Abstract
Growth at the shoot apical meristem (SAM) is essential for shoot architecture construction. The phytohormones gibberellins (GA) play a pivotal role in coordinating plant growth, but their role in the SAM remains mostly unknown. Here, we developed a ratiometric GA signaling biosensor by engineering one of the DELLA proteins, to suppress its master regulatory function in GA transcriptional responses while preserving its degradation upon GA sensing. We demonstrate that this degradation-based biosensor accurately reports on cellular changes in GA levels and perception during development. We used this biosensor to map GA signaling activity in the SAM. We show that high GA signaling is found primarily in cells located between organ primordia that are the precursors of internodes. By gain- and loss-of-function approaches, we further demonstrate that GAs regulate cell division plane orientation to establish the typical cellular organization of internodes, thus contributing to internode specification in the SAM.
Similar content being viewed by others
Introduction
The shoot apical meristem (SAM) at the tip of the shoot axes comprises a stem cell niche whose activity produces lateral organs and stem segments in a modular iterative fashion during the whole plant life. Each of these repetitive units or phytomere includes an internode and lateral organs at a node and an axillary meristem at the leaf axil1. The growth and organization of phytomeres change during development. In Arabidopsis thaliana, internode growth is inhibited during the vegetative phase and axillary meristems rest dormant at the axils of rosette leaves. Upon floral transition, the SAM turns into an inflorescence meristem, producing elongated internodes and axillary buds that form branches at the axils of cauline leaves, and later flowers without leaves2. While substantial progress has been made in our understanding of the mechanisms controlling the initiation of leaves, flowers, and branches, much less is known about how internodes are initiated.
Growth via cell division and expansion is essential for reiterative organogenesis at the SAM. The tetracyclic diterpenoid hormones gibberellins (GA) are key growth regulators3,4,5, with a crucial role in many embryonic and post-embryonic developmental processes6. Central to the GA signaling pathway are the five DELLA proteins, GIBBERELLIC ACID INSENSITIVE (GAI), REPRESSOR OF GA1-3 (RGA), and RGA-Like (RGL) 1-37. These nuclear proteins are composed of an N-terminal DELLA/TVHYNP domain and of a GRAS domain. The GRAS domain allows DELLAs to interact with diverse transcription factors and transcriptional regulators and to suppress growth by modulating their activity7. The binding of GA to the GIBBERELLIN INSENSITIVE DWARF1 (GID1) GA receptor promotes GID1 interaction with the N-terminal domain of DELLAs, triggering DELLA degradation by the ubiquitin-dependent proteasome pathway7,8,9,10, and in turn, de-repressing GA responses. Despite the relatively specialized role of the GRAS and DELLA/TVHYNP domains, residues required for DELLA degradation and partner protein interaction are widely distributed within the protein sequence.
The identification of genes encoding GA catabolic enzymes as direct targets of the class I KNOX meristem identity regulators has led to the proposal that GA levels are low in the SAM cells, while high GA concentrations trigger the growth of lateral organs3,4,5. Low GA levels could then contribute to SAM maintenance3. However, more recent analysis indicates also that GAs promote the increase in SAM size during the floral transition by regulating the division and expansion of inner SAM cells11,12, in a similar manner as in the root13,14. DELLAs were likewise found to limit meristem size by directly regulating the expression of the cell-cycle inhibitor KRP2 in the internal part of the SAM (the rib zone)12. Together, these findings support a role for GA in positively regulating cell division in the inner tissues of the SAM, and thus SAM size. At the same time, several genes encoding GA biosynthetic and catabolic enzymes are expressed specifically in lateral organs4,11, illustrating a complex spatiotemporal GA distribution in the SAM to likely fulfill different functions.
Accessing spatiotemporal GA distribution has been instrumental in better understanding the functions of these hormones in different tissues and at various developmental stages. Visualization of degradation of an RGA-GFP fusion expressed under its own promoter provided key information on the regulation of GA global levels in the root15,16. However, RGA is expressed differentially in tissues17 and this expression is regulated by GA18. Differential expression from the RGA promoter thus potentially contributes to the fluorescence pattern observed with RGA-GFP, making this approach not quantitative. Accumulation of GA in the root endodermis and regulation of their cellular level via GA transport was later discovered by using bioactive fluorescein (Fl)-tagged GA19,20. More recently, the nlsGPS1 GA FRET sensor revealed that GA levels are correlated with cell elongation in roots, stamen filaments, and dark-grown hypocotyls21. However, as we have seen, GA concentration is not the only parameter controlling GA signaling activity as it depends on a complex perception process. Here, building on the knowledge of DELLAs and the GA signaling pathway, we report on the engineering and characterization of a degradation-based GA signaling ratiometric biosensor. To design this quantitative biosensor, we used a mutated yet GA-sensitive RGA fused to a fluorescent protein and expressed ubiquitously in tissues, together with a GA-insensitive fluorescent protein. We show that the mutated RGA protein fusion does not interfere with endogenous GA signaling when expressed ubiquitously and that the biosensor allows quantifying the signaling activity resulting from the contribution of GA and of the perception machinery processing the GA signal with a high spatiotemporal resolution. We used this biosensor to map the spatiotemporal distribution of GA signaling activity and to quantitatively analyze how GAs regulates cell behavior in the SAM epidermis. We demonstrate that GAs regulate the orientation of division planes of SAM cells located between organ primordia, therefore specifying the typical cellular organization of internodes.
Results
Modifying the RGA protein for sensor construction
DELLA degradation results from the processing of the information from GA by a complex perception process involving GID1 receptors and ubiquitination and is then a readout of the GA signaling activity. We thus aimed at generating a degradation-based GA signaling biosensor by engineering a DELLA protein to meet two criteria, (i) a specific degradation upon GA perception; and (ii) a minimal interference with GA signaling. To do so, we modified DELLAs to preserve the interaction with GID1 while abolishing interactions with partner transcription factors, by introducing mutations in the GRAS domain. Among the 5 Arabidopsis DELLAs, RGA displays one of the highest GA-dependent degradation rate22, and RGA-GFP fusions are widely used as GA signaling reporter8. Leveraging on the results of GRAS domain mutant analyses in rice23 and Arabidopsis24,25, we generated four modified RGA versions (RGAm1: G218A, V219A, R220A; RGAm2: H471A, Y472A, Y473A; RGAm3: S578Stop; and RGAm4: S578D) and tested their ability to meet the above-defined criteria (Fig. 1a and Supplementary Fig. 1a). We first used a yeast-two-hybrid (Y2H) assay to test the binding capacity of these modified candidates with three well known DELLA-interacting partners, JAZ1, TCP14 and IDD226. While RGAm1 had a minor effect on interactions, RGAm2, RGAm3, and RGAm4 lost their capacity to interact with these partners (Fig. 1b and Supplementary Fig. 1b). In addition, RGAm2, RGAm3 and RGAm4 were able to bind GID1 in the presence of GA, thus suggesting that these DELLA candidates are still degraded in response to GA (Fig. 1c and Supplementary Fig. 1c). To assess this possibility, we explored GA-dependent degradation of RGAm2, RGAm3 and RGAm4 fused to GFP in transient expression assays. These three candidates were degraded after GA treatment (Fig. 1d and Supplementary Fig. 1d), RGAm2-GFP having the fastest degradation kinetics although it was slightly more stable than RGA-GFP. Noteworthy, RGAm2 harbors three amino acid substitutions in the GRAS PFYRE motif (Fig. 1a), which is highly conserved in all plant DELLAs26. Therefore, this variant is likely to have similar properties in plant species other than Arabidopsis.
a Schematic representation of the domain structure of a typical DELLA protein. Conserved histidine (H471), tyrosine (Y472), and tyrosine (Y473) residues were mutated into alanine (A) to obtain the modified RGAm2 protein. The nucleic acids and amino acids mutated in RGAm2 are indicated in red. b Yeast two-hybrid (Y2H) assays in which RGA and RGAm2 were tested pairwise with four known DELLA-interacting partners: JAZ1, TCP14, IDD2 and BZR1. Empty pGBKT7 and pGADT7 vectors were included as negative controls. Photos show the growth of the yeast on control media (GM) and on selective media (SM). c Pairwise Y2H interaction assays between RGA or RGAm2 and the three GA receptors GID1a, GID1b and GID1c. Photos show the growth of the yeast on control media (GM), selective media (SM), and SM media supplemented with 100 μM GA3. d Time-course analysis of GA-induced degradation of RGA (upper panel) and RGAm2 protein (lower panel). Immunodetection of RGA-GFP and RGAm2-GFP protein in 35S::RGA-GFP and 35S::RGAm2-GFP N. benthamiana agro-infiltrated leaves treated with 100 mM cycloheximide (CHX) and 100 µM GA3 for the indicated times. Numbers indicate RGA-GFP and RGAm2-GFP levels relative to actin levels, used as loading control. The experiment was repeated twice with similar results. e Co-immunoprecipitation assays between RGA or RGAm2 and IDD2 or TCP14. Protein extracts from different combinations of N. benthamiana agro-infiltrated leaves with 35S::RGA-GFP, 35 S::RGAm2-GFP, 35S::IDD2-RFP, and 35S::TCP14-RFP were immunoprecipitated with anti-GFP antibodies. The co-immunoprecipitated protein (IDD2-RFP and TCP14-RFP) was detected by anti-RFP antibodies. The experiment was repeated twice with similar results. f, g Effect of RGA and RGAm2 on BZR1 (f) and TCP14 (g) transcriptional activities in N. benthamiana agro-infiltrated leaves with a combination of BZR1, TCP14, RGA, and RGAm2 effector constructs and corresponding Luciferase/Renilla reporter constructs, as indicated (top panels). BZR1 and TCP14 have been fused to VP16 transcriptional activator domain in this experiment. Transcriptional activities are represented as the ratio of Luciferase and Renilla (used as internal control) activities, relative to the value obtained for the reporter construct alone that was set to 1. Data are means ± SD of three biological replicates. P-values were calculated in R using a two-tailed Welch t-test. Bottom panels: immunodetection of RGA-GFP, RGAm2-GFP, HA-VP16-BZR1, and HA-VP16-TCP14 from N. benthamiana agro-infiltrated leaves used for transcriptional activity assays. These experiments were repeated three times with similar results.
Based on the above results, we selected RGAm2 for further analysis. We next showed that RGAm2 is unable to bind with the BZR1 DELLA-interacting partner in yeast and a larger screen confirmed that these mutations abolish interactions with practically all known DELLA partners (Fig. 1b and Supplementary Table 1). Moreover, co-immunoprecipitation studies demonstrated that binding of RGAm2 to IDD2 or TCP14 is also strongly reduced in planta compared to RGA (Fig. 1e). Finally, transient expression assays confirmed that, while RGA represses BZR1 and TCP14 transcriptional activities, RGAm2 did not (Fig. 1f, g). Taken together, these data indicate that the expression of RGAm2 in plants, hereafter named mRGA for simplicity might have a limited impact on GA signaling. Thus, mRGA constitutes a suitable DELLA variant candidate for engineering a degradation-based biosensor that monitors GA signaling activity and more specifically the combinatorial effect of GA and of its complex perception machinery.
Engineering a GA signaling sensor
To create a ratiometric GA signaling sensor, we fused the mRGA protein to the fast-maturing yellow fluorescent protein VENUS27 and co-expressed this fusion protein together with a nuclear-localized non-degradable reference protein, TagBFP-NLS, under a 2.5 kb pUBQ1028 or pRPS5a29,30 constitutive promoter. We used the 2A self-cleaving peptide to allow for a stoichiometric production of both fluorescent proteins, enabling quantification of GA signaling activity using fluorescence intensity ratio between mRGA-VENUS and TagBFP31,32 (Fig. 2a). We named the sensor lines qmRGA (quantitative mRGA) and first analyzed their TagBFP fluorescence pattern in vegetative and reproductive tissues (Fig. 2b and Supplementary Fig. 2a, b). TagBFP fluorescence of pUBQ10::qmRGA lines was homogeneously distributed in hypocotyl, the vegetative shoot meristem, cotyledons, and roots (except in the root tip) but the TagBFP signal was unevenly distributed in the inflorescence SAM, with a stronger signal in organ boundaries (Supplementary Fig. 2a). Conversely, the TagBFP signal was strong in the root tip and the vegetative SAM in pRPS5a::qmRGA lines (Fig. 2b), and showed a homogenous distribution in the inflorescence SAM (Supplementary Fig. 2b). Hence, the choice of one of these constructs depends on the analyzed tissue: we used pUBQ10::qmRGA in subsequent experiments in seedlings except root tip, and pRPS5a::qmRGA for experiments performed in root tip and inflorescence SAM.
a Schematic representation of the qmRGA construct composed of two elements: the sensor module (mRGA-VENUS), and the normalization element (TagBFP fused with a nuclear localization signal (NLS)). The two elements are linked by a 2A self-cleaving peptide, and driven by the same promoter allowing stoichiometric expression. GA signaling activity is measured as 3 minus the ratio between VENUS and TagBFP signal intensities. b TagBFP expression pattern of pUBQ10::qmRGA and pRPS5a::qmRGA sensors monitored in cotyledon, vegetative SAM, hypocotyl, and root of 7-day-old seedlings. The experiment was repeated twice with similar results. c Boxplot representations of the hypocotyl length of wild-type (Col-0) and pUBQ10::qmRGA seedlings. Upper panel: hypocotyl length at 5 (Col-0, n = 33 biological independent seedlings; qmRGA, n = 36 biological independent seedlings) and 9 days (Col-0, n = 34 biological independent seedlings; qmRGA, n = 35 biological independent seedlings) after sowing (DAS). Lower panel: hypocotyl length of 9-day-old seedlings grown for 4 days on MS media and then transferred to MS media (Mock; Col-0, n = 34 biological independent seedlings; qmRGA, n = 35 biological independent seedlings), supplemented with 5 μM Pac (Col-0, n = 33 biological independent seedlings; qmRGA, n = 30 biological independent seedlings) or 10 μM GA3 (Col-0, n = 33 biological independent seedlings; qmRGA, n = 33 biological independent seedlings). Center lines show the medians and box limits indicate the 25th and 75th percentiles. Whiskers indicate minima and maxima as determined using the R software. P-values are determined with the R software using a two-tailed Welch t-test. d Boxplot representations of the number of rosette leaves (upper panel) and final plant height (lower panel) of wild-type (Col-0, n = 23) and pRPS5a::qmRGA adult plants (n = 22). Center lines show the medians and box limits indicate the 25th and 75th percentiles. Whiskers indicate minima and maxima as determined using the R software. P-values are from two-sided Student’s t-tests. e, f Time-course analysis of GA-induced RGA and mRGA degradation. 7-day-old pRPS5a::qRGA (e) and pRPS5a::qmRGA seedlings (f) were treated with 100 µM cycloheximide (CHX) and 100 µM GA3. At indicated time points, total proteins were extracted and analyzed by immunoblot using RGA antibodies. Numbers indicate the fold increase in RGA and mRGA protein levels relative to the blue-stained protein signal. The experiment was repeated twice with similar results. g Representative confocal images of mRGA-VENUS and TagBFP signals and corresponding heatmap representation of GA signaling activity in hypocotyls of 5-day-old pUBQ10::qmRGA seedlings treated with 10 μM GA3 for the time indicated (and mock control). The experiment was repeated twice with similar results. h Boxplot representation of GA signaling activity (Mock, n = 467 nuclei examined over ten independent seedlings; 1 h, n = 331 nuclei examined over eight independent seedlings; 2 h, n = 486 nuclei examined over nine independent seedlings; 2 h, n = 550 nuclei examined over nine independent seedlings) measured in pUBQ10::qmRGA seedling hypocotyls grown in the same conditions as in (g), indicated by different colors. Center lines show the medians and box limits indicate the 25th and 75th percentiles. Whiskers indicate minima and maxima as determined using the R software. P-values are from Kruskal–Wallis tests. The experiment was repeated twice with similar results. Scale bars = 100 µm.
To test that qmRGA activity is indeed not interfering with signaling activity and thus with plant growth, we first investigated the effects of exogenous GA and paclobutrazol (Pac; an inhibitor of GA biosynthesis) treatments on the growth of qmRGA plants. We found that the hypocotyl length of qmRGA seedlings was similar to that of wild-type under mock conditions and upon GA or Pac treatment, although hypocotyls were slightly longer after GA treatment (Fig. 2c). Similarly, shoot development and plant fertility were not significantly affected in qmRGA plants (Fig. 2d and Supplementary Fig. 2c). Last, we showed that when mRGA in the qmRGA construct is replaced by d17RGA, a mutant version of RGA that is fully insensitive to GA33, the resulting qd17RGA plants exhibited a severe dwarf phenotype reminiscent of GA-insensitive mutants. By contrast, qd17mRGA plants, expressing a mutated version of mRGA to which the d17 mutation was added, had similar rosette size and height to wild-type plants (Supplementary Fig. 2d–i). Altogether, these results demonstrate that qmRGA negligibly interferes with plant growth and GA responses.
Next, we assessed whether qmRGA can detect changes in signaling activity resulting from changes in GA levels. Consistent with the above transient expression assays, GA treatment induced the degradation of mRGA in pRPS5a::qmRGA seedlings, although the protein tends to be slightly more stable than RGA (Fig. 2e, f). Accordingly, while the TagBFP signal was unaffected in hypocotyls of pUBQ10::qmRGA seedlings upon GA application, fluorescence of the mRGA-VENUS sensor element was substantially reduced after this treatment (Fig. 2g). Similar results were observed in qmRGA root tips for which GA and Pac application respectively reduced and increased mRGA-VENUS signal (Supplementary Fig. 3a, b). In contrast, VENUS fluorescence was much less affected by the treatments in the RGAm3-VENUS and RGAm4-VENUS lines, consistently with the lower GA-dependent degradation rate of these variants (Supplementary Fig. 3c–f).
Since mRGA-VENUS is degraded upon GA sensing in qmRGA plants, mRGA-VENUS fluorescence is negatively related to GA signaling, while—mRGA-VENUS/TagBFP is positively related to GA signaling and accounts for possible variations in promoter activity. In further image analyses, we then used 3— (mRGA-Venus/TagBFP) to have a positive proxy for GA signaling activity that fully covers the range of values in VENUS/TagBFP fluorescence ratio that we measure (hereafter named “GA signaling”; Fig. 2a; see also Supplementary Methods). This quantitative approach confirmed statistically significant changes in GA signaling in hypocotyls, with an increase and decrease respectively upon GA and Pac treatments compared to untreated seedlings (Fig. 2g, h and Supplementary Fig. 4a–d). Exogenous GA and Pac treatments induced similar responses in the SAM, although the effect of Pac was less pronounced than in hypocotyl (Supplementary Fig. 5). Furthermore, GA signaling activity increased in the SAM with both exogenous GA concentration and treatment duration (Supplementary Fig. 4e–i), showing that qmRGA is suitable to be used as a GA signaling sensor in the SAM as in outer cell layers of hypocotyls and root tips (Supplementary Fig. 3a, b).
Finally, we asked whether qmRGA is able to report changes in endogenous GA levels using growing hypocotyls. We previously showed that nitrate promotes growth by increasing GA synthesis and in turn DELLA degradation34. Accordingly, we observed that hypocotyl length of pUBQ10::qmRGA seedlings grown on adequate nitrate supply (10 mM NO3−) was significantly longer compared to those grown on nitrate-deficient conditions (Supplementary Fig. 6a). Consistent with the growth response, GA signaling was higher in hypocotyls of seedlings grown with 10 mM NO3− compared with those grown in absence of nitrate (Supplementary Fig. 6b, c). Thus, qmRGA also allows monitoring changes in GA signaling resulting from endogenous changes in GA concentration.
qmRGA fluorescence depends on GA receptor activity
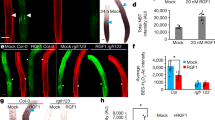
To ask if the GA signaling activity reported by qmRGA depends on both GA concentration and GA perception as expected from the sensor design, we analyzed the expression of the three GID1 receptors in vegetative and reproductive tissues. In seedlings, GID1-GUS reporter lines showed that GID1a and c are highly expressed in cotyledons (Fig. 3a–c). Moreover, all three receptors are expressed in leaves, lateral root primordia, root tip (excluding root cap for GID1b), and vasculature (Fig. 3a–c). In inflorescence SAM, we only detected a GUS signal for GID1b and 1c (Supplementary Fig. 7a–c). In situ hybridization confirmed these expression patterns and further demonstrated that GID1c is homogenously expressed at a low level in the SAM while GID1b shows higher expression at the SAM periphery (Supplementary Fig. 7d–l). A pGID1b::2xmTQ2-GID1b translational fusion further revealed a graded GID1b expression ranging from low or no expression in the SAM center to high expression in organ boundaries (Supplementary Fig. 7m). Thus, GID1 receptors are unevenly distributed across and within tissues. In a subsequent experiment, we also observed that overexpressing GID1 (pUBQ10::GID1a-mCherry) enhanced qmRGA sensitivity to external GA application in hypocotyls (Fig. 3d, e). By contrast, the fluorescence measured from qd17mRGA in hypocotyls was insensitive to GA3 treatment (Fig. 3f, g). For these two assays, seedlings were treated with a high concentration of GA (100 µM GA3) in order to assess the fast behavior of the sensor when the capacity to bind the GID1 receptors is enhanced or lost. Taken together, these results confirm that the qmRGA biosensor reports for the combinatorial action of GA and GA perception and suggest that differential expression of GID1 receptors notably can modulate the emission ratio of the sensor.
a–c Expression pattern of GID1a, GID1b and GID1c in shoots (upper panel) and root tips (lower panel) of 7-day-old pGID1a::GID1a-GUS (a), pGID1b::GID1b-GUS (b) and pGID1c:: GID1c-GUS (c) seedlings. d, f Overlay of VENUS and TagBFP maximum intensity projection (upper row), and corresponding heatmap representation of GA signaling activity (lower row) in hypocotyls of 5-day-old pUBQ10::qmRGA and pUBQ10::qmRGA pUBQ10::GID1a-mCherry seedlings treated with 100 μM GA3 for 30, 9,0 and 180 min (d), and of pUBQ10::qmRGA and pUBQ10::qd17mRGA seedlings treated with 100 μM GA3 for 1 and 2 h (f) and mock controls. e Boxplot representation of GA signaling activity (Mock, n = 65 nuclei examined over three independent seedlings; 100 µM GA3 for 30 min, n = 163 nuclei examined over four independent seedlings; 90 min, n = 129 nuclei examined over three independent seedlings; 180 min, n = 142 nuclei examined over three independent seedlings) measured in pUBQ10::qmRGA seedling hypocotyls, and (Mock, n = 150 nuclei examined over four independent seedlings; 100 µM GA3 for 30 min, n = 182 nuclei examined over four independent seedlings; 90 min, n = 251 nuclei examined over five independent seedlings; 180 min, n = 101 nuclei examined over three independent seedlings) measured in pUBQ10::qmRGA pUBQ10::qmRGA pUBQ10::GID1a-mCherry seedling hypocotyls grown in the same conditions as in (d), indicated by different colors. Center lines show the medians and box limits indicate the 25th and 75th percentiles. Whiskers indicate minima and maxima as determined using the R software. P-values are from Kruskal–Wallis tests. The experiment was repeated three times with similar results. g Boxplot representation of GA signaling activity (Mock, n = 332 nuclei examined over eight independent seedlings; 100 µM GA3 for 1 h, n = 329 nuclei examined over seven independent seedlings; 2 h, n = 343 nuclei examined over seven independent seedlings) measured in pUBQ10::qmRGA seedling hypocotyls, and (Mock, n = 293 nuclei examined over nine independent seedlings; 100 µM GA3 for 1 h, n = 421 nuclei examined over ten independent seedlings; 2 h, n = 343 nuclei examined over ten independent seedlings) measured in pUBQ10::qd17mRGA seedling hypocotyls grown in the same conditions as in (f), indicated by different colors. Center lines show the medians and box limits indicate the 25th and 75th percentiles. Whiskers indicate minima and maxima as determined using the R software. P-values are from Kruskal–Wallis tests. The experiment was repeated twice with similar results. Scale bars = 100 µm.
A GA signaling map in the shoot apical meristem
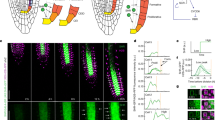
The distribution of GA signaling within the SAM has remained elusive so far. Thus, we used plants expressing qmRGA together with the pCLV3::mCherry-NLS35 stem cell reporter to compute a high-resolution quantitative map of GA signaling activity, focusing on the L1 layer (epidermis; Fig. 4a, b, see Methods and Supplementary Methods) given the key role of L1 in controlling growth at the SAM36. Here, the expression of pCLV3::mCherry-NLS provided a fixed geometric reference for analyzing the spatiotemporal distribution of GA signaling activity37. Although GAs were proposed to be required for lateral organ development4, we observed that GA signaling was lower in flower primordia (P) from the P3 stage onward (Fig. 4a, b), while young P1 and P2 primordia had intermediate activity similar to the one found in the central zone (Fig. 4a, b). Higher GA signaling activity was found in the boundaries of organ primordia, starting from P1/P2 (on the lateral sides of the boundary) and culminating from P4, and in all peripheral zone cells located between primordia (Fig. 4a, b and Supplementary Fig. 8a, b). This higher GA signaling activity was not only monitored in the epidermis but also in the L2 and upper L3 layers (Supplementary Fig. 8b). The GA signaling pattern detected with qmRGA in the SAM was also consistent over time (Supplementary Fig. 8c–f, k). While the qd17mRGA construct was systematically silenced in the SAM of T3 plants in 5 independent lines we characterized in depth, we could analyze the fluorescence pattern obtained with pRPS5a::VENUS-2A-TagBFP construct (Supplementary Fig. 8g–j, l). Only small variations in fluorescence ratio were detected in the SAM from this control line, except at the center of the SAM where we robustly observed an unexpected decrease in VENUS related to TagBFP. This confirms that the signaling pattern observed with qmRGA reflects GA-dependent degradation of mRGA-VENUS but also that qmRGA might overestimate GA signaling activity at the center of the meristem. Taken together, our results reveal a GA signaling pattern mostly mirroring primordia distribution. This inter-primordia-region (IPR) distribution results from the progressive establishment of a high GA signaling activity between developing primordia and the central zone, while in parallel GA signaling activity decreases in primordia (Fig. 4c, d).
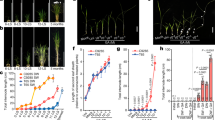
a Maximum intensity projection of three overlay channels showing the expression of pRPS5a::qmRGA (yellow for VENUS channel and blue for TagBFP channel) and pCLV3::mCherry-NLS (magenta) in the SAM. CZ, central zone; P, primordium. The subprint of P indicates the order of primordia with P1 being the youngest emerged primordium. b–e Maximum intensity projection of two overlay channels (b) showing the ratio expression of pRPS5a::qmRGA (yellow for VENUS channel and blue for TagBFP channel) and of three individual channels (c–e) showing the expression of mRGA-VENUS (c), TagBFP-NLS (d) and pCLV3::mCherry-NLS (e) in the SAM, separately. The experiment was repeated twice with similar results. f Heatmap representation of L1 GA signaling activity averaged from seven SAMs aligned using the CLV3 domain as a reference (see supplemental methods for more details). g, h Quantification of GA signaling activity in central zone (CZ, n = 699 nuclei examined over seven independent SAM samples), inter-primordia region (IPR, n = 1504 nuclei examined over seven independent SAM samples), and primordia (P, n = 1381 nuclei examined over seven independent SAM samples) indicated by different colors as shown in (g). P-values are from one-way ANOVA. i 3D visualization of nlsGPS1 emission ratio in the SAM. Primordia stages are estimated according to morphology. Arrows highlight a higher nlsGPS emission ratio in the boundaries and IPR. The experiment was repeated twice with similar results. j, k Fluorescence distribution in wild-type (Ler) SAMs treated with fluorescein (Fl, (j)) and GA4-Fl (k). l Comparison of the ratio of average fluorescence intensity in the IPR to that in the non-IPR (excluding primordia) between Fl (n = 4 independent plants) and GA4-Fl (n = 5 independent plants) treatment in the SAM. The P-value is from one-way ANOVA with Turkey’s test for multiple comparisons of means. The experiment was repeated twice with similar results. The center lines of boxes in (h, l) show the medians and box limits indicate the 25th and 75th percentiles. Whiskers indicate minima and maxima as determined using the R software. Scale bars = 20 μm.
The distribution of GID1b and GID1c receptors (see above) suggests that differential expression of GA receptors contributes to shaping the GA signaling activity pattern in the SAM. We wondered if the differential accumulation of GA could also be involved. To investigate this possibility, we used the nlsGPS1 GA FRET sensor21. An increased emission ratio was detected in nlsGPS1 SAMs treated with 10 µM GA4+7 for 100 min (Supplementary Fig. 9a–e), indicating that nlsGPS1 responds to changes in GA concentration in the SAM as it does in roots21. The spatial distribution of the nlsGPS1 emission ratio indicates that GA levels are relatively low in the SAM external layers, but it shows that they are elevated in the center of the SAM and in the boundaries (Fig. 4e and Supplementary Fig. 9a, c). This suggests that GAs are also distributed in the SAM with a spatial pattern comparable to the one revealed by qmRGA. As a complementary approach, we also treated SAMs with fluorescent GAs (GA3-, GA4-, GA7-Fl) or with Fl alone as a negative control. The Fl signal was distributed in the whole SAM, including the central zone and primordia, although with lower intensity (Fig. 4j and Supplementary Fig. 10d). In contrast, all the three GA-Fls specifically accumulated in primordia boundaries and, to different degrees, in part of the rest of the IPR, with GA7-Fl accumulating in the largest domain in the IPR (Fig. 4k and Supplementary Fig. 10a, b). Fluorescence intensity quantification demonstrated a higher IPR to non-IPR intensity ratio in the GA-Fl-treated SAMs, compared to Fl-treated SAMs (Fig. 4l and Supplementary Fig. 10c). Taken together, these results suggest that GAs are present at higher levels in the IPR cells closest to organ boundaries. This indicates that the SAM GA signaling activity pattern results from both differential expression of the GA receptors and from the differential accumulation of GA in IPR cells closest to organ boundaries. Our analysis thus identifies an unexpected spatiotemporal GA signaling pattern, with lower activity in the center of the SAM and in primordia, while activity is elevated in the IPR of the peripheral zone.
Correlation analyses suggest a role for GA signaling in cell division plane orientation in the shoot apical meristem
To understand the role of differential GA signaling activity at the SAM, we analyzed the correlation between GA signaling activity, cell expansion, and cell division using time-lapse live imaging of qmRGA pCLV3::mCherry-NLS SAMs. Given the role of GA in growth regulation, a positive correlation was expected with cell expansion parameters. We thus first compared maps of GA signaling activity to those of cell surface growth rate (as a proxy for cell expansion intensity for a given cell and daughter cells if it divides) and growth anisotropy, which measures the directionality of cell expansion (here also for a given cell and daughter cells if it divides; Fig. 5a, b, see Methods and Supplementary Methods). Our cell surface growth intensity map of the SAM was consistent with previous observations38,39, with a minimal growth rate in boundaries and a maximal rate in developing flowers (Fig. 5a). A principal component analysis (PCA) showed an anti-correlation between GA signaling activity and cell surface growth intensity (Fig. 5c). It further showed that the main axis of variability, encompassing GA signaling input and growth intensity, was orthogonal to the direction defined by high expression of CLV3, which argues in favor of excluding cells from the center of the SAM in the rest of the analysis. Spearman correlation analyses confirmed the PCA results (Fig. 5d), suggesting that higher GA signaling in the IPR does not lead to higher cell expansion. However, the correlation analysis demonstrated a mild positive association between GA signaling activity and growth anisotropy (Fig. 5c, d), suggesting that higher GA signaling in the IPR acts on cell growth orientation and possibly cell division plane positioning.
a, b Averaged surface growth (a) and growth anisotropy (b) heat maps (used as proxies for cell expansion intensity and direction, respectively) in the SAM averaged from seven independent plants. c PCA analysis including the following variables: GA signaling, surface growth intensity, surface growth anisotropy, and CLV3 expression. PCA component 1 is mostly associated negatively with surface growth intensity and positively with GA signaling. PCA component 2 is mostly associated positively with surface growth anisotropy and negatively with CLV3 expression. Percentages are the variations explained by each component. d Spearman correlation analysis between GA signaling, surface growth intensity, and surface growth anisotropy at the tissue scale but excluding CZ. The numbers on the right are Spearman’s rho values between two variables. Stars indicate when the correlation/anti-correlation is highly significant. e 3D visualization of Col-0 SAM L1 cells using confocal microscopy. New cell walls formed in the SAM (but not primordia) in 10 h are colored according to their angle values. The color bar is shown in the bottom-right corner. The insert shows the corresponding 3D image at 0 h. The experiment was repeated twice with similar results. f Boxplot representation of cell division frequency in the IPR and non-IPR of Col-0 SAM (n = 10 independent plants). Center lines show the medians and box limits indicate the 25th and 75th percentiles. Whiskers indicate minima and maxima as determined using the R software. P-value is from two-sided Welch’s t-test. g, h schematic diagram showing (g) how the angle of a new cell wall (magenta) relative to the radial direction from the center of the SAM (white dotted line) was measured (only acute angle values, i.e., 0–90°, were considered), and (h) the circumferential/transverse and radial orientations within the meristem. i Frequency histograms of division plane orientation of cells from the entire SAM (dark blue), the IPR (medium blue), and non-IPR (light blue), respectively. P-values are from two-sided Kolmogorov–Smirnov tests. The experiment was repeated twice with similar results. j Frequency histograms of division plane orientation of IPR cells around P3 (light green), P4 (mediate green), and P5 (dark green), respectively. P-values are from two-sided Kolmogorov–Smirnov tests. The experiment was repeated twice with similar results.
Thus, we next studied the correlation between GA signaling and cell division activity, by identifying newly formed cell walls during a time-course analysis (Fig. 5e). This methodology allows us to measure both the frequency and orientation of cell divisions. Strikingly, we found that cell division frequency was similar in the IPR and the rest of the SAM (non-IPR, Fig. 5f), showing that differences in GA signaling between IPR and non-IPR cells do not have a major effect on cell division. This, together with the positive correlation between GA signaling and growth anisotropy, led us to ask whether GA signaling activity could act on cell division plane orientation. We measured the orientation of new cell walls as the acute angle relative to the radial axis connecting the center of the meristem to the center of new cell walls (Fig. 5e–i), and observed that cells had a clear tendency to divide at angles closer to 90° relative to the radial axis, with the highest frequency observed at 70–80° (23.28%) and 80–90° (22.62%) (Fig. 5e, i), i.e., corresponding to cell divisions oriented in the circumferential/transverse direction (Fig. 5h). To explore the contribution of GA signaling to this cell division behavior, we analyzed separately the cell division parameters in the IPR and non-IPR (Fig. 5i). We observed that the distribution of cell division angles was different for IPR compared to non-IPR cells or the cells from the entire SAM, with IPR cells showing a higher proportion of transverse/circumferential cell divisions, i.e. 70–80° and 80–90° (the corresponding proportion is 33.86% and 30.71%, respectively) (Fig. 5i). Thus, our observations reveal a link between high GA signaling and cell division plane orientation close to the circumferential direction that parallels the correlation between GA signaling activity and growth anisotropy (Fig. 5c, d). To further establish spatial conservation of this link, we measured the orientation of division planes in IPR cells around primordia starting at stage P3, given that the highest GA signaling activity is detected in this region from stage P4 (Fig. 4). The division angles of the IPR around P3 and P4 did not show statistically significant differences, although an increase in the frequency of transverse cell divisions was observed in the IPR around P4 (Fig. 5j). Differences in cell division plane orientation were however statistically significant in IPR cells around P5, in which the frequency of transverse cell divisions was drastically increased (Fig. 5j). Taken together, these results suggest that GA signaling could control orientation of cell division in the SAM coherently with previous reports40,41, with high GA signaling likely inducing a transverse orientation of cell divisions in the IPR.
GA signaling activity positively regulates transverse cell divisions in the shoot apical meristem
Cells in the IPR are expected not to be incorporated into primordia but rather in the internodes2,42,43. A transverse orientation of cell divisions in the IPR could generate the typical organization in parallel longitudinal cell files of the internode epidermis. Our observations above indicate that GA signaling is likely to act in this process by regulating cell division orientation.
Loss-of-function of multiple DELLA genes leads to constitutive GA response, and thus della mutants could be used to test this hypothesis44. We first analyzed the expression patterns of the five DELLA genes in the SAM. Transcriptional fusion GUS lines45 showed that GAI, RGA, RGL1, and, to a much lesser extent, RGL2 are expressed in the SAM (Supplementary Fig. 11a–d). In situ hybridization further showed that GAI mRNA specifically accumulates in primordia and developing flowers (Supplementary Fig. 11e). RGL1 and RGL3 mRNA were detected throughout the SAM dome and in older flowers, while RGL2 mRNA was more abundant in the boundary regions (Supplementary Fig. 11f–h). Confocal imaging of pRGL3::RGL3-GFP SAM confirmed the expression observed with in situ hybridization and showed that the RGL3 protein accumulates in the central part of the SAM (Supplementary Fig. 11i). Using a pRGA::GFP-RGA line, we also found that the RGA protein accumulates in the SAM, but its abundance is reduced in boundaries starting from P4 (Supplementary Fig. 11j). Notably, the expression patterns of RGL3 and RGA are compatible with a higher GA signaling activity in the IPR, as detected with qmRGA (Fig. 4). In addition, these data indicate that all DELLAs are expressed in the SAM and that collectively, their expressions cover the entire SAM.
We next analyzed the cell division parameters in wild-type (Ler, control) and quintuple (global) gai-t6 rga-t2 rgl1-1 rgl2-1 rgl3-4 della mutant SAMs (Fig. 6a, b). Interestingly, we observed a statistically significant change in frequency distribution of cell division angles in the SAM of global della mutant compared to wild-type (Fig. 6c). This change in the global della mutant resulted from an increase in frequency of 80–90° angles (34.71 % vs 24.55 %) and to a lesser extent of 70–80° angles (23.78 % vs 20.18 %), i.e. corresponding to transverse cell divisions (Fig. 6c). The frequency of non-transverse divisions (0–60°) was also lower in global della mutant (Fig. 6c). The increased occurrence of transverse cell divisions was easily visible in the SAM of global della mutant (Fig. 6b). The frequency of transverse cell divisions in the IPR was also higher in global della mutant compared to wild-type (Fig. 6d). Outside of the IPR region, distribution of the angle of cell division was more homogeneous in wild-type, while in the global della mutant it was skewed toward tangential divisions, as in the IPR (Fig. 6e). We also quantified cell division orientation in the SAM of quintuple ga2-oxidase (ga2ox) mutants (ga2ox1-1, ga2ox2-1, ga2ox3-1, ga2ox4-1, and ga2ox6-2), a GA inactivation mutant background in which GA accumulates. Consistent with an increase in GA levels, the quintuple ga2ox mutant has a bigger inflorescence SAM than Col-0 (Supplementary Fig. 12a, b) and, compared to Col-0, the quintuple ga2ox SAMs showed a significantly different distribution of cell division angles with increases of the frequencies of angles from 50 to 90°, i.e., again skewed toward tangential divisions (Supplementary Fig. 12a–c). We thus show that both constitutive activation of GA signaling and accumulation of GA induce transverse cell division both in the IPR and the rest of the SAM.
a, b 3D visualization of the L1 layer of PI-stained Ler (a) and global della mutant (b) SAM using confocal microscopy. New cell walls formed in the SAM (but not primordia) in 10 h are shown and colored according to their angle values. Inserts show the SAM at 0 h. The color bars are shown in the bottom-right corners. Arrows in (b) highlight examples of aligned cell files in global della mutant. The experiment was repeated twice with similar results. c–e Comparison of the frequency distribution of division plane orientation of cells in the entire SAM (d), IPR (e), and non-IPR (f) between Ler and global della. P-values are from two-sided Kolmogorov–Smirnov tests. f, g 3D visualization of confocal image stacks of PI-stained SAMs of Col-0 (i) and pCUC2::gai-1-VENUS (j) transgenic plants. New cell walls formed in the SAM (but not primordia) in 10 h are shown as in (a, b). The experiment was repeated twice with similar results. h–j Comparison of the frequency distribution of division plane orientation of cells located in the entire SAM (h), IPR (i), and non-IPR (j) between Col-0 and pCUC2::gai-1-VENUS plants. P-values are from two-sided Kolmogorov–Smirnov tests.
We then tested the effect of inhibiting GA signaling specifically in the IPR. To do so, we used the CUP-SHAPED COTYLEDON 2 (CUC2) promoter to drive expression of the dominant negative gai-1 protein fused to VENUS (in a pCUC2::gai-1-VENUS line). CUC2 promoter drives expression in a large part of the IPR (including the boundary cells) in the SAM starting from P4 in wild-type SAMs and a similar specific expression was observed in pCUC2::gai-1-VENUS plants (see below). The distribution of cell division angles in the entire SAM or in the IPR of pCUC2::gai-1-VENUS plants did not show statistically significant differences with respect to the wild-type, although unexpectedly, we found in these plants a higher frequency of 80–90° divisions in non-IPR cells (Fig. 6f–j).
The orientation of cell division has been proposed to be influenced by the geometry of the SAM and notably by tensile stresses prescribed by the curvature of the tissue46. We thus asked whether the SAM shape of the global della mutant and pCUC2::gai-1-VENUS plants was changed. As previously shown12, the size of the global della mutant SAM was bigger than the wild-type (Supplementary Fig. 13a, b, d). CLV3 and STM RNA in situ hybridization confirmed the enlargement of the meristem in della mutants, further showing a lateral enlargement of the stem cell niche (Supplementary Fig. 13e, f, h, i). However, the SAM curvature was identical in the two genotypes (Supplementary Fig. 13k, m, n, p). We observed a comparable increase in size in the quadruple gai-t6 rga-t2 rgl1-1 rgl2-1 della mutant, again without modification of the curvature compared to wild-type (Supplementary Fig. 13c, d, g, j, l, o, p). The frequency of cell division orientation was also affected in the quadruple della mutant, but to a lesser extent than in the global della mutant (Supplementary Fig. 12d–f). This dosage effect, along with the absence of effects on curvature, suggests that the remaining RGL3 activity in quadruple della mutants limits the changes in cell division orientation caused by the loss of DELLA activity and that changes in the occurrence of transverse cell division depend on changes in GA signaling activity rather than in SAM geometry. As mentioned above, the CUC2 promoter drives expression in the IPR in the SAM starting from P4 (Supplementary Fig. 14a, b) and, by contrast, the size of pCUC2::gai-1-VENUS SAMs was reduced, whereas it had a much higher curvature (Supplementary Fig. 14c–h). This change in the morphology of pCUC2::gai-1-VENUS SAMs could generate a different mechanical stress distribution compared to wild-type, whereby high circumferential stress starts at a shorter distance from the SAM centre47. Alternatively, the change in morphology in pCUC2::gai-1-VENUS SAMs could result from changes in regional mechanical properties induced by the expression of the transgene48. In both cases, this could counteract in part the effect of GA signaling changes by increasing the probability of cell division with circumferential/transverse orientation, thus explaining our observations.
Taken together, our data support a positive role for higher GA signaling in the transverse orientation of the cell division plane in the IPR. They further suggest that the curvature of the meristem can also influence the orientation of the cell division plane in the IPR.
High GA signaling initiates internode specification in the shoot apical meristem
Transverse orientation of division planes in the IPR as a result of higher GA signaling activity opens the possibility that GAs pre-organize radial cell files in the epidermis within the SAM to specify the cellular organization later found in the internode epidermis. Indeed, such cell files are often visible in the SAM images of the global della mutant (Fig. 6b). Therefore, to further understand the developmental function of the GA signaling spatial pattern in the SAM, we used time-lapse imaging to analyze the cell spatial organization in the IPR in wild-type (Ler and Col-0), global della mutant and pCUC2::gai-1-VENUS transgenic plants.
We have seen that qmRGA shows that GA signaling activity in the IPR increases from P1/P2 and peaks from P4, a pattern that is consistent over time (Fig. 4a–f and Supplementary Fig. 8c–f, k). To analyze cell spatial organization when GA signaling increases in the IPR, we thus marked Ler IPR cells above and on the side of P4 according to their developmental fates analyzed 34 h after the first observation, i.e., more than two plastochrons and thus allowing to follow IPR cells during primordia development, from P1/P2 to P4. We used three different colors: yellow for those incorporated into the primordia in the vicinity of P4, green for those located in the IPR, and magenta for those contributing to both (Fig. 7a–c). At t0 (0 h), 1–2 layers of IPR cells were visible in front of P4 (Fig. 7a). As expected, when these cells divide, they mostly do it with a transverse division plane (Fig. 7a–c). Similar results were obtained with Col-0 SAMs (focusing on P3 that had a comparable folding at the boundary than P4 in Ler), although in this genotype the formation of the crease at the flower boundary hides the IPR cells more rapidly (Fig. 7g–i). Thus, the division pattern of IPR cells indeed pre-organizes cells in radial files as in the internode. The organization in radial files and the localization of IPR cells in between successive organs suggest that these cells are internode precursors.
a–l Time-lapse (0 h, 24 h, 34 h) visualization of the L1 of the SAM from confocal microscopy of Ler ((a–c) n = 4 independent plants), global della ((d–f) n = 5 independent plants), Col-0 ((g–i) n = 3 independent plants) and pCUC2::gai-1-VENUS transgenic ((j–l) n = 4 independent plants). The imaging time is indicated at the right-up corner of each panel. Cells outlined with yellow dotted lines are those incorporated into a primordium at 34 h, and cells in green are found in the IPR at 34 h. Those in magenta produce both primordium and IPR cells. White dotted lines mark the edge of the elder primordium (that have been removed at 34 h except in (c)). m–p Scanning electron microscopy images of the shoot apex of Ler (m), global della (n), Col-0 (o), and pCUC2::gai-1-VENUS (p) allowing to analyze the early establishment of internode just below the SAM. The different size of arrows indicates differences in early internode length in the different genetic backgrounds. The experiment was repeated twice with similar results. Scale bars = 20 μm (a–l), 50 μm (m–p).
Compared to Ler, 1-2 extra layers of IPR cells were observed in front of P4 at t0 (0 h) in the SAM of global della mutants. These cells divided several times in 34 h (Fig. 7d–f, compared to 7a–c), and in consequence, the mostly transverse divisions of IPR cells led to a higher population of cells organized in radial cell files (Fig. 7d–f, compared to 7a–c). This indicates that the higher GA signaling activity in global della mutant SAMs promotes internode specification. We conducted a similar analysis in pCUC2::gai-1-VENUS plants. Since expression of this transgene causes changes in SAM geometry (Supplementary Fig. 12m, n, p, q), we analyzed IPR cells above the first primordium showing a comparable folding at the boundary as Col-0 P3. In these plants, opposite to global della mutants, much fewer cell divisions occurred in the IPR and there was no clear sign of an organization in radial cell files (Fig. 7j–l), thus showing that inhibition of GA signaling in the IPR perturbs the specification of the cellular organization of internodes in the SAM. In line with these results, we were able to detect the appearance of internodes in the global della mutant just below the SAM using electron microscopy, while flowers remained compacted in Ler (Fig. 7m, n). By contrast, organs were much more compacted in the SAM of pCUC2::gai-1-VENUS than in Col-0 (Fig. 7o, p), consistent with the taller and shorter inflorescence stem of the global della mutant44,49 and pCUC2::gai-1-VENUS plants, respectively (Supplementary Fig. 15). Our results thus support the hypothesis that higher GA signaling activity in the IPR specifies the cellular organization of internodes in the SAM, through a regulation of the orientation of cell division planes (Supplementary Fig. 16).
Discussion
Here, we developed a ratiometric GA signaling biosensor, qmRGA, that provides information on GA function at the cellular level by allowing quantitative mapping of GA signaling activity that results from the combinatorial action of GA and GA receptors concentrations, with minimal interference with the endogenous signaling pathway. To this end, we have engineered a modified DELLA protein, mRGA, that has lost its capacity to bind DELLA-interacting partners but that remains sensitive to GA-induced proteolysis. qmRGA responds to both exogenous and endogenous changes in GA levels and its dynamic sensing properties allow the assessment of spatiotemporal changes in GA signaling activity during developmental processes. qmRGA is also a highly flexible tool as it can be adapted to a variety of tissues simply by changing, if needed, the promoter used for its expression, and is very likely transferable to other species given the conserved nature of the GA signaling pathway and of the PFYRE motif in angiosperms22. In line with this, the equivalent mutation in the rice DELLA protein SLR1 (HYY497AAA) has also been shown to inhibit SLR1 growth-repressing activity while only slightly reducing its GA-mediated degradation, which is similar to mRGA23. Noteworthy, recent work in Arabidopsis reports that a single amino acid mutation in the PFYRE domain (S474L) alters the transcriptional activity of RGA, without interfering with its ability to interact with transcription factor partners50. Although this mutation is in close proximity to the 3 amino acid substitutions present in mRGA, our work shows that the two mutations alter different characteristics in DELLAs. Despite that most of the transcription factor partners bind to the LHR1 and SAW domains of the DELLAs26,51, it is possible that some conserved amino acids in the PFYRE domain contribute to stabilizing these interactions.
Internode development is a key trait for plant architecture and crop improvement. qmRGA revealed a higher GA signaling activity specifically in cells of the IPR, which are the precursors of internodes. By combining quantitative image analysis and genetics, we show that the GA signaling pattern imposes circumferential/transverse cell division planes in the SAM epidermis, shaping the cell division organization required for internode development. Few developmental regulators of the orientation of the cell division plane during development have been identified52,53. Our work provides a striking example where GA signaling activity regulates this cellular parameter. DELLA can interact with the prefoldin complex41 and GA signaling could thus regulate the orientation of the cell division plane through a direct effect on cortical microtubule orientation40,41,54,55. The fact that we show that unexpectedly not cell elongation nor cell division but only growth anisotropy correlates in the SAM with higher GA signaling activity is coherent with a direct effect of GAs on cell division orientation in the IPR. However, we cannot eliminate the possibility that this effect could also be indirect, e.g. mediated by GA-induced softening of the cell wall56. Changes in cell wall properties induce mechanical stress57,58 that could also affect cell division plane orientation by acting on cortical microtubule orientation39,46,59. A combined effect of GA-induced mechanical stress and direct regulation by GA of microtubule orientation could then participate in creating the specific pattern of cell division orientation in the IPR to specify the internode and further work is needed to test this idea. Likewise, previous works have highlighted the importance of the DELLA-interacting proteins TCP14 and 15 in the control of internode patterning60,61 and these factors could convey GA action, together with BREVIPEDICELLUS (BP) and PENNYWISE (PNY), which regulate internode development and have been shown to affect GA signaling2,62. Given the fact that DELLA interacts with the signaling pathways of brassinosteroids, ethylene, jasmonic acid, and ABA63,64 and that these hormones can influence microtubule orientation65, the effects of GA on cell division orientation could also be mediated together with other hormones.
Early cytological studies showed that both the inner tissues and the peripheral zone of the SAM are required for internode development in Arabidopsis2,42. The fact that GAs positively regulate cell division in the inner tissues12 supports a dual function of GAs in regulating meristem size and internode at the SAM. Patterns of oriented cell division are also highly regulated in the inner SAM tissues, and this regulation is essential to stem growth52. It will be interesting to explore whether GAs also play a role in orienting cell division planes in the inner tissues of the SAM and thus synchronize internode specification and development within the SAM.
Methods
Growth conditions and plant material
Plants were grown on soil or in vitro on 1x Murashige-Skoog (MS) medium (Duchefa) supplemented with 1% sucrose and 1% agar (Sigma) under standard conditions (16 h photoperiod at 22 °C), except for hypocotyl and root growth experiments, for which the seedlings were grown on vertical plates under continuous light and 22 °C. For experiments with nitrate, plants were grown on MS-modified medium without nitrogen (bioWORLD plant media) supplemented with an adequate nitrate concentration (0 or 10 mM KNO3), 0.5 mM NH4-succinate, 1% sucrose and 1% type-A agar (Sigma) under long-day photoperiod.
The mutant and transgenic lines we used and all the plasmids and transgenic lines generated for this paper are listed in Supplementary Table 2. The plasmid construction and plant transformation were conducted as below. RGA cDNA (AGI code AT2G01570) and RGAm1, RGAm2, RGAm3, and RGAm4 mutant variants were obtained by PCR using specific primers numbered 1 to 8 in Supplementary Table 3, and inserted into pDONR221 (Thermo Fisher Scientific) by Gateway cloning and recombined with pB7WGF266 to generate p35S::RGA-GFP and p35S::RGAm1/m2/m3/m4-GFP. To generate pRPS5a::qRGA, pUBQ10:qRGA, pRPS5a::qmRGA, pUBQ10:qmRGA, pRPS5a::RGAm1/m3/m4-VENUS-2A-TagBFP, pRPS5a::VENUS-2A-TagBFP, pUBQ10::RGAm1/m3/m4-VENUS-2A-TagBFP, and pCUC2::gai-1-VENUS, the promoter region of pRPS5a (1.7 kb fragment), pUBQ10 (2.5 kb fragment) or pCUC2 (3.2 kb fragment) inserted into pDONR P4-P1R (Thermo Fisher Scientific), RGA-VENUS, mRGA-VENUS, RGAm1/m3/m4-VENUS, VENUS-N7 (for pRPS5a::VENUS-2A-TagBFP) or gai-1 cDNA inserted into pDONR221, and 2A-TagBFP-SV40nls or VENUS (for pCUC2::gai-1-VENUS) inserted into pDONR P2R-P3 (Thermo Fisher Scientific), were recombined into pB7m34GW66.
d17RGA (RGA deleted of the 17 amino acids DELLAVLGYKVRSSEMA composing the DELLA domain33) and d17mRGA mutant variant obtained by PCR using primers 1, 2, 5, and 6 (Supplementary Table 3) were inserted into pDONR221 and recombined into pB7m34GW with p35S (inserted into pDONR P4-P1R) and VENUS (inserted into pDONR P2R-P3) to generate p35S::d17RGA-VENUS and p35S::d17mRGA-VENUS. d17RGA-VENUS and d17mRGA-VENUS were then amplified by PCR using primers 1 and 12, inserted into pDONR221 and recombined as previously with pRPS5a or pUBQ10 and 2A-TagBFP- SV40nls to generate pRPS5a::qd17RGA, pUBQ10:qd17RGA, pRPS5a::qd17mRGA, pUBQ10:qd17mRGA.
To obtain pUBQ10::GID1a-mCherry, GID1a cDNA inserted into pDONR221 was recombined with pDONR P4-P1R-pUBQ10 and pDONR P2R-P3-mCherry into pB7m34GW. p35S:IDD2-RFP was obtained by recombining IDD2 cDNA inserted in pDONR221 into pB7RWG266. To get pGID1b::2xmTQ2-GID1b, a 3.9-kb fragment upstream of the coding region of GID1b and a 4.7-kb fragment including GID1b cDNA (1.3 kb) and terminator (3.4 kb) were first amplified using primers in Supplementary Table 3, then inserted into pDONR P4-P1R (Thermo Fisher Scientific) and pDONR P2R-P3 (Thermo Fisher Scientific), respectively, and finally recombined into pGreen 012567 destination vector with pDONR221 2xmTQ268 by Gateway cloning. To make pCUC2::LSSmOrange, the promoter sequence of CUC2 (3229 bp upstream the ATG), followed by the coding sequence for the large stokes shift mOrange (LSSmOrange)69 with an N7 nuclear localization signal, and the NOS transcription terminator, was assembled into the pGreen Kanamycin destination vector using 3 fragments Gateway recombination system (Invitrogen). Plant binary vectors were inserted into Agrobacterium tumefaciens GV3101 strain and respectively introduced into Nicotiana benthamiana leaves by agro-infiltration and Arabidopsis Col-0 by floral dip. pUBQ10::qmRGA pUBQ10::GID1a-mCherry and pCLV3::mCherry-NLS qmRGA were isolated from F3 and F1 progeny of the appropriate crosses, respectively.
Pharmacological treatments
Chemical treatments with GA (GA3, Sigma, or Duchefa) and paclobutrazol (Pac, Duchefa) were performed at the concentrations and time indicated in the figures. For Y2H assays, 100 µM GA3 was added in selective media to promote interaction between RGA and GID1. For RGA and RGAm1/m2/m3/m4 degradation kinetics, N. benthamiana agro-infiltrated leaf discs were incubated with 100 µM GA3 and 100 mM cycloheximide (Sigma) over 300 min. For hypocotyl length measurements and fluorescence analyses of qmRGA hypocotyls and roots, seedlings were grown for 5 days on MS agar medium and then transferred for 4 days on MS agar plates supplemented with 5 µM Pac or 10 µM GA3. For GA3 treatment on qmRGA, GA4+7 treatment on nlsGPS1, and GA-Fl treatment on Ler, dissected shoot apices were cultured into Apex Culture Medium (ACM, 1/2x MS medium (Duchefa), 1% sucrose, 1% agarose, 2 mM MES (Sigma), 1x vitamin solution (myo-inositol 100 mg/L, nicotinic acid 1 mg/L, pyridoxine hydrochloride 1 mg/L, thiamine hydrochloride 10 mg/L, glycine 2 mg/L), 200 nM N6-benzyladenine, pH 5.8) with indicated concentrations of GA/GA-Fl, and also immersed under 200 µL of GA/GA-Fl solution of indicated concentrations for a indicated period of time. For Pac treatment on qmRGA, 50 µM Pac (dissolved in ethanol and diluted in water) was sprayed on the whole inflorescence every two days for a period of 5 days before observation.
Yeast two-hybrid assays
For Y2H assays, both the full-length and the C-terminal part of RGA and RGAm1/m2/m3/m4 (named M5 version, amino acids 199 to 587; the N-terminal part is subject to self-activation in yeast70) inserted into pDONR207 were recombined into pGBKT7 (Clontech) to obtain BD-RGA, BD-RGAm1/m2/m3/m4, BD-M5RGA and BD-M5RGAm1/m2/m3/m4. On the other hand, JAZ1, TCP14, IDD2, BZR1, GID1a, GID1b and GID1c cDNAs inserted into pDONR221 were fused to the activation domain GAL4 (AD) after recombination into pGADT7 (Clontech).
Direct interaction assays were carried out following the Clontech procedures. BD-M5DELLA and AD-JAZ1, AD-TCP14, AD-IDD2, AD-BZR1 and, on the other hand, BD-DELLA (full-length) and AD-GID1 constructs were co-transformed in the yeast strain AH109 and interactions tests were surveyed on selective medium lacking tryptophan, leucine, and histidine. In some cases, the medium was supplemented with 3-amino-1, 2, and 4 triazole (3AT, Sigma) or with GA to promote interaction between DELLA and GID1.
To confirm that the RGAm2 mutation impaired interaction with all RGA protein partners, we used the C-terminal part of RGA and RGAm2 (amino acids 199 to 587), to probe the Arabidopsis transcription factors REGIA + REGULATORS (RR) arrayed library, following the protocol described in Castrillo et al.71 TFs in the RR library were fused to GAL4 activation domain of the pDEST22 vector and independently transformed into the yeast strain YM4271 in 96-well plates. The RGAm2 protein fused to the GAL4 BD in the pDEST32 vector was transformed into the pJ694 yeast strain and used as bait. Replicates of the library were grown overnight on SD-Trp solid media and inoculated together with 100 μL of an overnight RGAm2 culture grown on SD-Leu on microtiter plates containing 100 μL of YPDA per well. Plates were incubated for 2 days at 30 °C for mating, and diploid colonies were selected in new 96-well plates containing 200 μL of SD-Leu/Trp. 5 μL of the diploid cell cultures were lastly tested for protein interaction by placing them on solid SD medium lacking both Leu and Trp (positive growth control), and on SD medium lacking Leu, Trp and His, in the presence of 1 mM 3-aminotriazol (3-AT) (Sigma-Aldrich). Results were expressed in the form of a heat map for the strength of interaction according to the colony growth after five days of incubation at 30 °C.
Co-immunoprecipitation assays
CoIP assays were performed on N. benthamiana agro-infiltrated leaves with p35S::IDD2-RFP, p35S::TCP14-RFP, p35S::RGA-GFP or p35S::mRGA-GFP. Three days after infiltration, total proteins were extracted with the native extraction buffer [Tris-HCl (pH 7.5) 50 mM, glycerol 10%, nonidet P-40 0.1% supplemented with Complete Protease Inhibitors 1X (Roche)], and then incubated for 2 h at 4 °C with 50 µL of anti-GFP antibody conjugated with paramagnetic beads (Miltenyi Biotec, Catalog number 130-091-125). After incubation, samples were loaded onto a magnetic column system (µ columns; Miltenyi Biotec) to recover the immunoprotein complexes. The immunoprecipitated (RGA-GFP and mRGA-GFP) and co-immunoprecipitated (IDD2-RFP and TCP14-RFP) proteins were detected by western-blot with a 2000-fold dilution of anti-GFP (JL8; Clontech, Catalog number 632380) and anti-RFP (6G6 α-Red, Chromotek, Catalog number 51020014AB), respectively.
Immunodetection analyses
Plant materials were ground in the protein extraction buffer (Tris-base 62,5 mM pH6.8; urea 4 M; sodium dodecyl sulfate 3% (p/v); dithiothreitol (DTT); glycerol 10% (v/v), bromophenol blue 0,1% (p/v)), followed by heating at 95 °C for 5 min. After centrifugation at 13000 g for 5 min, total proteins were separated on 8.5% SDS-PAGE gel and transferred to an immobilon-P (PVDF) membrane (Millipore). Membranes are then saturated with blocking buffer (TBS 1x, tween-20 0.1%; milk 5%) and incubated with a 2000-fold dilution of anti-GFP (JL8; Clontech, Catalog number 632380), anti-RFP (6G6 α-Red, Chromotek, Catalog number 51020014AB), anti-RGA (Agrisera, Catalog number AS111630), anti-HA (Sigma, Catalog number H9658, clone HA-7) or anti-actin (Agrisera, Catalog number AS 132640) antibodies, and a 5000-fold dilution of peroxidase-conjugated goat anti-rabbit or mouse IgG (Invitrogen, Catalog number G21040 and G21234, respectively). Signals were detected using the Luminata Forte Western HRP Substrate (Millipore).
Transactivation assays
Transactivation assays were performed using the Dual-Glo Luciferase Assay System (Promega). N. benthamiana leaves were first agro-infiltrated with pTCP::LUC and pBRE::LUC reporter constructs66, p35S::3xHA-VP16-TCP14 or p35S::3xHA-VP16-BZR1 encoding effector proteins66, and p35S::RGA-GFP or p35S::mRGA-GFP. Three days after infiltration, total proteins were extracted in lysis buffer (Promega), then Firefly and control Renilla LUC activities were quantified with FLUOstar Omega luminometer (BMG Labtech) using OMEGA2 software version 5.50 R4. For loading control, protein levels were analyzed by immunodetection.
GUS staining
For GUS expression detection in inflorescence apices, 28-day-old plants grown under long-day (LD) conditions were used. After fixation in 90% cold acetone at room temperature for 20 min, shoot apices were transferred into a GUS staining solution containing 1 mM potassium ferrocyanide, 1 mM potassium ferricyanide, and 1 mg/mL 5-bromo-4-chloro-3-indolyl-ß-D-glucuronide (X-Gluc), vacuum-infiltrated on ice for 15 min, and incubated overnight at 37 °C in dark before washing with ethanol series and microscope detection. For the experiment with seedlings, 7-day-old plantlets grown in vitro under LD conditions were vacuum-infiltrated for 15 min in GUS staining solution containing 2 mM potassium ferrocyanide, 2 mM potassium ferricyanide and 0.25 mg/mL X-Gluc, and incubated at 37 °C for 24 h. Then GUS solution was replaced with ethanol 70% and seedlings were observed using an optical microscope.
In situ hybridizations
RNA in situ hybridizations were performed on shoot apices with ~1 cm stem72 that were collected and immediately fixed in FAA solution (3.7% formaldehyde, 5% acetic acid, 50% ethanol) precooled at 4 °C. After vacuum treatments for 2 × 15 min, the fixative was changed and the samples were incubated overnight. Antisense probes of the cDNA and 3’-UTR of GID1a, GID1b, GID1c, GAI, RGL1, RGL2, and RGL3 were synthesized as described in Rozier et al.73 using primers indicated in Supplementary Table 3. Immunodetection of the digoxigenin-labeled probes was performed using an anti-digoxigenin antibody (3000-fold dilution; Roche, Catalog number: 11 093 274 910), and sections were stained with 5-bromo-4-chloro-3-indoryl phosphate (BCIP, 250-fold dilution)/nitroblue tetrazolium (NBT, 200-fold dilution) solutions.
Microscopy
For confocal microscopy observations of hypocotyl and root, complete seedlings were put on slides, and images were obtained with a Zeiss LSM 780 confocal microscope. For SAM live imaging, dissected SAMs were let to recover overnight after dissection. Confocal images were then taken with a Zeiss LSM 710 or 700 confocal laser scanning microscope equipped with a water-dipping lens (W Plan-Apochromat 40x/1.0 DIC). To observe GFP or GFP with propidium iodide (PI), a laser of 488 nm was used to excite, and the emission for GFP was 500–520 nm (in some cases it was 500–540 nm), and 610–650 nm for PI. For imaging of qmRGA or qmRGA with mCherry and PI, lasers of 514 nm, 405 nm, 561 nm, and 488 nm were used for the excitation of VENUS, TagBFP, mCherry, and PI, respectively, and the corresponding emission wavelengths were 520–560 nm, 430–460 nm, 580–615 nm and 620–660 nm. mTURQUOISE2 (mTQ2) was excited by a 445 nm laser and the emission range was 470–510 nm. For FRET detection of nlsGPS1, an acquisition mode of spectral imaging (λ-scan) with emission wavelength from 461 nm to 597 nm was used with excitation at 458 nm.
For optical microscopy, photographs of plants were taken with a LEICA MZ12 stereoscopic microscope equipped with a ZEISS AxioCam ICc5 camera head or Canon camera.
For scanning electronic microscopy of the fresh shoot apex, a Hirox SH-3000 table-top microscope equipped with Coolstage (–20 °C to –30 °C) was used.
Image processing
Confocal stacks were processed in Fiji (fiji.sc) to get max projection or orthogonal views. Older primordia were also removed in Fiji. For 3D visualization of confocal stacks, we used the Zeiss ZEN2 software (Fig. 7) and a rendering using the VTK library74 (Figs. 5e and 6). In both cases, parameters were adjusted accordingly to show mainly the L1 cells.
Quantification and statistical analysis
Fluorescence quantification
For qmRGA quantification and visualization, images of hypocotyls and roots were analyzed using a Python script that performs nuclei detection and signal quantification in 3D. The details of the algorithms used in the analysis are provided in Supplementary Methods. The quantification of fluorescence ratios in the control lines was performed using the same computational pipeline. A modification was done to this pipeline to be able to compare quantitatively fluorescence ratio distributions between qmRGA and the pRPS5a::VENUS-2A-TagBFP control line (Supplementary Fig. 8). To transform the distributions onto comparable value ranges, we standardized the values of ratios obtained for each image by dividing them by their mean, in order to always have ratio distributions centered on 1.
For nlsGPS1 quantification and visualization, a series of Fiji macros based on a published plugin “3D ImageJ suite” were used75. Briefly, a “seg-auto.ijm” macro was run for the segmentation of nuclei based on the sum of signals from all 14 channels of the spectral imaging. After removing little objects corresponding to signal noise using a “object-screening.ijm” macro, the ratio of DxAm (channel 8) to DxDm (channel 3) of each segmented nucleus was calculated by running a “3D-ratio.ijm”, and from here, the 3D construction of the SAM, with every nucleus showing its ratio value, was also achieved and printed. Statistical analysis was done in R.
SAM image sequence analysis
To analyze time-lapse confocal acquisitions of SAMs and obtain quantitative measures of GA signaling as well as cellular growth parameters, we developed a computational pipeline. It consists of 3D watershed segmentation for cell identification from the PI staining, nuclei detection and qmRGA quantification, temporal registration using the PI image channel, and surface growth estimation based on expert cell lineages. Individual SAM sequences were then aligned in order to perform population-scale statistics. Extensive details on the algorithms used in this pipeline are provided in Supplementary Methods.
Hypocotyl length measurement
To measure hypocotyl length, seedlings were grown in vertical MS medium (Duchefa) with 0.8% (w/v) phytoagar and 1% sucrose for 5 days and transferred to MS, MS supplemented with 10 μM GA3 or 5 μM Pac for 4 days in continuous light at 22 °C. Plates were scanned and hypocotyl length was measured from the images using Fiji.
Cell division orientation quantification
New cell walls were identified by comparing images obtained at 0 h and 10 h, and masked with a manually drawn line in Fiji. Then, a macro was used to skeletonize and then to measure the angles of the drawn cell walls with an expert-defined center of the SAM. Further angle frequency distribution was done in Excel using the Pivot table. Statistical analyses of Kolmogorov–Smirnov tests were performed using an online tool (http://www.physics.csbsju.edu/stats/KS-test.n.plot_form.html) or in R.
Curvature quantification
The MorphoGraphX software was used to quantify the curvature of L1 cells of SAMs of different genetic backgrounds. Statistical analyses were done in R.
Meristem size measurement
To measure meristem size, the SAM radius was determined by drawing a circle that covers I1 and I2 and so that the center of the center is roughly overlapping with the geometrical center of the SAM surface using Fiji. Statistical analysis was done in R.
Synthesis and characterization of GA-fluorescein (GA-Fl)
Extensive details on the synthesis and characterization of GA-fluorescein (GA-Fl)19 are provided in Supplementary Methods.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated and analyzed during this study are either included in this published article (and its supplementary information files) or available for download from Zenodo (https://doi.org/10.5281/zenodo.10934411). Source data are provided in this paper.
Code availability
Quantitative image and geometry analysis algorithms are provided in Python libraries timagetk, cellcomplex, tissue_nukem_3d, and sam_atlas (https://gitlab.inria.fr/mosaic/) made publicly available under the CECILL-C license. The script used to process hypocotyl images is publicly available in the qrga_nuclei_quantification project (https://gitlab.inria.fr/gcerutti/). The pipelines used to analyze SAM image sequences and to produce map visualizations are provided in a separate project (https://gitlab.inria.fr/mosaic/publications/sam_spaghetti) as Python scripts.
References
Hay, A. & Tsiantis, M. KNOX genes: versatile regulators of plant development and diversity. Development 137, 3153–3165 (2010).
Smith, H. M. S. & Hake, S. The interaction of two homeobox genes, BREVIPEDICELLUS and PENNYWISE, regulates internode patterning in the Arabidopsis Inflorescence. Plant Cell 15, 1717–1727 (2003).
Hay, A. et al. The gibberellin pathway mediates KNOTTED1-Type homeobox function in plants with different body plans. Curr. Biol. 12, 1557–1565 (2002).
Jasinski, S. et al. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr. Biol. 15, 1560–1565 (2005).
Shani, E., Yanai, O. & Ori, N. The role of hormones in shoot apical meristem function. Curr. Opin. Plant Biol. 9, 484–489 (2006).
Hedden, P. & Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 34, 740–760 (2015).
Davière, J.-M. & Achard, P. Gibberellin signaling in plants. Development 140, 1147–1151 (2013).
Silverstone, A. L. et al. Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13, 1555–1566 (2001).
Fu, X. et al. Gibberellin-mediated proteasome-dependent degradation of the barley DELLA protein SLN1 repressor. Plant Cell 14, 3191–3200 (2002).
Ueguchi-Tanaka, M. et al. Gibberellin Insensitive dwarf1 encodes a soluble receptor for gibberellin. Nature 437, 693–698 (2005).
Kinoshita, A. et al. Regulation of shoot meristem shape by photoperiodic signaling and phytohormones during floral induction of Arabidopsis. eLife 9, e60661 (2020).
Serrano-Mislata, A. et al. DELLA genes restrict inflorescence meristem function independently of plant height. Nat. Plants 3, 749–754 (2017).
Achard, P. et al. Gibberellin signaling controls cell proliferation rate in Arabidopsis. Curr. Biol. 19, 1188–1193 (2009).
Ubeda-Tomás, S. et al. Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Curr. Biol. 19, 1194–1199 (2009).
Fu, X. & Harberd, N. P. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421, 740–743 (2003).
Achard, P. et al. Integration of plant responses to environmentally activated phytohormonal signals. Science 311, 91–94 (2006).
Gallego-Bartolomé, J. et al. Transcriptional diversification and functional conservation between DELLA proteins in Arabidopsis. Mol. Biol. Evol. 27, 1247–1256 (2010).
Middleton, A. M. et al. Mathematical modeling elucidates the role of transcriptional feedback in gibberellin signaling. Proc. Natl Acad. Sci. USA 109, 7571–7576 (2012).
Shani, E. et al. Gibberellins accumulate in the elongating endodermal cells of Arabidopsis root. Proc. Natl Acad. Sci. USA 110, 4834–4839 (2013).
Tal, I. et al. The Arabidopsis NPF3 protein is a GA transporter. Nat. Commun. 7, 11486 (2016).
Rizza, A., Walia, A., Lanquar, V., Frommer, W. B. & Jones, A. M. In vivo gibberellin gradients visualized in rapidly elongating tissues. Nat. Plants 3, 803–813 (2017).
Wang, F. et al. Biochemical insights on degradation of Arabidopsis DELLA proteins gained from a cell-free assay system. Plant Cell 21, 2378–2390 (2009).
Hirano, K. et al. Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice. Plant Cell 22, 2680–2696 (2010).
Hussain, A., Cao, D. & Peng, J. Identification of conserved tyrosine residues important for gibberellin sensitivity of Arabidopsis RGL2 protein. Planta 226, 475–483 (2007).
Hussain, A., Cao, D., Cheng, H., Wen, Z. & Peng, J. Identification of the conserved serine/threonine residues important for gibberellin-sensitivity of Arabidopsis RGL2 protein. Plant J. 44, 88–99 (2005).
Velde, K. V. D., Ruelens, P., Geuten, K., Rohde, A. & Straeten, D. V. D. Exploiting DELLA signaling in cereals. Trends Plant Sci. 22, 880–893 (2017).
Nagai, T. et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20, 87–90 (2002).
Norris, S. R., Meyer, S. E. & Callis, J. The intron of Arabidopsis thaliana polyubiquitin genes is conserved in location and is a quantitative determinant of chimeric gene expression. Plant Mol. Biol. 21, 895–906 (1993).
Weijers, D. et al. An Arabidopsis minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Dev. Camb. Engl. 128, 4289–4299 (2001).
Tol et al. Enhancement of Arabidopsis growth characteristics using genome interrogation with artificial transcription factors. PLoS ONE 12, e0174236 (2017).
Wend, S. et al. A quantitative ratiometric sensor for time-resolved analysis of auxin dynamics. Sci. Rep. 3, 2052 (2013).
Goedhart, J. et al. Quantitative co-expression of proteins at the single cell level – application to a multimeric FRET sensor. PLoS ONE 6, e27321 (2011).
Dill, A., Jung, H.-S. & Sun, T. The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc. Natl Acad. Sci. USA 98, 14162–14167 (2001).
Camut, L. et al. Nitrate signaling promotes plant growth by upregulating gibberellin biosynthesis and destabilization of DELLA proteins. Curr. Biol. https://doi.org/10.1016/j.cub.2021.09.024 (2021).
Ma, Y. et al. WUSCHEL acts as an auxin response rheostat to maintain apical stem cells in Arabidopsis. Nat. Commun. 10, 5093 (2019).
Savaldi-Goldstein, S., Peto, C. & Chory, J. The epidermis both drives and restricts plant shoot growth. Nature 446, 199–202 (2007).
Galvan-Ampudia, C. S. et al. Temporal integration of auxin information for the regulation of patterning. eLife 9, e55832 (2020).
Uyttewaal, M. et al. Mechanical stress acts via katanin to amplify differences in growth rate between adjacent cells in Arabidopsis. Cell 149, 439–451 (2012).
Burian, A. et al. A correlative microscopy approach relates microtubule behaviour, local organ geometry, and cell growth at the Arabidopsis shoot apical meristem. J. Exp. Bot. 64, 5753–5767 (2013).
Marc, J. & Hackett, W. P. Changes in the pattern of cell arrangement at the surface of the shoot apical meristem in Hedera helix L. following gibberellin treatment. Planta 186, 503–510 (1992).
Locascio, A., Blázquez, M. A. & Alabadí, D. Dynamic regulation of cortical microtubule organization through prefoldin-DELLA interaction. Curr. Biol. 23, 804–809 (2013).
Vaughan, J. G. The morphology and growth of the vegetative and reproductive apices of Arabidopsis thaliana (L.) Heynh., Capsella bursa-pastoris (L.) Medic. and Anagallis arvensis L. J. Linn. Soc. Lond. Bot. 55, 279–301 (1955).
Jackson, D., Veit, B. & Hake, S. Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120, 405–413 (1994).
Cheng, H. et al. Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131, 1055–1064 (2004).
Gallego‐Giraldo, C. et al. Role of the gibberellin receptors GID1 during fruit-set in Arabidopsis. Plant J. 79, 1020–1032 (2014).
Louveaux, M., Julien, J.-D., Mirabet, V., Boudaoud, A. & Hamant, O. Cell division plane orientation based on tensile stress in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 113, E4294–E4303 (2016).
Hamant, O. et al. Developmental patterning by mechanical signals in Arabidopsis. Science 322, 1650–1655 (2008).
Åhl, H., Zhang, Y. & Jönsson, H. High-throughput 3d phenotyping of plant shoot apical meristems from tissue-resolution data. Front. Plant Sci. 13, 827147 (2022).
Kumar, S. V. et al. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484, 242–245 (2012).
Huang, X. et al. The master growth regulator DELLA binding to histone H2A is essential for DELLA-mediated global transcription regulation. Nat. Plants 9, 1291–1305 (2023).
Fukazawa, J. et al. DELLAs function as coactivators of GAI-ASSOCIATED FACTOR1 in regulation of gibberellin homeostasis and signaling in Arabidopsis. Plant Cell 26, 2920–2938 (2014).
Bencivenga, S., Serrano-Mislata, A., Bush, M., Fox, S. & Sablowski, R. Control of oriented tissue growth through repression of organ boundary genes promotes stem morphogenesis. Dev. Cell 39, 198–208 (2016).
Yoshida, S. et al. Genetic control of plant development by overriding a geometric division rule. Dev. Cell 29, 75–87 (2014).
Chakrabortty, B. et al. A plausible microtubule-based mechanism for cell division orientation in plant embryogenesis. Curr. Biol. 28, 3031–3043.e2 (2018).
Wenzel, C. L., Williamson, R. E. & Wasteneys, G. O. Gibberellin-induced changes in growth anisotropy precede gibberellin-dependent changes in cortical microtubule orientation in developing epidermal cells of barley leaves. kinematic and cytological studies on a gibberellin-responsive dwarf mutant, M489. Plant Physiol. 124, 813–822 (2000).
Cao, D., Cheng, H., Wu, W., Soo, H. M. & Peng, J. Gibberellin mobilizes distinct DELLA-dependent transcriptomes to regulate seed germination and floral development in Arabidopsis. Plant Physiol. 142, 509–525 (2006).
Heisler, M. G. et al. Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol. 8, e1000516 (2010).
Bozorg, B., Krupinski, P. & Jönsson, H. Stress and strain provide positional and directional cues in development. PLoS Comput. Biol. 10, e1003410 (2014).
Rasmussen, C. G. & Bellinger, M. An overview of plant division-plane orientation. N. Phytol. 219, 505–512 (2018).
Davière, J.-M. et al. Class I TCP-DELLA interactions in inflorescence shoot apex determine plant heigh. Curr. Biol. 24, 1923–1928 (2014).
Kieffer, M., Master, V., Waites, R. & Davies, B. TCP14 and TCP15 affect internode length and leaf shape in Arabidopsis. Plant J. 68, 147–158 (2011).
Khan, M. et al. Repression of lateral organ boundary genes by PENNYWISE and POUND-FOOLISH is essential for meristem maintenance and flowering in Arabidopsis. Plant Physiol. 169, 2166–2186 (2015).
Ross, J. J., Miraghazadeh, A., Beckett, A. H., Quittenden, L. J. & McAdam, E. L. in Annual Plant Reviews. Vol. 49 229–252 (John Wiley & Sons, Ltd, 2016).
Weiss, D. & Ori, N. Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol. 144, 1240 (2007).
Shibaoka, H. Plant hormone-induced changes in the orientation of cortical microtubules: alterations in the cross-linking between microtubules and the plasma membrane. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45, 527–544 (1994).
Karimi, M., Inzé, D. & Depicker, A. GATEWAYTM vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7, 193–195 (2002).
Galinha, C. et al. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449, 1053–1057 (2007).
Simon, M. L. A. et al. A PtdIns(4)P-driven electrostatic field controls cell membrane identity and signalling in plants. Nat. Plants 2, 1–10 (2016).
Shcherbakova, D. M., Hink, M. A., Joosen, L., Gadella, T. W. J. & Verkhusha, V. V. An orange fluorescent protein with a large stokes shift for single-excitation multicolor FCCS and FRET imaging. J. Am. Chem. Soc. 134, 7913–7923 (2012).
de Lucas, M. et al. A molecular framework for light and gibberellin control of cell elongation. Nature 451, 480–484 (2008).
Castrillo, G. et al. Speeding cis-trans regulation discovery by phylogenomic analyses coupled with screenings of an arrayed library of Arabidopsis transcription factors. PLoS ONE 6, e21524 (2011).
Vernoux, T., Kronenberger, J., Grandjean, O., Laufs, P. & Traas, J. PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Dev. Camb. Engl. 127, 5157–5165 (2000).
Rozier, F., Mirabet, V., Vernoux, T. & Das, P. Analysis of 3D gene expression patterns in plants using whole-mount RNA in situ hybridization. Nat. Protoc. 9, 2464–2475 (2014).
Schroeder, W., Martin, K. & Lorensen, B. The Visualization Toolkit: an Object-oriented Approach to 3D Graphics. (Kitware, 2006).
Ollion, J., Cochennec, J., Loll, F., Escudé, C. & Boudier, T. TANGO: a generic tool for high-throughput 3D image analysis for studying nuclear organization. Bioinformatics 29, 1840–1841 (2013).
Acknowledgements
We thank the colleagues of the Laboratoire Reproduction et Développement des Plantes and the Institut de Biologie Moléculaire des Plantes for fruitful discussions on this work and for sharing material and advice. We thank Mathilde Sirlin-Josserand for her help with graphical representation and statistics in Figs. 1f, g, and 2c, and in Supplementary Fig. 2g. We also thank Miguel Perez-Amador, Jan Lohmann, Yvon Jaillais, Tai-ping Sun, Miguel Blazquez, David Alabadi, and the NASC for seeds and plasmids. We thank the personnel of SFR Biosciences (UMS3444/CNRS, US8/Inserm, ENS de Lyon, UCBL) facility PLATIM, and especially Jacques Brocard, for assistance with microscopy and image analysis. We also thank Claire Lionnet for her help with image analysis. This work was supported by the ANR-16-CE13-0014 (GrowthDynamics) grant to T.V. and P.A.; a European Research Council grant (GAtransport) to R.W.; a Guangdong Laboratory for Lingnan Modern Agriculture Grant NG2021001 to B.S.; a grant from the Israel Science Foundation (grant no. 1057/21) to R.W.
Author information
Authors and Affiliations
Contributions
P.A. and T.V. designed the study and supervised the work; B.S., A.F.-B., P.A., and T.V. designed the experiments. B.S., A.W., and A.M.J. performed the FRET analysis; A.N.-G. and S.P. conducted the large-scale two-hybrid analysis; S.L. and R.W. designed the synthesis strategy and synthesized the GA-Fl fluorescent probes. G.C. developed the pipeline for quantitative image analysis with the help of J.L., B.S., A.F.-B., J.M., and G.C. performed the image analysis. B.S., A.F.-B., C.G.-A., L.J., G.B., E.V., L.S.-A., and J.-M.D. performed all the other experiments; all authors were involved in data analysis; B.S., A.F.-B., P.A., and T.V. wrote the manuscript with inputs from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Keisuke Nagai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, B., Felipo-Benavent, A., Cerutti, G. et al. A quantitative gibberellin signaling biosensor reveals a role for gibberellins in internode specification at the shoot apical meristem. Nat Commun 15, 3895 (2024). https://doi.org/10.1038/s41467-024-48116-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-48116-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.