Abstract
Partially saturated, fluorine-containing rings are ubiquitous across the drug discovery spectrum. This capitalises upon the biological significance of the native structure and the physicochemical advantages conferred by fluorination. Motivated by the significance of aryl tetralins in bioactive small molecules, a reaction cascade has been validated to generate novel gem-difluorinated isosteres from 1,3-diaryl cyclobutanols in a single operation. Under the Brønsted acidity of the catalysis conditions, an acid-catalysed unmasking/fluorination sequence generates a homoallylic fluoride in situ. This species serves as the substrate for an I(I)/I(III) cycle and is processed, via a phenonium ion rearrangement, to an (isolable) 1,3,3-trifluoride. A final C(sp3)-F bond activation event, enabled by HFIP, forges the difluorinated tetralin scaffold. The cascade is highly modular, enabling the intermediates to be intercepted: this provides an expansive platform for the generation of structural diversity.
Similar content being viewed by others
Introduction
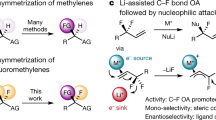
The development of enabling technologies to generate fluorinated analogues of bioactive leads is a core research endeavor in contemporary catalysis1,2,3,4,5,6,7,8,9,10,11. This reflects the clinical importance of fluorination in reconciling physicochemical limitations with promising bioactivity profiles12,13. Diversifying the existing drug discovery module portfolio, in a sustainable and atom economic fashion14,15, has created a fertile ground to advance main group catalysis-based fluorination reactions. In particular, the I(I)/I(III) catalysis manifold16,17,18,19 has proven to be well-suited to this challenge on account of the inexpensive nature of the aryl iodide organocatalyst and the availability of simple organic oxidants and amine•HF reagents20,21,22,23. More recently, efforts to leverage the intrinsic acidity of the catalysis conditions in multi-step processes have come into focus24. Compelling arguments to pursue this research line include (i) circumventing substrate limitations through direct in situ generation, and (ii) the possibility to increase structural complexity in post-catalysis events. Motivated by the prominence of aryl tetralins and fluorinated cycloalkyl motifs in bioactive small molecule discovery (Fig. 1A)25,26, it was envisaged that this conceptual framework may be advantageous in generating fluorinated analogues. A one-pot cascade was envisaged in which the direct conversion of 1,3-diarylcyclobutanols to gem-difluoro tetralins might be achieved via the merger of Brønsted acid activation and I(I)/I(III) catalysis in a single operation (Fig. 1B). Specifically, it was envisioned that, under the acidic I(I)/I(III) catalysis fluorination conditions with HF, dehydration of the cyclobutanol (1) would rupture the ring (I ↔ II) and generate the homoallylic fluoride III in situ. This would ultimately complement the elegant studies by Lanke and Marek on the generation of trans−1,2-disubstituted homoallylic fluorides, via cyclopropylcarbinyl/bicyclobutonium cation formation, from cyclopropyl carbinols27. In addition to the well-documented involvement of cyclopropylcarbinyl/bicyclobutonium cations27,28,29, direct fluorination of the proposed cyclobutonium species I would also account for the generation of homoallylic fluoride III. It is pertinent to note that Li and co-workers observed disparate reactivity when exposing aryl-substituted methylene cyclopropanes to Selectfluor® and HF: this triggered a Wagner-Meerwein rearrangement to generate difluorocyclobutanes30. In our postulated reaction sequence, the process of in situ substrate formation forges a 1,1-disubstituted alkene: this can then engage in an I(I)/I(III) catalysis cycle31, enabling a regioselective 1,1-difluorination32,33,34,35,36,37,38,39 to occur via a precedented phenonium ion rearrangement40.
Inspired by the seminal work of Paquin and co-workers on HFIP-enabled activation of benzylic C-F bonds41,42, it was reasoned that a Friedel-Crafts-type cyclisation would furnish the target scaffold and demonstrate the value of integrating I(I)/I(III) catalysis in cascade reaction design. If successful, this would logically lead to an exploration of alternative exogenous nucleophiles, thereby further enhancing the modularity of the paradigm (Fig. 1B, bottom).
Results and discussion
Transformation of cyclobutanols (1) to trifluorides (2)
To validate the working hypothesis (Fig. 1B), a process of reaction deconstruction43 was initiated beginning with the formation of the key trifluoride (major−1c → 2c, Table 1). It seemed likely that the Brønsted acidity of the conditions would facilitate dehydration of the 1,3-diarylcyclobutanol major−1c with concomitant rearrangement, via the transient cation (I ↔ II), to generate the allylic fluoride motif (III). This species would then be intercepted by the I(I)/I(III) catalysis cycle, initiating a phenonium ion rearrangement/fluorination sequence to enable three C(sp3)-F bonds to be forged in a single operation.

To explore the feasibility of this process, substrate major-1c (cis) was exposed to amine:HF (1:5) (see General Procedure D in the ESI for further information on the preparation of amine:HF mixtures) in CHCl3 together with p-TolI (20 mol%) and Selectfluor® (1.5 eq.) as the catalyst and oxidant, respectively44. It is pertinent to mention that crystals of the trans-isomer of the starting cyclobutanol (minor-1c, CCDC 2239011) could be isolated and subjected to X-ray diffraction analysis (see Table 1 legend). After 18 h at ambient temperature, product 2c was formed in 74% yield thereby providing confidence in the reaction design (entry 1).
Cognisant of the impact Brønsted acidity has on the regioselectivity of I(I)/I(III)-catalysed fluorination reactions45, the amine:HF ratio was adjusted stepwise to 1:6.5 (entries 2–4): this allowed the optimal ratio of 1:5.5 to be identified (entry 2, 81% yield by 19F NMR, 74% isolated yield). Neither changes of solvent (entries 5–8) nor catalyst (entries 9 and 10) led to further enhancements in efficiency. Moreover, a reduction in catalyst loading manifested itself in lower yield (entry 11).
Control reactions in the absence of the HF source (entry 12), p-TolI organocatalyst (entry 13), or oxidant (entry 14) were unsuccessful, and support the postulated I(I)/I(III) manifold. To explore the impact of the relative stereochemistry of the substrate on reaction efficiency, a diastereomeric mixture of 1c enriched with the minor trans-isomer (minor−1c) was exposed to the standard conditions. As expected, comparable outcomes were observed (entry 2 vs. entry 15).
Having established an optimised catalysis protocol, a series of 1,3,3-trifluorobutanes 2 were prepared from 1,3-diarylcyclobutanols with electronically modulated aryl rings (Fig. 2). During the course of this study, a general trend was noted: for electron rich systems, the amine:HF ratio had to be lowered for optimal efficiency, whereas electron deficient systems required higher amine:HF ratios. For the electron rich phenyl or fluorophenyl substituents, compounds 2a and 2b were obtained in 57% and 50%, respectively. Introducing halogens such as chlorine and bromine allowed the formation of the desired trifluorinated products 2c and 2d in higher yields (74% and 84% respectively). In the case of the deactivated CF3 derivative 2e, extending the reaction time to 42 h was required to generate the product in 67% yield. Next, the effect of varying the R2 substituent was investigated whilst keeping R1 = H constant. This enabled the halogenated series 2f, 2g and 2h to be generated as well as the trifluoromethoxy substituted product 2i (up to 77% yield). Efficient formation of the trifluoromethylated product 2j and nitrile 2k could also be realised (67% and 45% respectively) by slightly elevating the amine:HF ratio to 1:6.5. The introduction of a biphenyl substituent (2l) and inclusion of meta-substituents (2m) were also compatible with the protocol. In the case of ortho-substituents, extended reaction times were required (e.g. 2n). In a reversal of circumstances, the impact of modifying R2 whilst leaving R1 unchanged was investigated. Cascade processes to furnish the halogenated substrates 2o, 2p and 2q were successful (up to 67%). Moreover, the electron-deficient products 2r and 2s could be prepared with comparable efficiency (66% and 67% yield, respectively).
Cognisant of the synthetic utility of aryl bromides for subsequent downstream cross coupling, an additional series with R1 = Br was explored. Synthetically useful yields were obtained for products 2t (68%) and 2u (77%), as well as for the trifluoromethyl derivative 2v (71%) and triflate 2w (82%). Further substitution of the cyclobutanol by addition of a methyl group at C3 was tolerated and enabled the trifluoride 2x to be accessed (57% after 42 h). Finally, the scope of this transformation was found to be compatible with heterocycle-containing substrates as is evident from the 3-phenylpyridine-derivative 2y (63% yield).
The bis-trifluoromethyl derivative 2e was crystalline and it was possible to unequivocally establish the molecular connectivity created in this cascade by single crystal diffraction (Fig. 3, CCDC 2239010). A slight difference in C-F bond lengths was noted for the aliphatic and benzylic environments (1.377 and 1.376 Å versus 1.396 Å, respectively)46.
Transformation of cyclobutanols (1) to gem-difluorinated tetralins (3)
In the course of the optimisation of the 1,3,3-trifluorination reaction of major−1c, traces of the cyclised Friedel-Crafts product 3c were detected when the reactions were conducted at higher amine:HF ratios. This preliminary validation of the one-pot, multi-step conversion of major-1c → 3c provided an excellent foundation for reaction development (Table 2, entry 1). To identify an appropriate amine:HF mixture for benzylic fluoride activation, the impact of systematically adding Olah´s reagent and CHCl3 was investigated.
Gratifyingly, the addition of solvent and Olah´s reagent (0.75 and 1 mL each) yielded notable quantities of the desired 3,3-difluorotetrahydronaphthalene 3c (entries 2 and 3). Reducing the volume of CHCl3 had a beneficial impact on reaction efficiency, and its exclusion enabled the product to be generated in 72% from starting cyclobutanol major-1c (entry 5). Inspired by the seminal work of Paquin and co-workers on the activation of benzylic C-F bonds41,42, HFIP was investigated as a substitute for additional Olah´s reagent47,48. In this case, the addition of a mixture of HFIP and CHCl3 (2 mL, 1:1) led to the formation of desired product after 24 h (entry 6). Once again, eliminating the CHCl3 had a beneficial impact on the yield of 3c (55%, entry 7). Moreover, increasing the amount of HFIP to 2 mL led to higher yields (entry 8, 70% isolated yield): this is comparable to the yields attained using additional Olah´s reagent (entry 5, 72%). The advantage of this direct, one-pot protocol was immediately apparent following a comparison with the stepwise synthesis: this led to product 3c being generated in 70% and 48%, respectively (entry 9, see Stepwise Synthesis of 3c from major-1c in the ESI for further details).
To determine the scope and limitations of this fluorinative cascade to generate the target tetralins, a set of 1,3-diarylcyclobutanols were exposed to the standard conditions (Fig. 4). The reaction proved to be compatible with phenyl- and fluorophenyl substituents (3a and 3b), and halogenated substrates were particularly well-suited, enabling diversely halogenated scaffolds 3c and 3d to be generated (up to 76% yield). This latter observation is in line with the observations described in Fig. 2. Subsequently, R1 was varied whilst R2 remained constant (R2 = H). This one pot protocol enabled halogenated derivatives 3f, 3g and 3h to be forged, as well as the trifluoromethoxy species 3i. The inclusion of electron-rich biphenyl substituents (3l), as well as meta- and ortho-substitution patterns (3m and 3n), proved to be compatible with the reaction conditions. It is interesting to note that in the case of 3m, only the 7-Br regioisomer was isolated, presumably to mitigate destabilising non-bonding interactions. Next, the impact of varying R2 on reaction efficiency was explored. Gratifyingly, the halogenated tetralin series 3o, 3p and 3q were obtained efficiently (up to 62% yield). It was possible to generate the triflate 3s (56% yield) but required an extension of the reaction time to 72 h. Switching R1 to the valuable bromide handle enabled products 3t and 3u to be prepared in up to 71% yield.
Isolated yields are given in parentheses. Where possible, substrate 1 was used as a single diastereoisomer. See ESI for full details. aAn amine:HF mixture with a ratio of 1:4.5 was used. bAn amine:HF ratio of 1:6.5 was used. cFor the formation of intermediate 2n, the reaction time was extended to 42 h. dAn amine:HF ratio of 1:5.0 was used. eThe reaction was stirred for 72 h. fThe reaction was conducted at 40 ˚C.
Pushing the limits of the process revealed electron-deficient substrates to be challenging. However, the desired tetralins could be generated by a two-step compromise and the use of Olah´s reagent for the final activation/Friedel-Crafts cyclisation (Fig. 4, bottom). To that end, the isolated intermediates (2) were treated with a mixture of Olah´s reagent and CHCl3 (1:1) and stirred for 24 h at the specified temperature. Leveraging this platform, it was possible to access the bis- and mono-CF3 species 3e, 3j, 3r and 3v (up to 98% yield). To enable further functionalisation, tetralin 3w bearing orthogonal C(sp2)-Br and C(sp2)-OTf motifs was synthesised in 75%. Finally, the pyridine-containing tetralin 3y was accessed by this protocol in 68%.
Synthetic applications
The efficiency of the C-F bond activation, coupled with the modest nucleophilicity of the aryl rings in this study, provided an opportunity to expand the scope of the process by introducing superior, external nucleophiles. To provide preliminary validation of this notion, AcOH, MeOH and p-xylene were introduced to the one-pot cascade reaction with major-1c (cis) (Fig. 5). All three transformations successfully generated the desired products in up to 72% yield. The oxidative lability of many nucleophiles called for the development of a complementary stepwise process to encompass S- and N-based nucleophiles (7, 9 and 10). By treating 2c with 2-mercaptobenzothiazole in a mixture of HFIP:CHCl3 (3:1) at 40 ˚C for 66 h, thioether 7 was obtained in 86% yield. Facile oxidation of 7 with m-CPBA furnished the olefination precursor, sulfone 8. A similar strategy enabled the fluorinated thioether 9 to be forged in 93% yield. Amination was achieved using acetonitrile as the exogenous nucleophile49, where a subsequent Ritter-type reaction liberated the γ,γ-difluoroamine 10 in 78% yield.
Isolated yields in parentheses. One-pot reactions: 2c was prepared under standard reaction conditions: a) AcOH (1.0 mL), Olah’s reagent (1.0 mL), 24 h, rt. b) MeOH (1.0 mL), Olah’s reagent (2.0 mL), 48 h, rt. c) p-xylene (1.0 mL), Olah’s reagent (1.0 mL), 48 h, rt. Reaction conditions using isolated 2c: d) 2c (0.2 mmol), 2-mercaptobenzothiazole (0.4 mmol), HFIP (1.2 mL), CHCl3 (0.4 mL), 66 h, 40 ˚C. e) 7 (0.2 mmol), m-CPBA (0.44 mmol), CHCl3 (3.0 mL), 15 h, −10 ˚C to rt. f) 2c (0.2 mmol), 4-nitrobenzenethiol (1.0 mmol), HFIP (1.2 mL), CHCl3 (0.4 mL), 24 h, 40 ˚C. g) 2c (0.2 mmol), Olah’s reagent (1.0 mL), MeCN (1.0 mL), 20 h, 40 ˚C.
To demonstrate the synthetic utility of the fluorinated tetralins in the arenas of contemporary medicinal chemistry and organic materials design, selected synthetic modifications were conducted (Fig. 6). Given the importance of tetralins in drug discovery, a short synthesis of the difluorinated analogue of Nafenopin was executed from triflate 3s (Fig. 6A). Saponification using NEt4OH enabled phenol 11 to be prepared and processed to the target 12 via an alkylation / deprotection sequence.
A Synthesis and crystal structure of nafenopin derivative 12. Reaction conditions: a) 3s (0.58 mmol), NEt4OH 10 wt% in H2O (1.16 mmol), 1,4-dioxane (1.75 mL), 90 min, rt. b) 11 (0.30 mmol), tert-butyl 2-bromo-2-methylpropanoate (1.50 mmol), MgSO4 (0.30 mmol), K2CO3 (1.20 mmol), DMF (2.0 mL), 24 h, 100 ˚C. c) TFA (6.0 mmol), DCM (3 mL), 1 h, 0 ˚C to rt. Crystal structure of compound 12 (CCDC 2239012) showing the main conformation found in the asymmetric unit (84%). Thermal ellipsoids are shown at 50% probability. B Synthetic modifications of tetralin 3q. d) 3q (0.20 mmol), ethynyltriisopropylsilane (0.30 mmol), Pd(PPh3)2Cl2 (0.02 mmol), CuI (0.04 mmol), NH(i-Pr)2 (0.5 mL), 14 h, 70 ˚C. e) 3q (0.20 mmol), benzo[d][1,3]dioxol-5-ylboronic acid (0.22 mmol), Pd(PPh3)4 (0.005 mmol), PPh3 (0.02 mmol), Na2CO3 (0.24 mmol), EtOH/H2O (5:1, 0.72 mL), 14 h, 80 ˚C. f) 3q (0.20 mmol), indole (0.20 mmol), CuI (0.02 mmol), N,N’-dimethyldiaminoethane (0.08 mmol), K3PO4 (0.42 mmol), toluene (0.5 mL), 20 h, 110 ˚C. Isolated yields in parentheses.
Product 12 was crystalline and it was possible to determine the structure via X-ray analysis (CCDC 2239012). The near perfect half-chair of the central ring system further underscores the effectiveness of the gem-difluoro motif as an isosteric replacement for methylene groups. Finally, to demonstrate the suitability of a representative tetralin in the generation of carbon rich scaffolds, Sonogashira (13, 93%), Suzuki (benzodioxol 14, 83%) and Ullmann-type coupling (15, 97%) were successfully conducted (Fig. 6B).
Hypervalent iodine catalysis has significantly augmented the fluorination portfolio, enabling the direct installation of C(sp3)-F bonds in alkene substrates without the need for substrate pre-functionalisation. The fusion of this platform with simple HF sources confers an array of advantages for reaction design, not least the ability to leverage the intrinsic acidity of the reaction medium to unmask substrates in situ. In this study, the dehydration of easily accessible cyclobutanols has been validated as a platform to trigger a fluorinative skeletal rearrangement cascade to access biologically relevant aryl tetralins in a highly regioselective fashion. Subsequent benzylic fluorination forges a (1,1-disubstituted) styrenyl substrate that can be further intercepted by an I(I)/I(III) catalysis manifold. Activation of the alkene by the ephemeral hypervalent iodine centre triggers a phenonium ion rearrangement, ultimately generating the gem-difluoromethyl unit, which forms part of a trifluoro motif. This motif can be isolated or processed, via C(sp3)-F bond activation, to the desired tetralin. Moreover, by introducing a competitive exogenous (C- and O-based) nucleophile, it is possible to override the intramolecular process to further broaden the modularity of the process.
Methods
General procedure for the synthesis of (±)−2
Cyclobutanol derivative 1 (0.20 mmol, 1.0 eq.) and p-TolI (8.7 mg, 0.04 mmol, 20 mol%) were dissolved in CHCl3 (0.5 mL) in a Teflon®-vial (5 mL total volume). Subsequently, NEt3•3HF and Olah’s reagent were added with the appropriate ratio (0.5 mL total volume, for more information, see previous publication of this group36). Finally, Selectfluor® (106 mg, 0.3 mmol, 1.5 eq.) was added to the reaction mixture in one portion. The reaction mixture was stirred for 18 h at room temperature. The reaction mixture was diluted with DCM (2 mL) and poured in saturated aqueous NaHCO3 (100 mL). The aqueous layer was extracted with DCM (3 × 30 mL). The combined organic layers were dried over Na2SO4 and the solvent was removed under reduced pressure. The crude product was purified by flash column chromatography.
Caution
Olah’s reagent is highly toxic and corrosive. Direct exposure should be avoided. In the case of skin exposure, immediate treatment of the affected skin area with calcium gluconate gel is necessary to prevent serious chemical burns.
General procedure for the synthesis of (±)−3 (one pot procedure)
The 1,1,3-trifluorobutane derivative (±)−2 was prepared according to the general procedure on a 0.200 mmol-scale with the indicated amine•HF mixture. Instead of quenching the reaction after 18 h, HFIP (2.0 mL) was added and the reaction was stirred for another 24 h at room temperature. The reaction mixture was diluted with DCM (2 mL) and poured in saturated aqueous NaHCO3 (100 mL). The aqueous layer was extracted with DCM (3 × 30 mL). The combined organic layers were dried over Na2SO4 and the solvent was removed under reduced pressure. The crude product was purified by flash column chromatography.
Caution
Olah’s reagent is highly toxic and corrosive. Direct exposure should be avoided. In the case of skin exposure, immediate treatment of the affected skin area with calcium gluconate gel is necessary to prevent serious chemical burns.
General procedure for the synthesis of (±)−3 (stepwise procedure)
Isolated 1,1,3-trifluorobutane derivative (±)−2 (0.200 mmol, 1.0 eq.) was dissolved in CHCl3 (1.0 mL). Olah´s reagent (1.0 mL) was added and the reaction was stirred at the indicated temperature for the indicated time. The reaction mixture was diluted with DCM (2 mL) and poured in saturated aqueous NaHCO3 (200 mL). The aqueous layer was extracted with DCM (3×30 mL). The combined organic layers were dried over Na2SO4 and the solvent was removed under reduced pressure. The crude product was purified by flash column chromatography.
Caution
Olah’s reagent is highly toxic and corrosive. Direct exposure should be avoided. In the case of skin exposure, immediate treatment of the affected skin area with calcium gluconate gel is necessary to prevent serious chemical burns.
Data availability
CCDC 2239011 contains the supplementary crystallographic data for compound minor-1c (trans). CCDC 2239010 contains the supplementary crystallographic data for compound 2e. CCDC 2239012 contains the supplementary crystallographic data for compound 12. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre [http://www.ccdc.cam.ac.uk/data_request/cif]. Supplementary Information is available for this paper. All data are available in the main text or the supplementary materials. Correspondence and requests for materials should be addressed to Prof. Ryan Gilmour (ryan.gilmour@uni-muenster.de).
References
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008).
O´Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 37, 308–319 (2008).
O’Hagan, D. Fluorine in health care: organofluorine containing blockbuster drugs. J. Fluor. Chem. 131, 1071–1081 (2010).
Zimmer, L. E., Sparr, C. & Gilmour, R. Fluorine conformational effects in organocatalysis: an emerging strategy for molecular design. Angew. Chem. Int. Ed. 50, 11860–11871 (2011).
Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015).
Meanwell, N. A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 61, 5822–5880 (2018).
Aufiero, M. & Gilmour, R. Informing molecular design by stereoelectronic theory: the fluorine gauche effect in catalysis. Acc. Chem. Res. 51, 1701–1710 (2018).
Han, J. et al. Next generation organofluorine containing blockbuster drugs. J. Fluor. Chem. 239, 109639 (2020).
Inoue, M., Sumii, Y. & Shibata, N. Contribution of organofluorine compounds to pharmaceuticals. ACS Omega 5, 10633–10640 (2020).
Wender, P. A. & Miller, B. L. Synthesis at the molecular frontier. Nature 460, 197–201 (2009).
Shah, P. & Westwell, A. D. The role of fluorine in medicinal chemistry. J. Enzyme Inhib. Med. Chem. 22, 527–540 (2007).
Johnson, B. M., Shu, Y.-Z., Zhuo, X. & Meanwell, N. A. Metabolic and pharmaceutical aspects of fluorinated compounds. J. Med. Chem. 63, 6315–6386 (2020).
Trost, B. M. The atom economy-a search for synthetic efficiency. Science 254, 1471–1477 (1991).
Trost, B. M. Atom economy-a challenge for organic synthesis: homogeneous catalysis leads the way. Angew. Chem. Int. Ed. 34, 259–281 (1995).
Yoshimura, A. & Zhdankin, V. V. Advances in synthetic applications of hypervalent iodine compounds. Chem. Rev. 116, 3328–3435 (2016).
Li, X., Chen, P. & Liu, G. Recent advances in hypervalent iodine (III)-catalyzed functionalization of alkenes. Beilstein J. Org. Chem. 14, 1813–1825 (2018).
Claraz, A. & Masson, G. Asymmetric iodine catalysis-mediated enantioselective oxidative transformations. Org. Biomol. Chem. 16, 5386–5402 (2018).
Parra, A. Chiral hypervalent iodines: active players in asymmetric synthesis. Chem. Rev. 119, 12033–12088 (2019).
Cresswell, A. J., Eey, S. T.-C. & Denmark, S. E. Catalytic, stereoselective dihalogenation of alkenes: challenges and opportunities. Angew. Chem. Int. Ed. 54, 15642–15682 (2015).
Kohlhepp, S. V. & Gulder, T. Hypervalent iodine (III) fluorinations of alkenes and diazo compounds: new opportunities in fluorination chemistry. Chem. Soc. Rev. 45, 6270–6288 (2016).
Molnár, I. G., Thiehoff, C., Holland, M. C. & Gilmour, R. Catalytic, vicinal difluorination of olefins: Creating a hybrid, chiral bioisostere of the trifluoromethyl and ethyl groups. ACS Catal. 6, 7167–7173 (2016).
Meyer, S., Häfliger, J. & Gilmour, R. Expanding organofluorine chemical space: the design of chiral fluorinated isosteres enabled by I (I)/I (III) catalysis. Chem. Sci. 12, 10686–10695 (2021).
Okoromoba, O. E., Han, J., Hammond, G. B. & Xu, B. Designer HF-based fluorination reagent: highly regioselective synthesis of fluoroalkenes and gem-difluoromethylene compounds from alkynes. J. Am. Chem. Soc. 136, 14381–14384 (2016).
Mondal, R., Agbaria, M. & Nairoukh, Z. Fluorinated rings: conformation and application. Chem. Eur. J. 27, 7193–7213 (2021).
Grygorenko, O. O., Melnykov, K. P., Holovach, S. & Demchuk, O. Fluorinated cycloalkyl building blocks for drug discovery. ChemMedChem 17, e202200365 (2022).
Lanke, V. & Marek, I. Nucleophilic substitution at quaternary carbon stereocenters. J. Am. Chem. Soc. 142, 5543–5548 (2020).
Larmore, S. P. & Champagne, P. A. Cyclopropylcarbinyl-to-homoallyl carbocation equilibria influence the stereospecificity in the nucleophiliuc substitution of cyclopropylcarbinols. J. Org. Chem. https://doi.org/10.1021/acs.joc.3c00257 (2023).
Creary, X. 3-t-Butyl-methylcyclobutyl cation. Experimental versus computational insights into tertiary bicyclobutonium cations. J. Org. Chem. 85, 7086–7096 (2020).
Lin, P.-P. et al. gem-Difluorination of methylenecyclopropanes (MCPs) featuring a Wagner-Meerwein rearrangement: synthesis of 2-arylsubstituted gem-Difluorocyclobutanes. Org. Lett. 23, 3088–3093 (2021).
Sarie, J. et al. Deconstructing the catalytic, Vicinal difluorination of alkenes: HF-free synthesis and structural study of p-TolIF2. J. Org. Chem. 82, 11792–11798 (2017).
Banik, S. M., Medley, J. W. & Jacobsen, E. N. Catalytic, asymmetric difluorination of alkenes to generate difluoromethylated stereocenters. Science 353, 51–54 (2016).
Scheidt, F., Neufeld, J., Schäfer, M., Thiehoff, C. & Gilmour, R. Catalytic Geminal difluorination of styrenes for the construction of fluorine-rich Bioisosteres. Org. Lett. 20, 8073–8076 (2018).
Zhou, B., Haj, M. K., Jacobsen, E. N., Houk, K. N. & Xue, X.-S. Mechanism and origins of chemo-and stereoselectivities of aryl iodide-catalyzed asymmetric difluorinations of β-substituted styrenes. J. Am. Chem. Soc. 140, 15206–15218 (2018).
Levin, M. D., Ovian, J. M., Read, J. A., Sigman, M. S. & Jacobsen, E. N. Catalytic enantioselective synthesis of difluorinated alkyl bromides. J. Am. Chem. Soc. 142, 14831–14837 (2020).
Häfliger, J., Livingstone, K., Daniliuc, C. G. & Gilmour, R. Difluorination of α-(bromomethyl) styrenes via I (I)/I (III) catalysis: facile access to electrophilic linchpins for drug discovery. Chem. Sci. 12, 6148–6152 (2021).
Neufeld, J., Stünkel, T., Mück-Lichtenfeld, C., Daniliuc, C. G. & Gilmour, R. Trifluorinated tetralins via I(I)/I(III)-catalysed ring expansion: programming conformation by [CH2CH2] → [CF2CHF] isosterism. Angew. Chem. Int. Ed. 60, 13647–13651 (2021).
Meyer, S. et al. Cyclopropene activation via I (I)/I (III) catalysis: proof of principle and application in direct tetrafluorination. Tetrahedron 126, 132925 (2022).
Livingstone, K. et al. Skeletal ring contractions via I(I)/I(III) catalysis: stereoselective synthesis of cis-α,α-difluorocyclopropanes. ACS Catal. 12, 14507–14516 (2022).
Bykova, T., Al-Maharik, N., Slawin, A. M. Z. & O´Hagan, D. Synthesis of selectively fluorinated cyclohexanes: The observation of phenonium rearrangements during deoxyfluorination reactions on cyclohexane rings with a vicinal phenyl substituent. J. Fluor. Chem. 179, 188–192 (2015).
Champagne, P. A. et al. Enabling nucleophilic substitution reactions of activated alkyl fluorides through hydrogen bonding. Org. Lett. 15, 2210–2213 (2013).
Champagne, P. A., Benhassine, Y., Desroches, J. & Paquin, J.-F. Friedel–crafts reaction of benzyl fluorides: selective activation of C-F bonds as enabled by hydrogen bonding. Angew. Chem. Int. Ed. 53, 13835–13839 (2014).
Holland, M. C. & Gilmour, R. Deconstructing covalent organocatalysis. Angew. Chem. Int. Ed. 54, 3862–3871 (2015).
Molnár, I. G. & Gilmour, R. Catalytic difluorination of olefins. J. Am. Chem. Soc. 138, 5004–5007 (2016).
Scheidt, F. et al. Enantioselective, catalytic vicinal difluorination of alkenes. Angew. Chem. Int. Ed. 57, 16431–16435 (2018).
Schaefer, T., Schurko, R. W., Sebastian, R. & Hruska, F. E. Experimental and theoretical assessments of the substituent and medium dependence of the internal rotational potentials in benzyl fluoride. 3,5-Difluorobenzyl fluoride and 4-fluorobenzyl fluoride. Can. J. Chem. 73, 816–825 (1995).
Colomer, I., Chamberlain, A. E. R., Haughey, M. B. & Donohoe, T. J. Hexafluoroisopropanol as a highly versatile solvent. Nat. Rev. Chem. 1, 0088 (2017).
Arnold, A. M., Pöthig, A., Drees, M. & Gulder, T. NXS, morpholine, and HFIP: the ideal combination for biomimetic haliranium-induced polyene cyclizations. J. Am. Chem. Soc. 140, 4344–4353 (2018).
Yu, Y.-J., Schäfer, M., Daniliuc, C. G. & Gilmour, R. Catalytic, regioselective 1,4-fluorodifunctionalization of dienes. Angew. Chem. Int. Ed. 62, e202214906 (2023).
Acknowledgements
We gratefully acknowledge the support provided by the technical departments of the Institute for Organic Chemistry at the WWU Münster. We acknowledge financial support from the WWU Münster (R.G.), the Deutsche Forschungsgemeinschaft (SFB 858, R.G.) and the European Research Council (ERC Consolidator Grant RECON 818949, R.G.).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Initial project idea: J.H., R.G., Conceptualization: J.H., R.G., Methodology: J.H., R.G., Investigation: J.H., L.R., N.S., C.D., Funding acquisition: R.G., Project administration: R.G., Supervision: J.H., L.R., R.G., Writing: J.H., L.R., R.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Deepak Chopra, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Häfliger, J., Ruyet, L., Stübke, N. et al. Integrating I(I)/I(III) catalysis in reaction cascade design enables the synthesis of gem-difluorinated tetralins from cyclobutanols. Nat Commun 14, 3207 (2023). https://doi.org/10.1038/s41467-023-38957-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-38957-w
This article is cited by
-
Enyne difluorination
Nature Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.