Abstract
Nigeria is the most malaria-endemic country in the world. Vegetation and livestock practices have been linked to malaria transmission but little is known about these in Nigeria. The study aimed to evaluate the influence of vegetation and livestock as predictors of malaria transmission in Nigeria. Secondary data obtained from the Nigerian Demographic and Health Survey’s Geospatial Covariate Datasets Manual were used for the analysis. The survey was carried out successfully in 1389 clusters of thirty (30) households each using a two-stage stratified random sampling design. Hierarchical beta regression models were used to model the associations between malaria incidence, enhanced vegetation index (EVI), and livestock practices. The correlation coefficients for vegetation index and livestock-related variables ranged from − 0.063 to 0.074 and varied significantly with the incidence of malaria in Nigeria (P < 0.001). The model showed vegetation index, livestock goats, and sheep as positive predictors of malaria transmission. Conversely, livestock chicken and pigs were observed to reduce the risk of malaria. The study recommends the need to take into account local differences in transmission when developing malaria early warning systems that utilize environmental and livestock predictors.
Similar content being viewed by others
Introduction
Malaria is a disease with serious public health impacts and has significant implications on the quality of life and the economy1. The global cases of malaria and associated deaths were estimated at 247 million and 619,000 respectively, in 20212. The African Region of the WHO bears a disproportionately large share of the global malaria burden. In 2021, the region had 95% of the malaria cases and 96% of the malaria deaths. The number of malaria deaths in Nigeria in 2021 is estimated at around 200,000, which is 31.9% of the worldwide total. Annually, over 60 million people are infected with malaria, and US$1.1 billion is lost due to malaria-related absenteeism and productivity losses3.
Over the years, malaria intervention programs implemented at both national and global levels have proven successful in reducing malaria transmission within the endemic regions of most sub-Saharan African countries4. While the impact of certain sociocultural and environmental variables in malaria transmission has been evaluated5,6,7, other possible factors such as vegetation index and livestock practices are inadequately addressed in many African countries including Nigeria.
Despite Nigeria being the most populated country in Africa and with rich vegetation diversity and index, few reports have identified vegetation index as a predictor of malaria risk8. As previous studies have shown, vegetation cover can affect malaria transmission and mosquito abundance by providing an outdoor resting place for the vector9. In addition, there is a dart of information on the impact of livestock on malaria transmission in the country. Nigeria is the highest producer of livestock in Africa and livestock contributed significantly to the country’s GDP in the third quarter of the year 202210. The livestock sector is expected to undergo transformation as part of the Economic Recovery and Growth Plan, with the Agricultural Promotion Policy 2016–2020 prioritizing investments in the dairy and poultry sectors to commercialize output and close demand–supply gaps11. However, the long-term consequences of livestock sector change may have a severe influence on public health, environmental, and social outcomes due to some unknown and unpredictable elements defining its development trajectory11.
While transmission of zoonotic diseases is one of the most recorded public health implications of the livestock sector, the possibility of animal-human malarial transmission patterns is hardly emphasized in sub-Saharan African countries. As a result, malaria intervention programs are yet to integrate interventions in animals into the region’s operational implementation policies. Evidence from some malaria-endemic countries such as southern Tanzania, Indonesia, southern Malawi, and southwestern Ethiopia has revealed conflicting outcomes on the influence of livestock on malaria transmission and severity12,13,14,15. Specifically, certain medium-sized livestock such as goats, sheep, dogs, and pigs, and small-sized livestock like poultry have been reported to reduce malaria transmission13,16. There is evidence suggesting that cattle play a role in increasing mosquito density and the transmission of malaria17.
Unfortunately, no study is currently available in this regard in Nigeria despite being the most malaria-endemic country in the world and the highest livestock producer in Africa. Therefore, it becomes difficult to evaluate the contribution of livestock to malaria transmission in the country.
This study evaluated the contributions of vegetation index and livestock to the risk of malaria transmission in Nigeria. This becomes important as the study could provide useful information to support the current national policies on malaria control in Nigeria.
Materials and methods
Study area
The study was conducted in Nigeria. Nigeria is located between latitudes 4° 16′ and 13° 53′ North and longitudes 2° 40′ and 14° 41′ East. The country has a total land area of approximately 923,768 square kilometers and a population density of 212.04 people per square kilometer. Malaria is one of the country's most serious public health issues, and the country's climatic conditions make it ideal for recurrent malaria transmission6. In Nigeria, the common livestock animals are poultry birds, cattle, small ruminants (goats, sheep), pigs, and rabbits, and in some parts of the northern region of the country, donkeys, camels, and horses. The most commonly reared ones are chickens, cattle, goats, and sheep18. In the third quarter of 2022, the contribution of livestock production to Nigeria's GDP experienced an increase of 1.55 percent compared to the same period of the previous year10. Despite the close interaction between humans and livestock, their possible contribution to malaria transmission is yet to be explored in Nigeria.
Data source and sampling procedures
The study relied on the Nigerian Demographic and Health Survey’s Geospatial Covariate Datasets Manual, in which certain variables in the survey were aggregated at the cluster level to facilitate the computation of indices, incidence, and prevalence. Data access was granted upon request for download via the DHS program website, https://dhsprogram.com/. Malaria incidence (MIN), enhanced vegetation index (EVI), and livestock ownership indices for cattle (LSCI), chicken (LSCH), goat (LSGO), sheep (LSSH) and pig (LSPI) were the variables of interest in this study. The dependent variable is MIN, while the other variables are predictors. The survey was carried out successfully in 1389 clusters of thirty (30) households each using a two-stage stratified random sampling design.
Malaria incidence was computed by calculating the average number of people per cluster who had clinical symptoms of Plasmodium falciparum malaria during the survey year. Surface maps of livestock distribution that provide global livestock densities that have been adjusted to match Food and Agriculture Organization (FAO) national estimates for the reference year 2006 were obtained from the 2007 Gridded Livestock of the World (GLW) database to obtain livestock indices19. The surface maps were created using improved and detailed sub-national livestock data (derived from various national census reports and livestock surveys), new and higher resolution predictor variables, and revised modeling methods that included a more systematic evaluation of model accuracy and the representation of uncertainties associated with predictions. Wint and Robinson20 go into more detail about the modeling method, whereas Benjamin et al.21 provided detailed information on the variables in the DHS geospatial covariates datasets manual. These materials can be consulted for additional information.
Models
In this study, the evaluation of different livestock as risk factors for malaria incidence was carried out using a hierarchical Bayesian regression model which adjusted for the contextual and geographical effects in the data. The regression function was formulated as follows:
where \({\propto }_{i}\) are the coefficients of the linear model and \({e}_{i}\) stochastic error associated with the model.
Therefore, the general form of the linear model was obtained through the transformation of the above equations and expressed in the general form given as:
However, the general linear model specification is not appropriate in this case because the response variable is bounded and takes values between 0 and 1. The transformation (3) in a spatial generalized linear mixed model is given as;
The \(g\left(\mu \right)\) is the link function, \(X\beta\) is the linear predictor where \(\varphi\) is the random effect term. The invertible link function, \(g\left(\mu \right)\) is controlled by the statistical distribution of the response variable. As a custom in generalized linear model (GLM), the response variable must have its probability distribution in the exponential family. The dependent variable in the current study is a proportion and so a beta distribution was assigned and consequently logit link function was used. The probability density function and the logit link function are respectively given as;
The symbol \(\varphi\) was introduced to capture spatial variation because the data used in the study are measure at different spatial units (cluster in this case). This is in line with the common belief that measurements in neigbouring cluster have a tendency of having similar values of malaria incidence. The most popular way to represent spatial proximity in lattice data is to construct adjacent matrix in which two areas are defined to be spatially related if they have border that touch on the map and non-neighbour if there is no such relationship, resulting to a \(n\times n\) binary matrix. Several approaches are available in disease mapping to accommodate geographical relationship disease mapping. This study use Leroux global prior, one of the most popular and efficient global priors. With this prior, the spatial dependence effects is the conditional expectation \({\varphi }_{c}\), that is,\(E({\varphi }_{c}/{\varphi }_{-c})\). This is interpreted as the weighted average of the random effects in its adjacent clusters. The full conditional for all set \(C\) random effects produce a unique Gaussian Markov Random Field (GMRF) with a multivariate normal distribution given as;
The precision matrix, \(\sum\) for the Leroux prior is defined as;
The quantity \(G\) and \({\lambda }_{max}\) in Eq. (8) are matrix and eigenvalue, respectively. To ensure correct model specification, the matrix \(G\) is defined as follows;
Substituting Eq. (8) into Eq. (9) and simplifying the resulting expression yields;
The hyper-parameters \({\theta }_{1}={\text{log}}(\tau )\sim logGamma(\mathrm{1,0.001})\) and \({\theta }_{2}=logit(\rho )\sim N(\mathrm{0,0.1})\)
According to Gómez-Rubio22, “the structure of the precision matrix is a convex combination of an identity matrix \({I}_{n}\) (to represent \(i.i.d\) spatial effect) and the precision of an intrinsic CAR \(R\) (to represent a spatial pattern)”.
The model is implemented in R software using fast and accurate integrated nested Laplace approximation (INLA) through R-INLA package23. However, it should be noted that direct implementation of the model was not possible in R-INLA, but was achieved through the “generic1” latent effect with the condition that the first expression in Eq. (9) holds.
Results
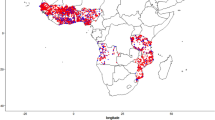
Table 1 shows the descriptive analysis of the variables such as malaria incidence (MIN), enhanced vegetation index (EVI), livestock cattle (LSCA), chicken (LSCH), goat (LSGO), pig (LSPI), and sheep (LSSH) under investigation in this study. The mean values of MIN, EVI, LSCA, LSCH, LSGO, LSPI, and LSSH are presented in Table 1. The correlation coefficients for enhanced vegetation index and livestock-related variables ranged from − 0.063 to 0.074 and varied significantly with the incidence of malaria in Nigeria (P < 0.001) (Table 2). The low-level values of the correlation coefficient between pairs of independent variables indicated the absence of multicollinearity, a serious problem in regression analysis. The posterior means and the 95% credible interval of each covariate and the intercept term is presented in Table 3. The exponentiated quantity can be interpreted as the relative risk of malaria incidence attributed to the specific independent variable. Consequently, an increase of 1 unit in livestock cattle, chicken, and pig is associated with a decrease of 0.03%, 3%, and 0.26% risk of malaria incidence, respectively. Whereas, an increase of 1-unit enhanced vegetation index, livestock goats and sheep are associated with corresponding increases of 85%, 0.1%, and 2% risk of malaria incidence, respectively. The specific relative risk of malaria incidence of each location considered in the study is mapped in Fig. 1. The gray, black, blue, green, and red bubbles are used to depict locations with a very low, low, moderate, high, and very high residual relative risk.
Discussion
Despite considerable resources invested in malaria control programs in Nigeria, their success has been limited24. Between 2017 and 2020, malaria cases increased by 5.3%, with a corresponding 4.7% rise in associated deaths among the at-risk population25. While environmental variables influencing malaria transmission have been somewhat explored in Nigeria, the impact of vegetation index remains poorly understood. To our knowledge, no study has assessed the relationship between livestock and malaria transmission in Nigeria, despite reported associations elsewhere in sub-Saharan Africa12,14,15.
Our study reveals vegetation index as a positive predictor of malaria transmission. This is consistent with findings from Eritrea, Ethiopia, and Uganda26,27,28. In addition to commonly assessed factors like temperature and rainfall, vegetation cover has emerged as a significant factor influencing malaria transmission, potentially by providing resting places for mosquito vectors29. The dense vegetation structure characteristic of tropical rainforests in southern Nigeria may contribute to heightened malaria transmission in the region. Conversely, in regions with limited moisture content, such as some parts of northern Nigeria, vegetation greenness may serve as an indirect indicator of rainfall, facilitating mosquito breeding and increasing malaria transmission risk27. Consequently, the interplay of vegetation and other drivers identified in our previous studies likely contributes to the widespread incidence of malaria across Nigeria's regions6.
The strategy of using environmental measures to mitigate malaria transmission, rooted in the concept of zooprophylaxis—redirecting malaria vectors away from humans—has long been advocated30. However, this approach can inadvertently heighten human exposure to malaria if it increases opportunities for mosquitoes to feed on alternative hosts. By fostering greater numbers of animals near mosquito breeding sites, blood meals become more accessible, potentially attracting more mosquitoes, enhancing their survival, and elevating the risk of disease transmission to humans, a phenomenon termed zoopotentiation12. Regional variations in these concepts have led to conflicting observations.
Our study, employing spatial beta regression models as detailed in Table 3, identifies livestock goats and sheep as positive predictors of malaria transmission in Nigeria, while livestock cattle, chicken, and pigs emerge as negative predictors. This suggests that the concept of zoopotentiation may apply to goats and sheep as malaria risk factors, although the impact of sheep was not statistically significant in this analysis. As previously noted, the presence of these livestock may elevate the relative abundance of mosquitoes carrying Plasmodium species31. The varied host selection by vectors targeting these livestock could heighten the potential for human exposure to Plasmodium. Moreover, keeping animals within or near households can attract more vectors due to the odors and heat they emit12. Furthermore, physical disturbances caused by large livestock such as cattle, such as puddles and hoof prints, may exacerbate zoopotentiation by creating additional larval habitats and thereby increasing adult vector density in close proximity to human residences13.
The significant negative influence of livestock cattle on malaria transmission underscores their potential for zooprophylaxis. The study by Finney et al.32 highlights that cattle and pigs contribute a notably higher proportion of blood meals compared to other livestock. Therefore, the heightened blood meal availability in cattle, pigs, and chickens, identified as having zooprophylactic potential, can reduce the risk of human exposure to malaria, as previously observed in larger livestock like cattle33. The zooprophylactic role of livestock in malaria risk reduction has also been documented in Malawi and Zambia11,34.
Advocacy for using livestock as bait to attract mosquitoes has emerged as a promising alternative to insecticide adoption. However, integrating zooprophylaxis with insecticide-treated livestock becomes more pertinent in areas where zoophilic vectors transmit the malaria parasite31. This approach aids in vector control without exacerbating the issue of mosquito resistance to insecticides35.
In this study, we employed hierarchical beta regression to forecast the impact of livestock on malaria transmission. This choice was driven by the necessity to incorporate the spatial aspect of the data and account for the response variable (malaria incidence), which is a proportion ranging between 0 and 1. Ordinary least squares regression, typically used for continuous responses, would be inappropriate in this context due to the nature of the response variable, resulting in inefficient parameter estimates.
Conclusion
This study examines the influence of vegetation and livestock on malaria transmission. The findings consistently indicate that the vegetation index increases the risk of malaria transmission in Nigeria in the tested model. Certain livestock species, such as goats and sheep, are identified as positive predictors of malaria transmission, whereas livestock like cattle, pigs, and chicken are negative predictors, suggesting their potential for prophylactic use.
The study underscores the importance of considering local variations in transmission patterns when devising malaria early warning systems that integrate environmental and livestock factors. Such tailored approaches can lead to more accurate early warning systems, crucial for effective malaria control. For instance, one potential strategy that can be recommended is insecticide-treated livestock into zooprophylaxis in areas where mosquitoes can feed on both animal and human hosts, thereby enhancing malaria control efforts. One limitation of the study lies in the utilization of secondary data for the findings, which may not fully extrapolate to real-life scenarios due to potential biases or constraints inherent in the dataset. Thus, it's suggested that a randomized controlled trial be conducted to authenticate and substantiate the modeling outcomes of livestock and malaria transmission. This approach would offer a more robust and reliable validation of the study's results, ensuring greater confidence in their applicability to practical settings.
Data availability
All data are included in the manuscript and further queries about sharing data can be directed to the corresponding author.
References
Tambo, E. et al. Scaling up impact of malaria control programmes: A tale of events in Sub-Saharan Africa and People’s Republic of China. Infect. Dis. Poverty. 1, 7 (2012).
World Health Organization (WHO). World Malaria Report. https://www.who.int/publications/i/item/9789240064898. Retrieved February 15, 2024 (2022).
World Malaria Day. Nigeria records 200,000 deaths, loses N646bn yearly to malaria. https://guardian.ng/news/world-malaria-day-nigeria-records-200000-deaths-loses-n646bn-yearly-to-malaria/. Retrieved 28 December 2022 (2022).
World Health Organization (WHO). Malaria control improves for vulnerable in Africa, but global progress off-track. https://www.who.int/news/item/13-12-2016-malaria-control-improves-for-vulnerable-in-africa-but-global-progress-off-track. Retrieved 24 December 2022 (2016).
Yadav, K. et al. Socio-economic determinants for malaria transmission risk in an endemic primary health centre in Assam. India. Infect. Dis. Poverty 3, 19. https://doi.org/10.1186/2049-9957-3-19 (2014).
Okunlola, O. A. & Oyeyemi, O. T. Spatio-temporal analysis of association between incidence of malaria and environmental predictors of malaria transmission in Nigeria. Sci. Rep. 9, 17500. https://doi.org/10.1038/s41598-019-53814-x (2019).
Uushona, S.I., Sheehama, J.A., & Iita, H. Sociocultural factors that influence the prevention of malaria in Ohangwena region, Namibia. Afr. J. Prim. Health Care Fam. Med. 30, 14. https://doi.org/10.4102/phcfm.v14i1.3524 (2022).
Adigun, A. B., Gajere, E. N., Oresanya, O. & Vounatsou, P. Malaria risk in Nigeria: Bayesian geostatistical modelling of 2010 malaria indicator survey data. Malar. J. 14, 156. https://doi.org/10.1186/s12936-015-0683-6 (2015).
Ricotta, E. E., Frese, S. A., Choobwe, C., Louis, T. A. & Shiff, C. J. Evaluating local vegetation cover as a risk factor for malaria transmission: A new analytical approach using ImageJ. Malar. J. 13, 1–7 (2014).
Statista Research Department. Livestock contribution growth to GDP in Nigeria 2019–2022. https://www.statista.com/statistics/1193513/livestock-contribution-growth-to-gdp-in-nigeria/. Retrieved 19 December 2022 (2022).
Food and Agriculture Organizer. Africa Sustainable Livestock 2050. https://www.fao.org/in-action/asl2050/countries/nga/en/. Retrieved December 26, 2022 (2022).
Mayagaya, V. S. et al. The impact of livestock on the abundance, resting behaviour and sporozoite rate of malaria vectors in southern Tanzania. Malar. J. 14, 17. https://doi.org/10.1186/s12936-014-0536-8 (2015).
Hasyim, H. et al. Does livestock protect from malaria or facilitate malaria prevalence? A cross-sectional study in endemic rural areas of Indonesia. Malar. J. 17, 302. https://doi.org/10.1186/s12936-018-2447-6 (2018).
Mburu, M. M. et al. Impact of cattle on the abundance of indoor and outdoor resting malaria vectors in southern Malawi. Malar. J. 20, 353. https://doi.org/10.1186/s12936-021-03885-x (2021).
Chan, K., Cano, J., Massebo, F. & Messenger, L. A. Cattle-related risk factors for malaria in southwest Ethiopia: A cross-sectional study. Malar. J. 21, 179. https://doi.org/10.1186/s12936-022-04202-w (2022).
Loha, E. Association between livestock ownership and malaria incidence in south-central ethiopia: A cohort study. Am. J. Trop. Med. Hyg. 108, 1145–1150. https://doi.org/10.4269/ajtmh.22-0719 (2023).
Tirados, I., Gibson, G., Young, S. & Torr, S. J. Are herders protected by their herds? An experimental analysis of zooprophylaxis against the malaria vector Anopheles arabiensis. Malar. J. 2011(10), 1–8 (2011).
Olajide, E., & Akpan, S. Livestock Production in Nigeria—A thriving Industry - One Health and Development Initiative (OHDI) (http://onehealthdev.org). Retrieved 19 December 2022 (2020).
Robinson, T. P. et al. Mapping the global distribution of livestock. PLoS ONE 9, e96084. https://doi.org/10.1371/journal.pone.0096084 (2014).
Wint, G. R. W. & Robinson, T. P. Gridded Livestock of the World 2007 (FAO, 2007).
Benjamin, M., Fish, T.D., Eitelberg, D., & Dontamsetti, T. The DHS Program Geospatial Covariate Datasets Manual (Second Edition) (ICF, Rockville, Maryland, USA, 2018).
Gómez-Rubio, V. Bayesian Inference with INLA (Chapman & Hall/CRC Press, 2020).
Rue, H., Martino, S., & Chopin, N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J. R. Stat. Soc. Ser. B (Stat. Method.) 71, 319–392 (2009).
Okunlola, O. A., Oyeyemi, O. T. & Lukman, A. F. Modeling the relationship between malaria prevalence and insecticide-treated bed net coverage in Nigeria using a Bayesian spatial generalized linear mixed model with a Leroux prior. Epidemiol. Health 43, e2021041. https://doi.org/10.4178/epih.e2021041 (2021).
World Health Organization - World malaria report. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021. Retrieved 24 December 2022.
Ceccato, P. et al. Malaria stratification, climate, and epidemic early warning in Eritrea. Am. J. Trop. Med. Hyg. 77, 61–68 (2007).
Midekisa, A., Senay, G., Henebry, G. M., Semuniguse, P. & Wimberly, M. C. Remote sensing-based time series models for malaria early warning in the highlands of Ethiopia. Malar. J. 11, 165. https://doi.org/10.1186/1475-2875-11-165 (2012).
Kigozi, R. et al. Assessing temporal associations between environmental factors and malaria morbidity at varying transmission settings in Uganda. Malar. J. 15, 511. https://doi.org/10.1186/s12936-016-1549-2 (2016).
Ricotta, E. E., Frese, S. A., Choobwe, C., Louis, T. A. & Shiff, C. J. Evaluating local vegetation cover as a risk factor for malaria transmission: A new analytical approach using ImageJ. Malar. J. 13, 1–7. https://doi.org/10.1186/1475-2875-13-94 (2014).
Saul, A. Zooprophylaxis or zoopotentiation: the outcome of introducing animals on vector transmission is highly dependent on the mosquito mortality while searching. Malar. J. 2, 32. https://doi.org/10.1186/1475-2875-2-32 (2003).
Franco, A. O., Gomes, M. G., Rowland, M., Coleman, P. G. & Davies, C. R. Controlling malaria using livestock-based interventions: A one health approach. PLoS ONE 9, e101699. https://doi.org/10.1371/journal.pone.0101699 (2014).
Finney, M. & McKenzie, B. A. Widespread zoophagy and detection of Plasmodium spp. in Anopheles mosquitoes in southeastern Madagascar. Malar. J. 20, 25. https://doi.org/10.1186/s12936-020-03539-4 (2021).
Iwashita, H. et al. Push by a net, pull by a cow: can zooprophylaxis enhance the impact of insecticide treated bed nets on malaria control?. Parasit. Vectors 7, 52. https://doi.org/10.1186/1756-3305-7-52 (2014).
Bulterys, P. L., Mharakurwa, S. & Thuma, P. E. Cattle, other domestic animal ownership, and distance between dwelling structures are associated with reduced risk of recurrent Plasmodium falciparum infection in Southern Zambia. Trop. Med. Int. Health. 14, 522–528. https://doi.org/10.1111/j.1365-3156.2009.02270.x (2009).
Kawaguchi, I., Sasaki, A. & Mogi, M. Combining zooprophylaxis and insecticide spraying: A malaria-control strategy limiting the development of insecticide resistance in vector mosquitoes. Proc. Biol. Sci. 2004(271), 301–309. https://doi.org/10.1098/rspb.2003.2575 (2004).
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
O.Oy.: conceptualization. O.Ok., S.O., A.E. & O.Oy.: methodology. O.Ok., S.O. and A.E.: data analysis. O.Ok. and O.Oy.: writing of manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okunlola, O., Oloja, S., Ebiwonjumi, A. et al. Vegetation index and livestock practices as predictors of malaria transmission in Nigeria. Sci Rep 14, 9565 (2024). https://doi.org/10.1038/s41598-024-60385-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60385-z
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.