Abstract
Biological interpretation of metabolomic datasets often ends at a pathway analysis step to find the over-represented metabolic pathways in the list of statistically significant metabolites. However, definitions of biochemical pathways and metabolite coverage vary among different curated databases, leading to missed interpretations. For the lists of genes, transcripts and proteins, Gene Ontology (GO) terms over-presentation analysis has become a standardized approach for biological interpretation. But, GO analysis has not been achieved for metabolomic datasets. We present a new knowledgebase (KB) and the online tool, Gene Ontology Analysis by the Integrated Data Science Laboratory for Metabolomics and Exposomics (IDSL.GOA) to conduct GO over-representation analysis for a metabolite list. The IDSL.GOA KB covers 2393 metabolic GO terms and associated 3144 genes, 1,492 EC annotations, and 2621 metabolites. IDSL.GOA analysis of a case study of older versus young female brain cortex metabolome highlighted 82 GO terms being significantly overrepresented (FDR < 0.05). We showed how IDSL.GOA identified key and relevant GO metabolic processes that were not yet covered in other pathway databases. Overall, we suggest that interpretation of metabolite lists should not be limited to only pathway maps and can also leverage GO terms as well. IDSL.GOA provides a useful tool for this purpose, allowing for a more comprehensive and accurate analysis of metabolite pathway data. IDSL.GOA tool can be accessed at https://goa.idsl.me/.
Similar content being viewed by others
Introduction
Metabolomics enables the simultaneous study of multiple metabolic processes, including pathways, transport, and reactions. Metabolomics assays are diverse and complex in terms of their analytical conditions, but they can generate quantitative and semi-quantitative data for hundreds of endogenous metabolites1. Recently reported datasets can have between 1500 and 2000 named metabolites and several thousand unidentified metabolites1,2. These metabolites originate from overlapping pathways of catabolic and anabolic reactions and can also be biomarkers for metabolic processes3. Environmental, genetic, or biological factors can alter the regulatory, signaling, and enzyme kinetic mechanisms in one or more metabolic pathways and processes, leading to altered levels of related metabolites in cells, tissues or body fluids4,5. For example, aging reprograms carbohydrate and lipid metabolism pathways in the liver6, tobacco smoke exposure alters the nucleotide and reactive oxidative stress species metabolism7, and FADS gene polymorphisms alter the levels of circulating PUFAs8. We can expect to see a continuous growth in the number of named metabolites in metabolomic datasets due to new advances9,10 in analytical techniques and computational methods and resources.
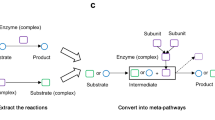
One of the key challenges in utilizing metabolomic datasets is how to interpret these large chemical lists for mechanistic insights11. Pathway and network analysis can provide mechanistic insights into the biological pathways linked to the altered metabolites12. Interestingly, metabolomic datasets often have metabolites that are yet to be connected to a biochemical reaction and pathway13,14. To also include these poorly studied metabolites, hybrid approaches of the atomic mapping of reaction and chemical similarity network (MetaMapp) and enrichment analysis (ChemRICH) can be used13,14. Transcripts and protein lists are also often interpreted using gene ontology (GO) term enrichment analysis15, which covers terms that relate to pathways as well as other biological processes such as cell cycle or apoptosis, or even pathways that are not yet included in other biochemical databases. However, there is not yet a single tool developed that can perform a GO analysis for a metabolite list.
We have developed a new tool named 'IDSL.GOA' (Gene Ontology Analysis by the Integrated Data Science Laboratory for Metabolomics and Exposomics) to perform GO enrichment analysis for a list of metabolites. The tool is supported by a knowledge base of genes, enzymes, and reactants (metabolites) that are directly sources from National Center for Biotechnology Information (NCBI), Expasy and GO consortium databases. We present a case study of an aging mouse metabolic atlas to highlight the metabolic processes that were suggested to be related to the aging process and were only identified by the IDSL.GOA based GO analysis method. The online tool is available at https://goa.idsl.me/ site.
Material and methods
IDSL.GOA knowledgebase
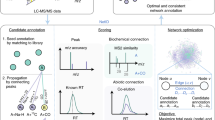
We assembled and integrated information from a diverse set of data sources, including genes, enzymes, compounds, gene ontology terms and the relationships among them. Table 1 provides the web addresses for the publicly available data sources and their respective locations. To focus specifically on metabolism, we restricted our gene selection to those related to GO term GO: 0,008,152 (metabolic process) and linked with the human genome. Only the downstream entities for these metabolic genes were included in the knowledgebase. Utilized identifiers for creating the knowledgebase were—NCBI Gene, NCBI Protein, NCBI Nucleotide, GO Term, Enzyme Commission Number (EC) and InChiKeys. Linkages among these entities were extracted or accessed from the resources listed in Table 1.
Over-representation statistics
For the GO analysis, we employed an overrepresentation analysis (ORA) test using the hypergeometric distribution. This statistical test is a widely accepted method for determining whether a set of molecular entities (gene or proteins or metabolites) is significantly overrepresented in a particular biological pathway or process, given a background database. We also applied filters (1) overlap > = 3, (2) at least three genes in the GO process (3) The set size < 5% of total compounds (4) FDR < 0.05 and (5) the overlap should be > 5% of the total set size for a GO term. The overlap represents how many out of the input InChiKey list are found among the InChiKey identifiers linked with a GO term. Only the first 14 characters of an InChiKey, which represent the two-dimensional structure were used to find the overlap. These filters narrow down the list of GO terms to only the most relevant ones.
We have used “phyper” function in R to compute the hypergeometric test. The parameter for the test were – phyper(x-1,y,a,b, lower.tail = FALSE), where x is the overlap between the input list of InChiKey and compounds linked with a GO term, y is the count of all compounds(2D structures) linked with the GO term, a is the count of all compounds (2D structures) not linked with the GO-term (1,856-y), b is the count of the InChiKey from the input list that were found in the knowledgebase. By default, the phyper function in R calculates the probability of drawing less than or equal to x for a GO term. Use of the parameters “x-1” and “lower.tail = FALSE” returns the probability of drawing more than or equal to x for a GO term. The total number of compounds (2D structures) linked with GO terms was 1856. For example, for the Nucleoside salvage (GO:0,043,174), x was 12, y was 58, b was 73, and a was 1798 (1,856–58) for the test study’s results. The p-value of this GO term was computed as ‘phyper(11,58,1784,73, lower.tail = FALSE)’ which returns 1.158242e-06.
The IDSL.GOA tool uses the False Discovery Rate (FDR) cutoff of 0.05 to control the proportion of false positives in multiple hypothesis testing in GO analysis. We repeated this test for all metabolically relevant 2,392 GO-terms.
Case study and its analysis
Our test study was based on publicly available data from the Aging Mouse Brain Metabolome Atlas1, a comprehensive resource that provides information on the metabolites found in the different regions of brain of aging mice. Specifically, we compared the brain metabolome of the cortex region in an older female mouse against that of a young mouse. To identify the significantly different metabolites, we used the student t-test. We used InChiKey identifiers for the compounds that had a p-value of less than 0.05 in the student t-test.
IDSL.GOA online tool
The online tool was developed using the ReactJS JavaScript framework (https://reactjs.org/), which is known for its efficient rendering of dynamic user interfaces. To facilitate data visualization, we utilized the Google Chart (https://developers.google.com/chart ) and Cytoscape JS plugins (https://github.com/plotly/react-cytoscapejs), specifically designed to work with ReactJS. Cytoscape online version is a lightweight and user-friendly tool that allows users to perform basic network visualization and analysis tasks without the need to install the software locally. For small networks, the online version may be sufficient, but for larger and complex network, it is recommended to download the Cytoscape SIF (Simple Interaction Format) file and use the local version of Cytoscape software to create high resolution graphics. Instructions to use the IDSL.GOA tool are provided on the landing page.
Results
Creating the IDSL.GOA metabolic knowledgebase: To perform IDSL.GOA over-representation analysis, we first needed to create a database of relationships among metabolic entities. This database was designed to capture the heterogenous relationships among genes, enzymes, compounds, and gene ontology terms. The source data for these relationships were obtained from various publicly available key databases, including the NCBI, Expasy – SIB Swiss Institute of Bioinformatics, and the Gene Ontology Consortium (Table 1). We restricted the knowledgebase to only human genes and their products in the first version of the KB. The resulting version 1 of the IDSL.GOA database contained a total of 3,144 genes, 1,492 enzyme commission numbers, 2,621 compounds, 1,856 2D chemical structures and 2,393 gene ontology terms for metabolic processes (Fig. 1). Overall, the IDSL.GOA database provided a comprehensive resource for performing GO over-representation analysis for metabolite lists.
Aging mouse brain metabolomics- a case study
In this study, we aimed to investigate the changes in metabolite levels in the brain cortex of old and young mice using a metabolomic atlas that contained close to 1,547 identified compounds. We identified 557 metabolites that were significantly different between the old (59 weeks) and young (3 weeks) female mouse brain cortex (Table S1). InChiKeys for these significant metabolites were used as input for IDSL.GOA analysis. The GO analysis results suggested a total of 82 GO processes that were over-represented in the input list at an FDR cutoff of 0.05 (Table S2). The GO network and the impact plot visualization suggested that processes in nucleotide and amino acid metabolism (GO:0,043,174, GO:0,046,415 and GO:0,006,166) were significantly affected during the aging process (Figs. 2 and 3, Table S2).
IDSL.GOA impact plot to show the most overrepresented GO terms by their specificity. A small set size shows more specific metabolic processes. For clarity, only the top metabolic processes are labelled but Table S2 has the names for all the significant metabolic processes.
IDSL.GOA online tool
The IDSL.GOA online tool is a user-friendly resource for identifying overrepresented metabolic processes in a list of metabolites. The online interface offers features including analysis, query, explore, statistic and download options. The ‘Run Go Analysis’ option on the landing page allows users to input a list of InChiKeys and obtain results in various formats, including Cytoscape SIF, Microsoft Excel, and CSV. The InChiKeys for only the significant compounds (p < 0.05) in a statistical test should be used as input. The Cytoscape SIF and node attribute files are useful for creating high-resolution figures in the Cytoscape desktop software16. The primary analysis results are visualized in a ‘GO Ontology network’ graph using Cytoscape JS library, which provides an intuitive and interactive way to explore the data. This view is analogous to the pathway ontology visualization in the Reactome database17. The size of the node in the graph reflects the significance of the term, with larger nodes indicating more significant terms in the hypergeometric test. Additionally, an impact plot shows how specific the GO terms are for the input list, by plotting the set size versus -log(p-value). The explore option allows users to navigate the GO ontology tree. Clicking on a GO term in the main analysis, query or explore options provide the GO-term specific InChikeys that overlap with the input list. The query option allows users to query a single compound, reaction, gene, protein and transcript to retrieve the associated metabolic GO terms. All GO network visualization has a basic set of layouts (views) implemented which can be explored by a user to find the most readable and helpful views for a GO ontology network that can aid in the biological interpretation of metabolite lists. Finally, the statistics and download tabs provide updates on the database version and download links, and the landing page offers Instructions for using the database.
Discussion
IDSL.GOA is the first bioinformatics tool that used GO terms for over-representation analysis of metabolomic datasets. By mapping the metabolites to their associated GO terms, IDSL.GOA can improve the mechanistic interpretation of metabolomics data by providing a functional annotation of the metabolites based on their associated metabolic processes and pathways in the Gene Ontology database. It is a more sensitive and accurate tool for data with larger lists (> 1000 named metabolites)1,2. This can lead to the identification of key regulatory pathways and molecular mechanisms that are involved in the observed changes and can guide further experimentation and hypothesis testing. By leveraging the new IDSL.GOA knowledgebase, we were able to identify the overrepresented metabolic pathways and processes in our case study dataset and gain new insights into the underlying mechanisms that govern metabolic activity in aging brain tissue.
Advantages of using GO terms for metabolomics data interpretation
There are several advantages of GO analysis over traditional pathway analysis. GO analysis provides a more comprehensive annotation system for genes and their products than pathway analysis, allowing for a broader range of metabolic processes and pathways to be analyzed18. Unlike pathway analysis, GO analysis is not limited to hand-drawn pathway maps which tend to differ from one database to another, making it more flexible and adaptable to different experimental conditions. Depending on the background pathway database, the interpretation of metabolite lists can differ and may be inaccurate, leading to contradicting results and less impact3. On contrast, GO analysis allows for a more detailed and accurate interpretation of results, as it provides a broader context for the function and regulation of metabolite levels. Because the GO system is standardized, it allows for greater consistency and comparability between different studies and datasets. GO terms not only covers the known pathway maps but also covers additional metabolic processes that are not yet included in the pathway databases.
Key strengths of IDSL.GOA tool
The IDSL.GOA tool is a free, user-friendly and web-based platform that utilizes Gene Ontology (GO) terms for the analysis of metabolomics data. It offers an intuitive interface that allows users to perform GO enrichment analysis for an input metabolite list. The tool has a range of useful features to facilitate the interpretation and has a wide range of capabilities, including query, explore, statistics, and download options. The use of GO terms provides an improved biological interpretation of metabolomics data, which can help researchers identify novel and metabolically relevant pathways and processes. The tool is built on a robust knowledgebase that contains relationships among metabolic entities, obtained from various sources including NCBI, Expasy and the Gene Ontology Consortium databases. The tool allows for a more comprehensive and accurate analysis of metabolomics data by identifying not only the predefined pathways but also relevant metabolic processes that are not included in the commonly used pathway databases. It is the first of its kind tool for metabolomics data.
Future plan
In the follow up work, we plan to improve IDSL.GOA by expanding the underlying database of GO to metabolite relationships. For this, we will curate and map the annotated compounds in the publicly available metabolomic datasets to the enzyme activity annotations and subsequently to the GO terms. There is also a need to harmonize the chemical information across reaction databases and metabolomics reports. Since the mapping between GO terms to genes, proteins and transcripts is available, the future version of IDSL.GOA may also facilitate a multi-omics GO analysis.
Limitations
Few limitations should be noted. The IDSL.GOA tool relies on the availability of InChiKey-linked metabolite data, and the coverage of metabolite curation may vary across different metabolomics laboratories. Not all annotated compounds in the metabolomic datasets have been linked with EC numbers in the biochemical databases. The GO hierarchy and associated annotations may contain biases or inaccuracies due to incomplete or outdated information. There is some redundancy in GO term names which may inflate the over-representation analysis results. The mechanistic interpretation still needs to be validated by additional experimentation. By discussing these limitations, we can provide a more balanced view of the capabilities and potential drawbacks of the IDSL.GOA tool for GO analysis in metabolomics.
Conclusions
In summary, the IDSL.GOA tool can enable a comprehensive and accurate biological interpretation of metabolomics data. A much-needed transition from pathway maps to GO terms for interpreting metabolomic datasets can be supported by the IDSL.GOA tool. It is more sensitive in identifying significantly enriched GO terms that are relevant for metabolic processes. By providing a comprehensive view of the underlying biology, this approach can facilitate the identification of key regulatory pathways and biomarkers that may be useful for diagnosis, prognosis, and therapeutic targeting.
Data availability
The Aging Mouse Metabolome Atlas dataset can be accessed at https://doi.org/https://doi.org/10.21228/M8C68D IDSL.GOA knowledgebase elements and relationships are available at https://zenodo.org/records/10223649. IDSL.GOA tool can be accessed at https://goa.idsl.me/ site and https://github.com/idslme/IDSL.GOA.
References:
Ding, J. et al. A metabolome atlas of the aging mouse brain. Nat. Commun. 12(1), 6021. https://doi.org/10.1038/s41467-021-26310-y (2021).
Byeon, S. K. et al. Development of a multiomics model for identification of predictive biomarkers for COVID-19 severity: A retrospective cohort study. Lancet Digit. Health 4(9), e632–e645. https://doi.org/10.1016/S2589-7500(22)00112-1 (2022).
Wieder, C. et al. Pathway analysis in metabolomics: Recommendations for the use of over-representation analysis. PLoS Comput. Biol. 17(9), e1009105. https://doi.org/10.1371/journal.pcbi.1009105 (2021).
Sarkar, A. et al. Intermittent fasting induces rapid hepatocyte proliferation to restore the hepatostat in the mouse liver. Elife https://doi.org/10.7554/eLife.82311 (2023).
Tanes, C. et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microb. 29(3), 394–407. https://doi.org/10.1016/j.chom.2020.12.012 (2021).
Hunt, N. J., Kang, S. W. S., Lockwood, G. P., Le Couteur, D. G. & Cogger, V. C. Hallmarks of aging in the liver. Comput. Struct. Biotechnol. J. 17, 1151–1161. https://doi.org/10.1016/j.csbj.2019.07.021 (2019).
Yuan, J. M. et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res. 71(21), 6749–6757. https://doi.org/10.1158/0008-5472.CAN-11-0209 (2011).
Surendran, P. et al. Rare and common genetic determinants of metabolic individuality and their effects on human health. Nat. Med. 28(11), 2321–2332. https://doi.org/10.1038/s41591-022-02046-0 (2022).
Vasilopoulou, C. G. et al. Trapped ion mobility spectrometry and PASEF enable in-depth lipidomics from minimal sample amounts. Nat. Commun. 11(1), 331. https://doi.org/10.1038/s41467-019-14044-x (2020).
Koopman, J. & Grimme, S. From QCEIMS to QCxMS: A tool to routinely calculate CID mass spectra using molecular dynamics. J. Am. Soc. Mass Spectrom. 32(7), 1735–1751. https://doi.org/10.1021/jasms.1c00098 (2021).
Barupal, D. K., Fan, S. & Fiehn, O. Integrating bioinformatics approaches for a comprehensive interpretation of metabolomic datasets. Curr. Opin. Biotechnol. 54, 1–9. https://doi.org/10.1016/j.copbio.2018.01.010 (2018).
Lind, L., Fall, T., Arnlov, J., Elmstahl, S. & Sundstrom, J. Large-scale metabolomics and the incidence of cardiovascular disease. J. Am. Heart Assoc. 12(2), e026885. https://doi.org/10.1161/JAHA.122.026885 (2023).
Barupal, D. K. & Fiehn, O. Chemical similarity enrichment analysis (ChemRICH) as alternative to biochemical pathway mapping for metabolomic datasets. Sci. Rep. 7(1), 14567. https://doi.org/10.1038/s41598-017-15231-w (2017).
Barupal, D. K. et al. MetaMapp: Mapping and visualizing metabolomic data by integrating information from biochemical pathways and chemical and mass spectral similarity. BMC Bioinf. 13, 99. https://doi.org/10.1186/1471-2105-13-99 (2012).
Gene Ontology, C. The gene ontology resource: Enriching a GOld mine. Nucl. Acids Res. 49(D1), D325–D334. https://doi.org/10.1093/nar/gkaa1113 (2021).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13(11), 2498–2504. https://doi.org/10.1101/gr.1239303 (2003).
Gillespie, M. et al. The reactome pathway knowledgebase 2022. Nucl. Acids Res. 50(D1), D687–D692. https://doi.org/10.1093/nar/gkab1028 (2022).
Gu, Y. et al. Multi-omics profiling visualizes dynamics of cardiac development and functions. Cell. Rep. 41(13), 111891. https://doi.org/10.1016/j.celrep.2022.111891 (2022).
Funding
NIH (U24ES035386, U2CES026561, R01ES032831, R35ES030435, U2CES026555 P30ES023515, K12ES033594, U2CES030859, UL1TR004419).
Author information
Authors and Affiliations
Contributions
P.M. and D.K.B. developed the database and online interface, prepared the figures and wrote the main manuscript text. O.F. provided the test data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
DKB has been a consultant for the Brightseed Bio, South San Francisco, California. The remaining author has no competing interest to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahajan, P., Fiehn, O. & Barupal, D. IDSL.GOA: gene ontology analysis for interpreting metabolomic datasets. Sci Rep 14, 1299 (2024). https://doi.org/10.1038/s41598-024-51992-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51992-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.