Abstract
Background
Pathological myopia (PM) is a major cause of worldwide blindness and represents a serious threat to eye health globally. Artificial intelligence (AI)-based methods are gaining traction in ophthalmology as highly sensitive and specific tools for screening and diagnosis of many eye diseases. However, there is currently a lack of high-quality evidence for their use in the diagnosis of PM.
Methods
A systematic review and meta-analysis of studies evaluating the diagnostic performance of AI-based tools in PM was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance. Five electronic databases were searched, results were assessed against the inclusion criteria and a quality assessment was conducted for included studies. Model sensitivity and specificity were pooled using the DerSimonian and Laird (random-effects) model. Subgroup analysis and meta-regression were performed.
Results
Of 1021 citations identified, 17 studies were included in the systematic review and 11 studies, evaluating 165,787 eyes, were included in the meta-analysis. The area under the summary receiver operator curve (SROC) was 0.9905. The pooled sensitivity was 95.9% [95.5%-96.2%], and the overall pooled specificity was 96.5% [96.3%-96.6%]. The pooled diagnostic odds ratio (DOR) for detection of PM was 841.26 [418.37–1691.61].
Conclusions
This systematic review and meta-analysis provides robust early evidence that AI-based, particularly deep-learning based, diagnostic tools are a highly specific and sensitive modality for the detection of PM. There is potential for such tools to be incorporated into ophthalmic public health screening programmes, particularly in resource-poor areas with a substantial prevalence of high myopia.
Similar content being viewed by others
Introduction
Myopia is one of the most common ocular conditions worldwide, with global prevalence predicted to increase from nearly 2.8 billion in the year 2020 to almost 5 billion—~49.8% of the world’s population—by the year 2050 [1]. High myopia, generally defined as a refractive error of −6 dioptres (D) or greater, can predispose individuals to sight-threatening sequelae such as glaucoma, cataract, retinal tears or detachment.
Pathological myopia (PM)—which occurs as a result of structural changes in the posterior segment of the eye due to significant axial elongation [2], is one of the major causes of irreversible visual impairment worldwide [2,3,4,5], affecting ~3% of the world population and as many as 50–70% of high myopics to some degree [6]. Reduced visual acuity due to PM can result in a considerable negative impact on quality of life, including social and emotional health and functional ability [7]. The potential economic impact of PM is also profound; a 2015 meta-analysis estimated the global productivity loss caused by myopic macular degeneration to be around US $6 billion worldwide [8].
The prevalence of myopia—the main risk factor for PM development—is extreme in many areas; in one study of 23,616 males in South Korea, 96.5% were myopic [9]. Evidence suggests that treatment failure in the correction of myopia is common and that long-term efficacy (of importance in reducing the risk of PM) is often limited [10]. As a result, a significant number of individuals, particularly in highly myopic populations, are still likely to develop PM, underscoring the need for cost-effective, reliable and scalable screening programmes to identify and monitor patients with PM and follow-up those at high risk of developing sight-threatening complications.
The diagnosis of PM is made qualitatively on fundal examination. Qualitative diagnosis can be subject to inter-observer variability between practitioners, and requires considerable clinical expertise to perform accurately. While optical coherence tomography (OCT) may also be used in the diagnosis of PM, retinal fundus photography remains the most widely accessible form of ophthalmic imaging for screening purposes. Hence, at present, retinal fundus images are likely to be the most useful modality to test the efficacy of new diagnostic aids and tools.
Several ‘classical’ features of PM may be visualised on fundus imaging, including a ‘tesselated’ atrophy of the retinal pigment epithelium, peripapillary atrophy, temporal flattening of the optic disc, lacquer cracks, posterior staphyloma and Fuch’s spot. A common cause of blindness in PM is myopic choroidal neovascularisation (CNV), which carries an extremely poor prognosis if untreated [2, 11].
Artificial intelligence (AI)-based diagnostic tools seek to reduce the need for expert interpretation by learning the features of normal and abnormal examples, with the aim of being able to label images autonomously. AI-aided diagnosis is no longer a novel concept in ophthalmology, and has been the subject of much evaluation for the screening of multiple ocular diseases, such as age-related macular degeneration, glaucoma, diabetic retinopathy, papilloedema and retinopathy of prematurity [12,13,14,15,16]. However, no systematic review or meta-analysis to date has sought to collate and evaluate the efficacy of these methods for the diagnosis of PM.
Therefore, the aim of the present systematic review and meta-analysis is to assess the diagnostic accuracy of artificial intelligence-based methods for the detection of PM using colour fundus images.
Methods
Study registration
This study was registered on PROSPERO with registration number CRD42022309830.
Search strategy and inclusion/exclusion criteria
According to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA), and using a search strategy designed by JP (Supplementary Table 1), the MEDLINE, EMBASE, CINAHL, Web of Science and IEEEExplore databases were searched. Reference lists of included studies were subsequently hand searched to identify additional studies that met the predefined inclusion criteria.
Studies were included if they reported the effectiveness of machine learning- or artificial intelligence-based detection algorithms in detecting PM; used indices such as area under the receiver-operator curve (AUROC), sensitivity and specificity to report on algorithm performance; evaluated colour fundus images; provided information about the size of the dataset and the reference standard; included a validation set at least 10% of the size of the training set; were in English and were published in a peer-reviewed journal. Reviews and conference abstracts were not included.
Study selection
Both reviewers independently screened all citations (and subsequently the full texts of included citations) for inclusion in a blinded process. Disagreements were resolved via mutual discussion, and details of these disagreements, along with final decisions on inclusion, are included in Supplementary Table 7.
Data extraction and quality assessment
A single reviewer (NT) extracted data from the included studies (Table 1, 2). Extracted data were directly checked against study data by a second reviewer (JP). Attempts were made to contact study authors for any missing information. Risk of bias assessment was performed using a novel, multi-step approach combining the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2 [17]) checklist and the Checklist for Artificial Intelligence in Medical Imaging (CLAIM [18]). Each reviewer performed quality assessment independently using both checklists. Areas of conflict were highlighted and are included in Supplementary Table 7.
Data analysis
2 × 2 contingency tables and statistics were generated using the best available data from study manuscripts (Table 1). Using the 2 × 2 contingency tables extracted for each study, sensitivity, specificity, positive predictive value, negative predictive value, negative likelihood ratio and positive likelihood ratio were calculated. Ungradable images were excluded from all analyses.
Where studies reported testing on both a primary, held-out test set and one or more external validation sets, the 2 × 2 contingency tables for each were combined to obtain a single contingency table. The combined contingency table was used in the meta-analysis. The contingency tables and model performance for the individual sets were recorded.
The presence of heterogeneity between included studies was assessed using the chi-square test and quantified by Higgins’ I2 [19], where significant heterogeneity was considered to be I2 ≥ 50%. The presence of threshold effects was assessed using the Spearman correlation coefficient between the logit of the true positive rate (TPR) and the false positive rate (FPR).
Where the heterogeneity among the included studies exceeded the stated threshold of 50%, measures were pooled using the DerSimonian and Laird (random-effects) model [20]. Where heterogeneity among the studies did not include the threshold, we planned to use a Mantel–Haenszel (fixed-effects) model [21].
Heterogeneity was investigated using subgroup analyses and meta-regression. Subgroup analyses were pre-specified in the study protocol, and included the type of model used and country of study/dataset origin. Sensitivity analyses were performed to assess the relationship between reviewer-assessed study quality and diagnostic accuracy and heterogeneity, in line with methods described by Higgins et al. [19]. Meta-regression was performed to analyse the relative effect of the size of the training set in each study.
The SROC curve was used to visually describe the relationship between the TPR and FPR in the included studies. The area under the SROC curve (AUROC) was calculated to demonstrate the probability of a classifier correctly classifying a randomly selected negative and positive example. Fagan nomograms were generated to describe the pre-test (prior) and post-test (posterior) probability for included studies, enabling direct translation of our results to the clinical setting.
Statistical analyses were performed using the Meta-Disc v1.4 software [22] and Review Manager 5.4 (The Cochrane Collaboration, 2020). Publication bias was assessed using the test described by Deeks et al. [23], implemented in R v4.1.3 using the meta package [24]. Fagan nomograms were generated in R v4.1.3 using the TeachingDemos package.
Results
Study selection
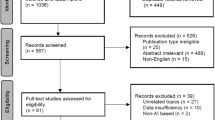
Databases were initially searched for studies from inception to 20/01/22; searches were re-run on 05/05/22 to identify newly published studies. 1021 citations were identified via the database search. After duplicate removal, 394 citations underwent abstract screening for eligibility. Thirty full text articles were screened, and met the inclusion criteria (Fig. 1). Five studies resulted in reviewer disagreements at the abstract screening stage, and are reported in Supplementary Table 7. There were no disagreements between reviewers at the full-text screening stage. Eleven studies were included in the meta-analysis [25,26,27,28,29,30,31,32,33,34,35], and a further six [35,36,37,38,39,40] in the systematic review.
Study quality assessment and publication bias
The results of the quality assessment are reported in Fig. 2, and Supplementary Tables 2 and 3. The quality of included studies was fair, with all studies achieving either moderate or high quality. There were no disagreements between reviewers on the quality of included studies. No articles were excluded on the basis of poor quality.
Using the quantitative funnel plot test described by Deeks et al. [23], it was determined that publication bias was unlikely for studies included in the meta-analysis (t = −1.53, p = 0.1607). The qualitative funnel plot is shown in Supplementary Fig. 1.
Study characteristics
All 17 included studies described and evaluated an AI-based method to identify pathological myopia from colour fundus images. Fourteen studies (82.4%) used convolutional neural network-based methods, with one of these studies also using a support vector machine (SVM) method and another using a k-nearest neighbours method for classification. Two studies (11.8%) [32, 38] used SVM for classification, and another [40] used joint sparse multi-task learning. Six studies (35.3%) used publicly available datasets, and two studies (11.8%) used some publicly available data. Demographic information, where reported, is presented in Supplementary Table 8. Eight studies (47.1%) used the META-PM definition for pathological myopia to guide annotation; the remainder did not use a formal definition. Eight studies (47.1%) included an external validation set. Seven studies (41.2%) compared the performance of the algorithm with that of one or more human graders, reported in Supplementary Table 10.
Fourteen studies (82.4%) used direct labelling by expert ophthalmologists or retinal specialists only as the reference standard. One study (5.9%) also used labelling by expert ophthalmologists and non-medical expert graders. One study (5.9%) used self-labelling methods for the training data and health record data for the test set. Two studies (11.8%) used health record data to generate labels.
Performance of AI in detection of pathological myopia
Eleven studies were included in the meta-analysis. The area under the SROC curve was 0.9905. The range of sensitivities reported was 0.850–1.000. The range of specificities reported was 0.900–1.000. All except three studies (27.3%) had a sensitivity and specificity above 0.900 [25, 30, 32]. The pooled sensitivity was 0.959 (95% CI 0.955–0.962, I2 97.1%). The pooled specificity was 0.965 (95% CI 0.963–0.966, I2 99.4%) (Fig. 3). Diagnostic odds ratios for included studies ranged from 51.00–22702.90 (Supplementary Table 6). The pooled diagnostic odds ratio (DOR) for detection of PM was 841.26 [95% CI 418.37–1691.61]. Fagan nomograms are used (Supplementary Fig. 4) to demonstrate the post-test probabilities of the included models, which ranged from 54.73% to 99.15%.
Forest plot of sensitivities (a) and specificities (b) and SROC curve for overall diagnostic performance (c) for all studies included in meta-analysis. CI indicates confidence interval; SROC, Summary receiver operating characteristic, AUC indicates area under the curve, SE indicates standard error; Q* indicates where sensitivity = specificity (intersection of diagonal with SROC curve).
Individual contingency tables for included studies are reported in Supplementary Table 5.
Performance comparison with human graders
7 of 11 studies included in the meta-analysis (63.6%) also reported a comparison with human graders, and are reported in Supplementary Table 10. Where reported, the mean sensitivities and specificities of the human graders ranged from 0.719–0.986 and 0.972–0.998, respectively. The corresponding proposed model sensitivities and specificities ranged from 0.908–0.991 and 0.925–1.000, respectively.
Heterogeneity analysis
Heterogeneity across the included studies was substantial (Fig. 3). We sought to explain this heterogeneity using threshold analysis, meta-regression and subgroup analysis.
Threshold analysis
A threshold analysis showed no significant effect (Spearman correlation coefficient = −0.112, p = 0.729), indicating that the heterogeneity observed between the included studies was unlikely to be due to a threshold effect.
Subgroup and sensitivity analyses
We performed subgroup analyses as pre-specified in the study protocol. Subgroup analysis by publication year was deemed inappropriate due to the recent publication date of all except one study.
A subgroup analysis by classification algorithm was performed. Two studies [25, 31] used an SVM-based model for classification and were considered as a separate subgroup to studies using CNNs. Fifteen studies used a CNN-based approach. Although the two non-CNN studies had a significantly lower pooled sensitivity (0.878 [95% CI 0.832–0.915], I2 = 0.0%) than the CNN studies (0.960 [95% CI 0.957–0.964], I2 = 97.4%), the pooled specificity was higher (0.999 [95% CI 0.998–1.000], I2 = 94.3%) than that of the CNN studies (0.961 [95% CI 0.959–0.962], I2 = 99.3%). The AUROC for the CNN studies was 0.9925; as there were only two non-CNN studies, AUROC was not estimable for this subgroup. Subgroup analysis by study country (studies originating from China vs. other regions) and showed lower sensitivities, specificities and AUROC for studies originating outside China (Supplementary Figs. 2 and 3). Heterogeneity generally remained high (I2 > 90%) in subgroups, limiting interpretation.
Sensitivity analyses based on reviewer-assessed study quality using QUADAS-2 (Fig. 2) showed a significantly lower AUROC (0.859), sensitivity (0.888 [95% CI 0.867–0.907], I2 = 16.8%) and specificity (0.959 [95% CI 0.944–0.971], I2 = 56.1%) for studies with a ‘moderate’ risk of bias than sensitivity and specificity for studies with a ‘low’ risk of bias (0.993, 0.966 [95% CI 0.962–0.969], I2 = 97.1%, 0.965 [95% CI 0.966–0.963], I2 = 99.6%).
Similarly, sensitivity was higher in studies with an external validation group (0.960 [95% CI 0.956–0.964], I2 = 98.1%) versus without (0.908 [95% CI 0.875–0.935], I2 = 72.5%) (Table 2). However, specificity was higher in studies without an external validation group (0.999 [95% CI 0.998–1.000], I2 = 85.6%, vs 0.961 [95% CI 0.959–0.962], I2 = 99.5%)
Meta-regression
Univariate meta-regression (Table 2) showed no statistically significant effect of training set size to account for the observed heterogeneity.
Discussion
To our knowledge, this is the first systematic review and meta-analysis to show that AI-based screening methods are highly sensitive and specific for the diagnosis of pathological myopia from fundus images. The area under the SROC curve was 0.9905, suggesting excellent classification performance of included models, as well as high diagnostic odds ratios (pooled DOR = 841.26) suggesting that included models generally possessed robust discriminative ability. However, the presence of unexplained statistical heterogeneity means that results should be interpreted with caution.
The majority of included studies used CNN-based models to detect PM. Three of the studies reported lower sensitivities [25, 30, 31], two of which used SVM to classify images, which would be expected to have lower discriminative ability than a deep learning-based approach. While one study employing SVM [37] was excluded from the quantitative analysis, it also showed a lower discriminative ability relative to CNN-based models.
Fagan nomograms were used to describe the likelihood of a patient having PM if the diagnostic tool deemed them to be a positive case (post-test probability); these demonstrated generally high (>85%) post-test probabilities, suggesting that diagnostic decisions made by AI-based tools may offer clinicians a high degree of clinical certainty.
The potential application of AI models to diagnose PM in practice is multi-fold. Firstly, these analyses show a universally high observed diagnostic accuracy of AI-based models, reinforcing their capability as powerful screening tools for PM.
Secondly, our analyses reveal that the diagnostic accuracy and discriminative capability of these models is comparable to that provided by ophthalmologists, highlighting their capability as decision aids. In all seven studies comparing algorithm and grader performance, algorithm sensitivity and specificity was comparable with that of human graders, offering support for the use of such algorithms as screening or triage tools. The use of AI in an assistive capacity may reduce uncertainty in diagnosis and reduce the variability in the diagnosis made between healthcare professionals [41].
Tools based on AI models can work with clinicians to guide triage and referral decisions in general practice or non-specialist centres with a high case burden, as described by De Fauw et al. [42]. This may have particular benefit for clinicians in training, or in regions with reduced incidence of PM, who may derive benefit from decision support in selecting cases of PM [43], or in areas with poor access to healthcare services [44].
Identification and close follow-up of patients with uncomplicated PM is crucial in enabling early management of treatable complications such as myopic CNV—for example with anti-VEGF therapy [45]—optimisation of visual acuity and stabilisation of progressive myopia. As novel treatments, such as stem cell therapy, gain prominence in the management of retinal disease [46], timely and targeted intervention is likely to be effective in reducing the public health burden of PM. Identification of PM can enable prognostication and careful multidisciplinary planning to mitigate the social, economic [47] and cognitive [48] impacts of progressive visual loss on the individual.
No included studies reported on the implementation of AI-based screening methods in clinical practice. While this review highlights the potential of AI to make highly specific and sensitive judgements on the presence or absence of pathological myopia, consideration must be given to generalisability across populations, explainability of screening decisions [49], and patient and healthcare professional acceptability [50].
Several existing reviews and meta-analyses examine the sensitivity and specificity of AI-based methods for the detection of other ophthalmic conditions from fundus photographs. Dong et al. [12] performed a systematic review and meta-analysis of AI algorithms used for the diagnosis of age-related macular degeneration, finding a pooled sensitivity and specificity of 0.88 and 0.90 respectively. Chaurasia et al. [13] demonstrated a pooled sensitivity and specificity of 0.92 and 0.94 respectively for the diagnosis of glaucoma from fundus images using AI algorithms. Finally, a meta-analysis by Wu et al. [51] observed a combined AUROC of 0.97–0.99 for the use of AI in diabetic retinopathy screening.
Examples are present in the literature of the use of AI for the detection of PM from optical coherence tomography (OCT) images [32, 52]. However, this was beyond the scope of this study. At the time of writing, OCT machines remain expensive, rendering them inaccessible in many regions. Fundus imaging, however, is widespread, and screening tools based on fundus photography may have a more significant clinical impact in less economically developed regions.
Future research assessing the diagnostic performance of AI models using OCT images for detection of PM may be useful in regions where the technology is widely used.
Strengths and weaknesses
The present study has several strengths. We used robust meta-analytic methodology to assess the pooled diagnostic accuracy of included studies, according to the PRISMA guidelines. Rigorous risk of bias assessment was performed, using two checklists, to identify studies which did not meet quality standards for inclusion, and heterogeneity and publication bias were comprehensively assessed using established methods.
Several limitations to this analysis are noted. First, there was significant statistical heterogeneity between the included studies, which was not entirely explained by analysis of threshold effects, study origin, training set size, study quality, the presence of external validation or the algorithm used.
A sensitivity analysis showed that studies deemed to have a moderate risk of bias reported a lower sensitivity and specificity, and studies without an external validation set reported a lower sensitivity—explaining some of the observed heterogeneity. Notably, there was considerable variation in the case-mix of positive to negative cases between studies, with PM prevalence varying from 3.78% in the test set used by Demir et al. to over 50% in other studies, potentially contributing to spectrum bias (where the discriminative ability of a diagnostic test varies according to the population in which it is used).
Meta-analyses of diagnostic accuracy of AI-based tools for the diagnosis of other ophthalmic conditions also demonstrate high unexplained heterogeneity [53], suggesting that variation in study and model design may have contributed to heterogeneity. However, it was not possible to assess the effects of variation in study design in detail (beyond subgroup analysis based on model type) due to the limited amount of methodological data provided in some studies. Regardless, the included studies spanned a diverse range of techniques and approaches, which—in the context of universally high diagnostic accuracy—suggests that AI-based techniques may possess excellent external validity for this purpose.
Secondly, several studies did not include an external validation set, limiting the generalisation ability of the algorithms reported. Ophthalmic patient populations are diverse, underlining the need for external validation, and meaning that the results of these studies should be interpreted with caution.
Third, there is a small degree of overlap between images contained within public datasets used in the studies, which could result in inflated estimates of diagnostic accuracy when comparing studies. Fourth, a retrospective study design was employed by all included studies. Retrospective design can lead to a selection bias, and have been shown to lead to overestimation of diagnostic accuracy [54]. Fifth, there was a preponderance of studies from East Asian countries, particularly China, in this review and meta-analysis. Planned subgroup analysis assessing studies by country of origin showed a small increase in sensitivity and specificity for studies originating from China, however it is unknown whether this is due to model factors or ethnic differences in fundus appearance [55].
Finally, due to the limitations of the available data, we did not include an analysis of model performance by severity of pathological myopia. It is therefore unknown how disease severity affects diagnostic accuracy in this case. Evidence suggests that use of severe cases may result in somewhat inflated estimates of diagnostic accuracy, and this would be an appropriate area for future research [54].
Conclusion
This systematic review and meta-analysis provide robust early evidence for the diagnostic accuracy of AI-based tools in the diagnosis of PM. Such tools are likely to have significant impact in screening, triage, assisted diagnosis and monitoring of myopic patients, and may enable earlier diagnosis and improve clinical outcomes for patients at risk of developing PM.
Summary
What was known before
-
Pathological myopia (PM) is an increasingly prevalent sight-threatening complication of high myopia, which requires close follow-up once identified to mitigate visual loss.
-
The identification of PM from fundus images generally relies upon qualitative diagnosis by a healthcare professional.
-
Artificial intelligence-based diagnostic tools have shown promise in ophthalmic diagnosis, but have not been specifically validated for use in PM.
What this study adds
-
Artificial intelligence-based algorithms are highly sensitive and specific for the diagnosis of PM from colour fundus images.
-
These tools may hold potential for use in resource-constrained healthcare settings with a high prevalence of PM.
Data availability
All data analysed within this study was obtained from publicly available studies and/or public anonymised datasets. Figures and graphs were generated using freely available software packages. All studies, datasets and software packages used are referenced in full within the study and the Supplementary Material.
References
World Health Organisation, Brien Holden Vision Institute. Report of the Joint World Health Organisation-Brien Holden Vision Institute global scientific meeting on myopia: the impact of myopia and high myopia. [Internet]. 2015. Available from: https://myopiainstitute.org/wp-content/uploads/2020/10/Myopia_report_020517.pdf. Accessed 30 Oct 2022.
Ohno-Matsui K. Pathologic Myopia. Asia Pac J Ophthalmol. 2016;5:415–23.
Rose KA, Morgan IG, Smith W, Burlutsky G, Mitchell P, Saw S-M. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol. 2008;126:527–30.
Varma R, Kim JS, Burkemper BS, Wen G, Torres M, Hsu C, et al. Prevalence and causes of visual impairment and blindness in chinese american adults: the chinese american eye study. JAMA Ophthalmol. 2016;134:785–93.
Cotter SA, Varma R, Ying-Lai M, Azen SP, Klein R, Los Angeles Latino Eye Study Group. Causes of low vision and blindness in adult Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2006;113:1574–82.
Takashima T, Yokoyama T, Futagami S, Ohno-Matsui K, Tanaka H, Tokoro T, et al. The quality of life in patients with pathologic myopia. Jpn J Ophthalmol. 2001;45:84–92.
Naidoo KS, Fricke TR, Frick KD, Jong M, Naduvilath TJ, Resnikoff S, et al. Potential lost productivity resulting from the global burden of myopia: systematic review, meta-analysis, and modeling. Ophthalmology. 2019;126:338–46.
Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CMG, Saw S-M, Verhoeven VJM, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159:877–83.e7.
Chen Y, Han X, Gordon I, Safi S, Lingham G, Evans J, et al. A systematic review of clinical practice guidelines for myopic macular degeneration. J Glob Health. 2022;12:04026.
Prousali E, Haidich A-B, Fontalis A, Ziakas N, Brazitikos P, Mataftsi A. Efficacy and safety of interventions to control myopia progression in children: an overview of systematic reviews and meta-analyses. BMC Ophthalmol. 2019;19:106.
Matsumura S, Ching-Yu C, Saw S-M. Global epidemiology of myopia. In: Ang M, Wong TY, editors. Updates on myopia: a clinical perspective. Singapore: Springer Singapore; 2020. 27–51.
Dong L, Yang Q, Zhang RH, Wei WB. Artificial intelligence for the detection of age-related macular degeneration in color fundus photographs: a systematic review and meta-analysis. EClinicalMedicine. 2021;35:100875.
Chaurasia AK, Greatbatch CJ, Hewitt AW. Diagnostic accuracy of artificial intelligence in glaucoma screening and clinical practice. J Glaucoma. 2022;31:285–99.
Islam MM, Yang H-C, Poly TN, Jian W-S. Jack Li Y-C. Deep learning algorithms for detection of diabetic retinopathy in retinal fundus photographs: a systematic review and meta-analysis. Comput Methods Programs Biomed. 2020;191:105320.
Milea D, Najjar RP, Zhubo J, Ting D, Vasseneix C, Xu X, et al. Artificial intelligence to detect papilledema from ocular fundus photographs. N Engl J Med. 2020;382:1687–95.
Scruggs BA, Chan RVP, Kalpathy-Cramer J, Chiang MF, Campbell JP. Artificial intelligence in retinopathy of prematurity diagnosis. Transl Vis Sci Technol. 2020;9:5.
University of Bristol. QUADAS-2 | Bristol Medical School: Population Health Sciences | University of Bristol [Internet]. Available from: https://www.bristol.ac.uk/population-health-sciences/projects/quadas/quadas-2/. Accessed 22 Oct 2022.
Mongan J, Moy L, Kahn CE. Checklist for artificial intelligence in medical imaging (CLAIM): a guide for authors and reviewers. Radio Artif Intell. 2020;2:e200029.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in metaanalyses. BMJ. 2003;327:557–60.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for metaanalysis of test accuracy data. BMC Med Res Methodol. 2006;6:31.
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–93.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60.
Demir F, Taşcı B. An Effective and robust approach based on R-CNN + LSTM model and NCAR feature selection for ophthalmological disease detection from fundus images. J Pers Med. 2021;11:1276.
Tan T-E, Anees A, Chen C, Li S, Xu X, Li Z, et al. Retinal photograph-based deep learning algorithms for myopia and a blockchain platform to facilitate artificial intelligence medical research: a retrospective multicohort study. Lancet Digit Health. 2021;3:e317–29.
Lu L, Ren P, Tang X, Yang M, Yuan M, Yu W, et al. AI-model for identifying pathologic myopia based on deep learning algorithms of myopic maculopathy classification and “plus” lesion detection in fundus images. Front Cell Dev Biol. 2021;9:719262.
Guo C, Yu M, Li J. Prediction of different eye diseases based on fundus photography via deep transfer learning. J Clin Med. 2021;10:5481.
Lu L, Zhou E, Yu W, Chen B, Ren P, Lu Q, et al. Development of deep learning-based detecting systems for pathologic myopia using retinal fundus images. Commun Biol. 2021;4:1225.
Du R, Xie S, Fang Y, Igarashi-Yokoi T, Moriyama M, Ogata S, et al. Deep learning approach for automated detection of myopic maculopathy and pathologic myopia in fundus images. Ophthalmol Retina. 2021;5:1235–44.
Li B, Chen H, Zhang B, Yuan M, Jin X, Lei B, et al. Development and evaluation of a deep learning model for the detection of multiple fundus diseases based on colour fundus photography. Br J Ophthalmol. 2022;106:1079–86.
Liu J, Wong D, Lim J, Tan N, Zhang Z, Li H, et al. Detection of pathological myopia by PAMELA with texture-based features through an SVM approach. J Healthc Eng. 2010;1:1–12.
Li J, Wang L, Gao Y, et al. Automated detection of myopic maculopathy from color fundus photographs using deep convolutional neural networks. Eye Vis. 2022;9:13. https://doi.org/10.1186/s40662-022-00285-3.
Tang J, Yuan M, Tian K, Wang Y, Wang D, Yang J, et al. An artificial-intelligence-based automated grading and lesions segmentation system for myopic maculopathy based on color fundus photographs. Transl Vis Sci Technol. 2022;11:16.
Cen L-P, Ji J, Lin J-W, Ju S-T, Lin H-J, Li T-P, et al. Automatic detection of 39 fundus diseases and conditions in retinal photographs using deep neural networks. Nat Commun. 2021;12(Aug):4828.
Himami ZR, Bustamam A, Anki P. Deep learning in image classification using dense networks and residual networks for pathologic myopia detection. In: Proc. international conference on artificial intelligence and big data analytics. IEEE; 2021. p. 1–6.
Hemelings R, Elen B, Blaschko MB, Jacob J, Stalmans I, De Boever P. Pathological myopia classification with simultaneous lesion segmentation using deep learning. Comput Methods Programs Biomed. 2021;199:105920.
Zhang Z, Xu Y, Liu J, Wong DWK, Kwoh CK, Saw S-M, et al. Automatic diagnosis of pathological myopia from heterogeneous biomedical data. PLoS ONE. 2013;8:e65736.
Rauf N, Gilani SO, Waris A. Automatic detection of pathological myopia using machine learning. Sci Rep. 2021;11:16570.
Chen X, Xu Y, Yin F, Zhang Z, Wong DWK, Wong TY, et al. Multiple ocular diseases detection based on joint sparse multi-task learning. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:5260–3.
Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44–56.
De Fauw J, Ledsam JR, Romera-Paredes B, Nikolov S, Tomasev N, Blackwell S, et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med. 2018;24:1342–50.
Fang Z, Xu Z, He X, Han W. Artificial intelligence-based pathologic myopia identification system in the ophthalmology residency training program. Front Cell Dev Biol. 2022;10:1053079.
Wahl B, Cossy-Gantner A, Germann S, Schwalbe NR. Artificial intelligence (AI) and global health: how can AI contribute to health in resource-poor settings? BMJ Glob Health. 2018;3:e000798.
Tan CS, Sadda SR. Anti-vascular endothelial growth factor therapy for the treatment of myopic choroidal neovascularization. Clin Ophthalmol. 2017;11:1741–6.
Zarbin M. Cell-based therapy for retinal disease: the new frontier. Methods Mol Biol. 2019;1834:367–81.
Chua SYL, Foster PJ. The economic and societal impact of myopia and high myopia. In: Ang M, Wong TY, editors. Updates on myopia: a clinical perspective. Singapore: Springer Singapore; 2020. 53–63.
Ong S-Y, Ikram MK, Haaland BA, Cheng C-Y, Saw S-M, Wong TY, et al. Myopia and cognitive dysfunction: the singapore malay eye study. Investig Ophthalmol Vis Sci. 2013;54:799–803.
Kelly CJ, Karthikesalingam A, Suleyman M, Corrado G, King D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 2019;17:195.
Singh RP, Hom GL, Abramoff MD, Campbell JP, Chiang MF, Task AAO. Force on artificial intelligence. Current challenges and barriers to real-world artificial intelligence adoption for the healthcare system, provider, and the patient. Transl Vis Sci Technol. 2020;9:45.
Wu J-H, Liu TYA, Hsu W-T, Ho JH-C, Lee C-C. Performance and limitation of machine learning algorithms for diabetic retinopathy screening: meta-analysis. J Med Internet Res. 2021;23:e23863.
Ye X, Wang J, Chen Y, Lv Z, He S, Mao J, et al. Automatic screening and identifying myopic maculopathy on optical coherence tomography images using deep learning. Transl Vis Sci Technol. 2021;10:10.
Aggarwal R, Sounderajah V, Martin G, Ting DSW, Karthikesalingam A, King D, et al. Diagnostic accuracy of deep learning in medical imaging: a systematic review and metaanalysis. npj Digital Med. 2021;4:65.
Rutjes AWS, Reitsma JB, Di Nisio M, Smidt N, van Rijn JC, Bossuyt PMM. Evidence of bias and variation in diagnostic accuracy studies. CMAJ. 2006;174:469–76.
Wolf-Schnurrbusch UEK, Röösli N, Weyermann E, Heldner MR, Höhne K, Wolf S. Ethnic differences in macular pigment density and distribution. Investig Ophthalmol Vis Sci. 2007;48:3783–7.
Diabetic retinopathy detection [Internet]. Kaggle. Available from: https://www.kaggle.com/c/diabetic-retinopathy-detection/data. Accessed 5 Nov 2022.
Guillaume PATRY GGAUTHIER. Messidor-2 [Internet]. ADCIS. 2022. Available from: https://www.adcis.net/en/third-party/messidor2/. Accessed 5 Nov 2022.
Fu H, Li F, Orlando JI, Bogunović H, Sun X, Liao J, et al PALM: PAthoLogic Myopia Challenge [Internet]. IEEE Dataport; (2019). Available from: https://doi.org/10.21227/55pk-8z03.
Odir-2019—Grand Challenge [Internet]. grand. Available from: https://odir2019.grand-challenge.org/dataset/. Accessed 5 Nov 2022.
Foong A, Saw S, Loo J, Shen S, Loon SC, Rosman M, et al. Rationale and methodology for a population-based study of eye diseases in malay people: the Singapore Malay Eye Study (SiMES). Ophthalmic Epidemiol. 2007;14:25–35. https://doi.org/10.1080/09286580600878844.
Lavanya R, Jeganathan V, Zheng Y, Raju P, Cheung N, Shyong Tai E, et al. Methodology of the Singapore Indian Chinese Cohort (SICC) eye study: quantifying ethnic variations in the epidemiology of eye diseases in Asians. Ophthalmic Epidemiol. 2009;16:325–36. https://doi.org/10.3109/09286580903144738.
Linchundan. 1000 fundus images with 39 categories [Internet]. Kaggle. 2019. Available from: https://www.kaggle.com/linchundan/fundusimage1000. Accessed 5 Nov 2022.
Li X, Hu X, Qi X, Yu L, Zhao W, Heng P-A, et al. Rotation-oriented collaborative self-supervised learning for retinal disease diagnosis. IEEE Trans Med Imaging. 2021;40:2284–94.
Acknowledgements
The authors would like to thank Emily Patterson and Megan Vaughan for their kind review of the manuscript.
Author information
Authors and Affiliations
Contributions
Both JP and NT are joint first authors, and developed the concept for the study. NT led the screening and article review process, and JP led the analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the articleʼs Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the articleʼs Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prashar, J., Tay, N. Performance of artificial intelligence for the detection of pathological myopia from colour fundus images: a systematic review and meta-analysis. Eye 38, 303–314 (2024). https://doi.org/10.1038/s41433-023-02680-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02680-z