aphids from insect pathogens [14,15,16]. However, the mechanism of this protection remains unclear. Here, we found that aphid pigments do not only affect body color, but also preserve the aphid from infection by insect pathogens. Recently, we reported that a colorless aphid, Cryptomyzus sp., harbors unpigmented polyketides with cytotoxic activity [17] We hypothesize that a large majority of aphids have polyketides (pigments or unpigmented molecules) that may function as chemopreventive agents. And we have another question. How did aphids acquire the enzymes required for polyketide synthesis (polyketide synthetase) through evolution? Did bacterial endosymbionts affect this evolutionary process? Further investigations are needed.
Experimental section
General experimental procedures
UV spectra were measured on a JASCO V-650 spectrophotometer. Optical rotations were measured on JASCO P-1030 polarimeters. IR spectra were measured on a JASCO FT/IR-410 spectrophotometer. 1H NMR spectra were acquired with Varian Unity-600 (600 MHz) and Varian Unity-500 (500 MHz) spectrophotometers with tetramethysilane as the internal standard in methanol-d4. 13C NMR spectra were measured on Varian Unity-600 (150 MHz) and Varian Unity-500 (125 MHz) spectrophotometers; chemical shifts were referenced to the residual solvent signal (methanol-d4: δC 49.0 ppm). Signal multiplicities were established via distortionless enhancement by polarization transfer. Mass spectra, including high-resolution mass spectra, were acquired with a JEOL JMS-700 spectrophotometer. The TLC analysis was performed with Merck pre-coated silica gel plates (60 F). Column chromatography was conducted with silica gel 60 N (Kanto Chemical Co. Inc, 63–210 μm). Preparative HPLC was carried out on a JASCO 880-PU pump unit equipped with an 880-UV detector (λ 254 nm) on a normal-phase column (Cosmosil 5SL-II, 28 × 250 mm); the column was eluted with CHCl3:methanol (7:1) at a flow rate of 10.0 mL/min.
Reagents
Naringinase (from Aspergillus niger) was purchased from Sigma-Aldrich Inc. Amberlite IRA96SB was purchased from Organo Co. Ltd. Ethanol, dimethyl sulfoxide (DMSO), and D-( + )-glucose were purchased from Wako Pure Chemical Industries, Ltd. Methanol and dichloromethane were purchased from Kanto Co. Ltd. Acetic anhydride, pyridine, n-butanol, ether, chloroform (for HPLC), methanol (for HPLC), toluene, ethyl acetate, dioxane, 0.5 M H2SO4 aq., 2 M HCl aq., Na2SO4, and phthalate pH standard solution (pH 4.01) were purchased from Nacalai Tesque Inc. Pyridine was used after distillation from CaH2. Agar was purchased from Kyokuto Pharmaceutical Industrial Co. Ltd. BactoTM Peptone was produced by Difco and purchased from Wako Pure Chemical Industries, Ltd.
Material. Insect
Red goldenrod aphids (U. nigrotuberculatum) were collected as they fed on the Canadian goldenrod (S. altissima) in Tokushima Prefecture, Japan, in May 2010. A voucher specimen for this aphid was prepared by Sigeru Takahashi and deposited in the Faculty of Agriculture of Utsunomiya University under code no. 10005.
Material. Entomopathogenic fungi
C. obscurus was isolated from a diseased aphid (species unknown) on Sonchus oleraceus at Tsukuba, Ibaraki, Japan on May 28, 2010, using Sabouraud’s dextrose agar supplemented with 1% yeast extract and subcultured on Nemoto’s medium (200 mL Sabouraud’s dextrose agar with one egg yolk). Lecanicillium sp. was isolated from a diseased U. nigrotuberculatum on S. altissima at Tsukuba, Ibaraki, Japan on June 24, 1996, using Sabouraud’s dextrose agar supplemented with 1% yeast extract and maintained in distilled water at 5 °C.
Extraction, isolation and spedtroscopic analyses of compounds 3 and 4
U. nigrotuberculatum was removed from S. altissima with a soft paintbrush and collected in a plastic Erlenmeyer flask equipped with a plastic funnel. The aphids (24.8 g) were crushed with a pestle and washed repeatedly with ethanol. The ethanol supernatant was separated by decanting. The residue was washed with several aliquots of fresh ethanol. The combined ethanol solutions were evaporated under reduced pressure to yield crude extracts (3.0 g), which were subjected to silica gel column chromatography using CHCl3:ethanol (20:1, 10:1, 6:1) and repeated HPLC using CHCl3:methanol (7:1) to yield red pigments 3 (88 mg) and 4 (13 mg). Aphids (0.435 g) were immersed in 6.0 mL of ethanol. Since the total volume of the mixture was 6.41 mL, aphid density is calculated to be ~1.06 g/mL.
Uroleuconaphin A1 glucoside (3): red amorphous powder; [α]21D + 770 (c 0.017, methanol); UV (methanol) λmax (log ε) 273 (4.27), 494 (3.64) nm; IR (neat) νmax 3396 (-OH), 1668, 1635, 1612, 1443, 1409, 1282 cm−1; NMR data were provided in Table 1; fast atom bombardment mass spectrometry (FAB-MS) m/z 749 [M + Na]+; FAB-HRMS m/z 749.2062 [M + Na]+ (calcd for C36H38O16Na, 749.2058).
Uroleuconaphin B1 glucoside (4); red amorphous powder; [α]19D + 995 (c 0.094, methanol); UV (methanol) λmax (log ε) 274 (4.27), 492 (3.62) nm; IR (neat) νmax 3364 (-OH), 1666, 1608, 1514, 1445, 1410, 1384 cm−1; NMR data were provided in Table 1; FAB-MS m/z 765 [M + Na]+; FAB-HRMS m/z 765.2001 [M + Na]+ (calcd for C36H38O17Na, 765.2007).
Determination of the sugar structure of molecule 3
A solution of 3 (9.2 mg) in 0.5 M H2SO4 aq. (1 mL) and dioxane (1 mL) was heated at 100 °C for 1.5 h. After cooling, the reaction mixture was neutralized with Amberlite IRA96SB. The resin was filtered off and washed with a small amount of water. n-Butanol (1 mL) was added to the filtrate and the mixture was evaporated. The residue was dissolved in H2O (3 mL) and the mixture was washed with ethyl acetate and separated. A small amount of n-butanol was added to the aqueous layer and the resulting solution was evaporated in vacuo into syrup. A pyridine (400 µL) solution of the syrup was treated with 100 µL (large excess) acetic anhydride at ambient temperature for 24 h. After the addition of 2 M HCl aq. (2 mL), the mixture was extracted with CH2Cl2 (three extractions with 3 mL) and the combined organic layers were dried over Na2SO4. After evaporation, the residue was purified via silica gel column chromatography (2 g, n-hexane:ethyl acetate = 3:1) to yield 1.4 mg of acetate as a colorless powder. This acetate was identified as D-glucose pentaacetate by comparison with a standard sample (1H NMR and [α]D value [α]20D + 35.7 (c 0.15, CHCl3)) and a pentaacetate of standard L-glucose ([α]22D −43.8 (c 2.1, CHCl3)).
Determination of the sugar structure of molecule 4
The sugar structure of molecule 4 was determined via the method described for molecule 3. Molecule 4 (5.4 mg) yielded 0.6 mg of D-glucose pentaacetate as a colorless powder ([α]20D + 26.8 (c 0.17, CHCl3)) and was compared with a pentaacetate of standard L-glucose ([α]22D −43.8 (c 2.1, CHCl3)).
Hydrolysis of glucosides 3 and 4 using naringinase
Toluene (10 mL) and naringinase (200 mg) were added to a mixture of 3 (6.4 mg) and phthalate buffer (pH 4.01, 15 mL); the resulting mixture was heated at 37 °C for 2 h. After cooling, the organic layer was separated. The aqueous layer was extracted with toluene (two extractions with 2 mL). The combined organic layers were washed with water (3 mL) and evaporated in vacuo to yield aglycone 1 quantitatively as a red solid. Aglycone 2 was obtained from 4 via the same procedure, with 84% yield. The structures of these compounds were established by comparison with samples that had been isolated previously from the ether extract of aphids.
Inhibition of the growth of entomopathogenic fungi
The activities of each pigment were evaluated by measuring the inhibition of growth of fungal colonies on medium mixed with each pigment. Pigments were dissolved in a very small volume of DMSO, diluted with distilled water, and sterilized by filtration through a 0.2-µm membrane filter, Sabouraud’s dextrose agar (10 g Bacto Peptone, 40 g dextrose, 15 g agar in 1000 mL distilled water) was autoclaved for 15 min and cooled to 50 °C. The pigment solution was mixed in the medium before the agar solidified to generate agar with the indicated final concentrations, poured into a 35 × 10 mm culture dish, and solidified. Sabouraud’s dextrose agar without pigments was used as a control.
Each fungal species was shaker-cultured at 25 °C for 5 days to obtain a mycelial suspension. Using a 2-mm platinum loop, the mycelial suspension was inoculated onto the center of a plate of agar containing pigment. Six replicates were performed for each concentration of each pigment. After inoculation, the dishes were sealed with Parafilm and incubated at 25 °C in total darkness for 10 days.
Two perpendicular diameters of the colony were measured with a caliper. The difference between the diameter of the colony without inoculum and the diameter of the colony with inoculum was determined.
References
Horikawa M, et al. Furanaphin: a novel naphtho[2,3-c]furan-4(1H)-one derivative from the aphid Aphis spiraecola Patch. Tetrahedron. 2004;60:1229–34.
Nishimura T, et al. A total synthesis of yellowish aphid pigment furanaphin through fries rearrangement assisted by boron trifluoride-acetic acid complex. Synlett. 2012;23:1789–92.
Horikawa M, et al. Uroleuconaphins A1 and B1, two red pigments from the aphid Uroleucon nigrotuberculatum (Olive). Tetrahedron. 2006;62:9072–6.
Horikawa M, Tanaka M, Kaku H, Nishii T, Tsunoda T. Uroleuconaphins A2a, A2b, B2a, and B2b: four yellowish pigments from the aphid Uroleucon nigrotuberculatum (Olive). Tetrahedron. 2008;64:5515–8.
Nishimura T, et al. Xanthouroleuconaphin: a yellowish pigment from the aphid Uroleucon nigrotuberculatum and its total synthesis. Tetrahedron. 2013;69:1808–14.
Suzuki S, et al. Pigments from uroleucon nigrotuberculatum induce apoptosis in hl60 human leukemia cells, implicating intracellular oxidative stress and activation of caspases. Biol Pharm Bull. 2006;29:2383–7.
Horikawa M, et al. Viridaphin A1 glucoside, a green pigment possessing cytotoxic and antibacterial activity from the aphid Megoura crassicauda. J Nat Prod. 2011;74:1812–16.
Tsuchida T, et al. Symbiotic bacterium modifies aphid body color. Science. 2010;330:1102–5.
Horikawa M, et al. Megouraphin glucosides: two yellowish pigments from the aphid megoura crassicauda. Heterocycles. 2012;85:95–101.
Losey JE, Ives AR, Harmon J, Ballantyne F, Brown C. A polymorphism maintained by opposite patterns of parasitism and predation. Nature. 1997;388:269–72.
Feng M-G, Johnson JB, Kish LP. Survey of entomopathogenic fungi naturally infecting cereal aphids (Homoptera: Aphididae) of irrigated grain crops in Southwestern Idaho. Environ Entomol. 1990;19:1534–42.
Matsuo Y, Akagi N, Hashimoto C, Tachikawa F, Mimaki Y. Steroidal glycosides from the bulbs of Bessera elegans and their cytotoxic activities. Phytochemistry. 2013;96:244–56.
Cameron DW, Sawyer WH, Trikojus VM. Colouring matters of the Aphidoidea XLII. purification and properties of the cyclising enzyme [protoaphin dehydratase (cyclising)] concerned with pigment transformations in the Woolly Aphid Eriosoma lanigerum Hausmann (Hemiptera: Insecta). Aust J Biol Sci. 1977;30:173–82.
Scarborough CL, Ferrari J, Godfray HCJ. Aphid protected from pathogen by endosymbiont. Science. 2005;310:1781.
Ferrari J, Darby AC, Daniell TJ, Godfray HCJ, Douglas AE. Linking the bacterial community in pea aphids with host-plant use and natural enemy resistance. Ecol Entomol. 2004;29:60–5.
Parker BJ, Spragg CJ, Altincicek B, Gerardo NM. Symbiont-mediated protection against fungal pathogens in Pea aphids: a role for pathogen specificity. Appl Environ Microbiol. 2013;79:2455–8.
Horikawa M, et al. Isolation and total syntheses of cytotoxic cryptolactones A1, A2, B1, and B2: α,β-unsaturated δ-lactones from a Cryptomyzus sp. aphid. J Nat Prod. 2014;77:2459–64.
Acknowledgements
This work was supported partially by a Grant-in-Aid for Scientific Research (C, 22590032 and 25460030) from MEXT (the Ministry of Education, Culture, Sports, Science and Technology of Japan). We are also thankful to the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2008–2012. We are grateful to M. Tanaka, Ph.D. (Faculty of Pharmaceutical Sciences, Tokushima Bunri University) for assistance with NMR spectroscopy. We thank Prof. S. Takahashi (Faculty of Agriculture, Utsunomiya University) for the identification of the aphid.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Horikawa, M., Shimazu, M., Aibe, M. et al. A role of uroleuconaphins, polyketide red pigments in aphid, as a chemopreventor in the host defense system against infection with entomopathogenic fungi. J Antibiot 71, 992–999 (2018). https://doi.org/10.1038/s41429-018-0093-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-018-0093-4